Abstract
Background
Late embryogenesis abundant (LEA) proteins are a group of highly hydrophilic glycine-rich proteins, which accumulate in the late stage of seed maturation and are associated with many abiotic stresses. However, few peanut LEA genes had been reported, and the research on the number, location, structure, molecular phylogeny and expression of AhLEAs was very limited.
Results
In this study, 126 LEA genes were identified in the peanut genome through genome-wide analysis and were further divided into eight groups. Sequence analysis showed that most of the AhLEAs (85.7%) had no or only one intron. LEA genes were randomly distributed on 20 chromosomes. Compared with tandem duplication, segmental duplication played a more critical role in AhLEAs amplication, and 93 segmental duplication AhLEAs and 5 pairs of tandem duplication genes were identified. Synteny analysis showed that some AhLEAs genes come from a common ancestor, and genome rearrangement and translocation occurred among these genomes. Almost all promoters of LEAs contain ABRE, MYB recognition sites, MYC recognition sites, and ERE cis-acting elements, suggesting that the LEA genes were involved in stress response. Gene transcription analyses revealed that most of the LEAs were expressed in the late stages of peanut embryonic development. LEA3 (AH16G06810.1, AH06G03960.1), and Dehydrin (AH07G18700.1, AH17G19710.1) were highly expressed in roots, stems, leaves and flowers. Moreover, 100 AhLEAs were involved in response to drought, low-temperature, or Al stresses. Some LEAs that were regulated by different abiotic stresses were also regulated by hormones including ABA, brassinolide, ethylene and salicylic acid. Interestingly, AhLEAs that were up-regulated by ethylene and salicylic acid showed obvious subfamily preferences. Furthermore, three AhLEA genes, AhLEA1, AhLEA3-1, and AhLEA3-3, which were up-regulated by drought, low-temperature, or Al stresses was proved to enhance cold and Al tolerance in yeast, and AhLEA3-1 enhanced the drought tolerance in yeast.
Conclusions
AhLEAs are involved in abiotic stress response, and segmental duplication plays an important role in the evolution and amplification of AhLEAs. The genome-wide identification, classification, evolutionary and transcription analyses of the AhLEA gene family provide a foundation for further exploring the LEA genes’ function in response to abiotic stress in peanuts.
Similar content being viewed by others
Background
Plant in nature often encounters various abiotic stresses including drought, cold, high temperature, and salinity, which affect growth and development, reduce its yield and survival rate. Plants have evolved many mechanisms to cope with various environmental stresses. It is known that the late embryogenesis abundant (LEA) proteins play important roles in protecting cells under abiotic stresses, and many LEAs are induced by cold, drought, salinity, abscisic acid (ABA), and ethylene [1–3]. Moreover, it has been confirmed that AdDHN1, a member of the Dehydrin family, can improve the drought resistance of transgenic Arabidopsis, but it is more sensitive to nematodes (Mota et al., 2018), which indicated that some of the LEAs may respond to abiotic stress as well as biotic stress.
LEA proteins are highly hydrophilic glycine-rich proteins, which accumulate largely in the later stage of seed maturation and fade away following germination [4, 5]. As water-binding molecules, the role of LEA proteins is enhancing the stability of protein and membrane. Subcellular localization analysis has indicated that LEA proteins are mainly located in nuclear regions and the cytoplasm [6]. LEA proteins have been observed in the roots, leaves, buds, and seedlings, although they mainly appear in seeds of plants [7].
LEA protein families were identified in many plant species by genome-wide identification and analysis, such as Arabidopsis thaliana [8], Populus trichocarpa [5], Camellia sinensis [9], Brassica napus [10], and Triticum aestivum [11]. During the growth and development of plant, LEA proteins are considered to play important roles. It was reported that Medicago falcate LEA3 conferred multiple abiotic stress tolerance by involving the protection of catalase activity [12]. A heterologous expression of a barley LEA3 protein gene, HVA1, improved tolerance to water stress in rice and wheat [13, 14]. AtLEA5 protects yeast cells against oxidative stress [15]. Escherichia coli can grow in high salt and extreme temperature conditions due to the over-expression of soybean PM2 protein (LEA3) [16, 17]. ABA can regulate the expression of many LEA proteins, and it was proved that the expression of LEA4 subfamily members was upregulated by exogenous ABA [18].
Peanut is one of the main oils and cash crops all over the world. Peanut is a rainfed crop, but it is sensitive to water deficit stress in the flowering and pegging stages, which would impact the yield of peanuts [19]. Also, Al stress inhibition of growth reduces peanut yield in acid soil [20]. To date, the function of the LEA gene family in peanuts has little been reported. In this study, we identified the LEAs in peanut and analyzed the structure, evolution, and chromosome location of peanut LEAs. Our findings provide a foundation for the evolutionary and functional characterization of LEA gene families in peanut and other plant species.
Results
Identification and characteristics of AhLEA gene in peanut
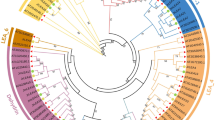
By using the publicly available peanut genome sequence data, the genome-wide identification of LEAs in peanuts based on sequence homology with 51 Arabidopsis LEAs was performed [21] (Table 1 and Additional file 1: Table S1). Proteins that contain a conserved LEA domain were screened by the NCBI-BLAST online tool. Eventually, 126 AhLEAs were identified. All of these genes were grouped with 51 AtLEAs by phylogenetic analyses. The AhLEAs were classified into eight subfamilies including LEA1, LEA2, LEA3, LEA4, LEA5, PvLEA18, SMP, and Dehydrin (Fig. 1). The LEA2 family was the largest, with 78 members. The LEA3s and LEA5s had 14 and 10 members, respectively. The LEA1s had 8 members, SMP had 6 members and PvLEA18 had 4 members. The LEA4 and Dehydrin families had 3 members. The species-specific group (AtM) of Arabidopsis was absent in the peanut.
Chromosomal locations, gene duplication and synteny analysis of the AhLEAs
The identified 126 AhLEAs were further located on the 20 chromosomes (Fig. 2). The largest number of AhLEAs was found on chromosome 12, fourteen genes, followed by chromosome 14 (eleven genes). The lowest loci density was observed on chromosome 8, with only two genes. Eight genes were found located on chromosomes 13 and 15, seven genes on chromosome 6. Five chromosomes (chr1, chr2, chr3, chr4, and chr16) carried six AhLEAs and four chromosomes (chr5, chr7, chr19, and chr20) carried five AhLEAs. Chromosomes 9, 10, and 11 contained four AhLEAs, and chromosomes 17, 18 contained three AhLEAs. The AhLEAs were distributed unevenly among the 20 chromosomes in peanut. All chromosomes contained the LEA2s, and all of the LEAs on chromosomes 3, 8, 10, 13, 18, and 20 belonged to the LEA2s. Chromosomal location analysis of AhLEAs indicated that eight subfamilies were distributed unevenly in the genome (Fig. 2).
The generation and evolution of gene families may be caused by tandem duplication and segmental duplication [22, 23]. To investigate the evolutionary relationships of the AhLEA gene family, we analyzed the duplication events of AhLEAs (Fig. 3). In this study, five pairs of tandem duplication and 93 pairs of segmental duplication were identified (Fig. 3, Additional file 1: Table S2). Five tandem duplication pairs belong to the LEA5s and LEA2s, and were located on chromosomes 12 and 15. The segmental duplication genes were mainly distributed on chromosome 12. All members of the LEA1s, SMPs, and PvLEA18s were segmental duplication genes, followed by LEA2s (79.5%) and LEA3s (71.4%). The Ka/Ks values of all the tandem duplication gene pairs were less than 1. Except for four segmental duplication gene pairs whose Ka/Ks values could not be calculated, the Ka/Ks values of the most segmental duplication gene pairs were less than 1, and only two pairs (2.2%) were more than 1 (Fig. 4, Additional file 1: Table S2). The divergence time of tandem duplication events was mainly 0–10 million years ago (MYA), and 49.5% (46/93) of segmental duplication events occurred between 0–5 MYA (Fig. 5, Additional file 1: Table S2).
To explore the evolutionary process of the peanut LEA genes, we performed synteny analysis among peanut, Arabidopsis, and soybean. AhLEAs showed a more syntenic to soybean than Arabidopsis (Fig. 6, Additional file 1: Table S3). Thirteen orthologous pairs exhibited single gene correspondences between peanut and Arabidopsis, and five orthologous pairs exhibited single gene correspondences peanut and soybean. Five AhLEAs were associated with multiple AtLEAs, and fourteen AhLEAs were associated with GmLEAs. Additionally, there were nine cases that peanut segmental duplications that corresponded to a single Arabidopsis gene, and eleven cases that AhLEAs corresponded to a single soybean gene. Finally, some genes showed more-to-more correspondence.
Analysis of gene structure and protein motifs of LEAs in peanut
To examine the structural characteristics of AhLEAs, an unrooted phylogenetic tree that combines the UTR-CDS structures and motifs were constructed based on the full lengths of the 126 peanut LEA genes sequence by using the Maximum-Likelihood method (Fig. 7). The majority of the AhLEAs contained zero or one intron, with 55 and 53, respectively. Sixteen genes had two introns. One gene, AH19G03360.1, contained three introns, and one gene, AH12G35940.1, contained seven introns. All the LEA1s and Dehydrins contained only one intron, and the main members of the LEA3 and LEA5 subfamilies had one intron. The majority of the LEA2s had no intron. To identify the conserved protein motifs, the MEME (http://meme-suite.org/tools/meme) online software was used to predict putative motifs of these proteins, with a maximum number of the different motifs at 20. Motif analysis indicated that members of each subfamily had the group-specific conserved domain, and AhLEAs with closer evolutionary relationships had more similar motif numbers. MEME analysis revealed that most AhLEAs contained motif 3 and all the LEA4s and LEA1s had motif 13. The LEA2s had the greatest number of motifs, which were 7 motifs, while other subfamily members had 1 to 4 motifs.
Phylogenetic relationships, gene structures, and compositions of the conserved protein motifs of the AhLEAs.I: LEA2; II: Dehydrin; III: LEA3; IV: SMP; V: LEA5; VI: PvLEA18; VII: LEA4; VIII: LEA1; a: Phylogenetic relationships, b: conversed motif, c: UTR–CDS organization, black lines represent intron
Analysis of cis-acting elements in promoters of AhLEAs
To investigate the cis-acting elements of AhLEAs, 2 kb region upstream of the translation initiation sites of all the LEA genes were obtained from the peanut genome database. Many cis-acting regulatory elements that may be involved in the response to environmental stresses in plant, including ABRE, WRE3, ERE, MYB recognition sites, MYC recognition sites, TC-rich repeats, STRE, and MRE, were detected (Fig. 8). The promoter of subfamily LEA2 contained the most cis-acting elements, followed by subfamily LEA3, LEA5, and LEA1. The promoter of subfamily LEA4, SMP, PvLEA18, and Dehydrin contained the least elements. Among the identified cis-acting elements, ABRE (22.2%), ERE (55.6%), MYB recognition sites (65.9%), and MYC recognition sites (70.6%) cis-acting elements were over-represented.
Expression profiles of AhLEAs in different tissues and at different stages of embryo development
To investigate the expression profiles of AhLEAs across different stages of embryo development and different tissues, the transcriptomic data of a cultivated variety (A. hypogaea L.) in gene bank were further scrutinized (http://peanutgr.fafu.edu.cn/Transcriptome.php) (Fig. 9, Additional file 1: Table S4). Not all AhLEAs were expressed at the four embryo development stages. Meanwhile, twenty-seven genes were not detected at any tested stages. Sixty-eight LEAs had different transcription levels among the four stages. In the early embryo development stages, most LEA3s were up-regulated. Among them, three LEA3s (AH01G27080.1, AH01G27080.2, and AH11G30560.1) exhibited very high transcription levels in the early stages, which showed up to tenfold higher than those in the late stages. Nevertheless, AhLEA1s, AhLEA4s, and AhLEA5s were up-regulated mainly in the late stages. Four genes including two LEA5s (AH12G24910.1 and AH12G24920.1) and two LEA1s (AH06G01030.1 and AH16G03650.1) exhibited very high transcription levels in the late stages. Two genes of the Dehydrins expressed at a high level in stages I, and II, while another Dehydrin (AH17G19580.1) expressed at a high level in stages III and IV. The transcription of most AhLEA2s was not changed among the four tested stages, while the transcription level of one LEA2 (AH12G34850.1) in the early stages showed up to 26-fold higher than those in the later stages.
As shown in Fig. 10, the expression profiles of eight subfamilies, including LEA1s, LEA2s, LEA3s, LEA4s LEA5s, SMPs, PvLEA18s, and Dehydrins, were similar in roots, stems, leaves, and flowers. Among them, the members of LEA2s, LEA3s, and Dehydrins were expressed at a high level in all four tissues. Twenty-four LEAs were highly expressed in roots, 21 in stems, 15 in leaves, and 20 in flowers. Two Dehydrins (AH07G18700.1 and AH17G19710.1) and two LEA3s (AH16G06810.1 and AH06G03960.1) had the highest transcription levels in the stem (Additional file 1: Table S5).
Expression profiles of AhLEAs in response to drought and low-temperature stresses.
To investigate the transcriptional changes of the AhLEAs under cold and drought stresses, the expression profiles of these genes were examined by using transcriptomic data (Fig. 11). Under drought treatment, 28.6% (36 out of 126) of the AhLEAs were up-regulated more than twofold compared with the control, while the transcription levels of 21.4% (27 out of 126) genes were down-regulated more than twofold. Among the 27 genes that down-regulated more than twofold, 24 genes belonged to the LEA2 subfamily. Two LEA3s (AH01G27080.1, and AH01G27080.2) showed the highest transcription levels under drought stress (Additional file 1: Table S6).
Under low-temperature treatment, 28.6% (36 out of 126) of the AhLEAs were up-regulated more than twofold compared with the control, while the transcription levels of 14.3% (18 out of 126) genes were down-regulated more than twofold. It was found that 21 genes of LEA2s were up-regulated and 11 genes were down-regulated. It is noteworthy that all Dehydrins were up-regulated under drought and low-temperature stresses. Interestingly, the genes expressed the highest under low-temperature stress were also two LEA3 subfamily genes (AH16G06810.1, AH06G03960.1) (Fig. 11: Additional file 1: Table S6).
Expression profiles of AhLEA genes in response to hormone
To understand the expression changes of the AhLEAs under different hormones, the responses of 126 AhLEAs to four stress-related hormones (abscisic acid, brassinolide, ethylene, and salicylic acid) were investigated (Fig. 12). The expression profiles of these genes were examined by using transcriptomic data. After ABA treatment, 8 LEAs were induced more than twofold, while 19 LEAs were down-regulated more than twofold. After brassinolide treatment, 5 genes were up-regulated more than twofold, while and 31 genes were down-regulated more than twofold. The transcription of 13 AhLEAs was up-regulated more than twofold after ethylene treatment, while 28 genes were down-regulated more than twofold. The transcription of 10 AhLEAs was up-regulated more than twofold after salicylic acid treatment, while 16 genes were down-regulated more than twofold. Although the main AhLEAs were down-regulated by these four hormones, half of the LEA3s (7 out of 14) were up-regulated more than twofold after ethylene treatment, and all members of LEA4s were induced by salicylic acid. Moreover, the transcription of five AhLEAs was up-/down-regulated more than twofold by all four tested hormones. These genes included four LEA2s (AH06G19190.1, AH16G23780.1, AH20G34490.1, and AH16G06810.1) which were down-regulated after hormone treatment and a PvLEA18 (AH11G11350.1) that was up-regulated (Additional file 1: Table S7).
Expression pattern of AhLEAs under Al stress
To gain a broader understanding of the putative functions of peanut LEAs in response to Al stress, the expression profiles of these genes were examined by using the RNA-Seq data which was generated from the root tips of two peanut cultivars that exhibited different Al sensitivity and had already been deposited in NCBI [24]. ZH2 is known as an Al sensitive peanut cultivar and 99–1507 is proved as an Al tolerant peanut cultivar [25]. Here, a total of 50 AhLEAs were found to be aluminum stress-responsive genes (Fig. 13, Additional file 1: Table S8). LEA2s which included twenty-three DEGs had the most aluminum stress-responsive genes. All of the members in LEA4s and Dehydrins were aluminum stress-responsive genes, and both of these two subfamilies were composed of three genes. The aluminum stress-responsive genes accounted for 75% (3 out of 4), 60% (6 out of 10), 50% (3 out of 6, and 4 out of 8), and 35.7% (5 out of 14) of the members in PvLEA18s, LEA5s, SMPs (and LEA1s), and LEA3s, respectively.
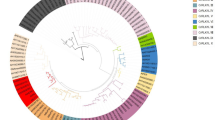
Expression profiles of Al-responsive AhLEAs in two varieties. The blue words represent not detected in the RNA-Seq dataset, and the orange words represent LEA genes were not expressed, the red words represent 50 differentially expressed genes. I: LEA2; II: Dehydrin; III: LEA3; IV: SMP; V: LEA5; VI: PvLEA18; VII: LEA4; VIII: LEA1
Five AhLEAs were significantly up-regulated after 8 h of Al treatment in ZH2. Seventeen AhLEAs were significantly up-regulated after 8 h of Al treatment in 99–1507, including 66% of LEA4s and Dehydrins (2 out of 3) and 50% of SMPs (3 out of 6). Nineteen AhLEAs were significantly up-regulated after 24 h of Al treatment in ZH2, including all members of LEA5s and PvLEA18s and half of LEA1s (4 out of 8) and SMPs (3 out of 6). Nine AhLEAs were significantly up-regulated after 24 h of Al treatment in 99–1507. Twelve AhLEAs were down-regulated after 8 h of Al treatment in ZH2, and all PvLEA18s were down-regulated. Two AhLEAs were down-regulated after 8 h of Al treatment in 99–1507. Seven AhLEAs were down-regulated after 24 h of Al treatment in ZH2. Eight AhLEAs were down-regulated after 24 h of Al treatment in 99–1507.
As ZH2 is a widely used commercial variety, eight differentially expressed LEA genes that were up-regulated greatly in ZH2 were selected for further qPCR analysis. As shown in Fig. 14, except for AH16G03650.1, the qPCR expression trends of the remaining seven genes were consistent with the transcriptomic data and were up-regulated after 24 h of aluminum treatment.
AhLEAs overexpression enhanced saccharomyces cerevisiae BY4741 growth under drought and Al stress
Furthermore, we compared the DEGs in Al stress and the genes that were up-/down-regulated more than twofold under drought and low-temperature stresses. As shown in Fig S1, a total of 100 AhLEAs were regulated under drought, low temperature, and Al stresses. Among these genes, 35 common AhLEAs were involved in the responses to low-temperature and drought stresses, 29 common AhLEAs that were involved in the responses to drought and Al stresses, and 22 common AhLEAs that were involved in the responses to low-temperature and Al stresses. Sixteen AhLEAs were overlaps among the three abiotic stresses (Additional file 2: Fig S1).
To investigate the potential function of AhLEAs under stress conditions, the CDS sequence of three LEA genes, AhLEA1, AhLEA3-1, and AhLEA3-3, which were regulated under drought, low temperature, and Al stress were cloned. The function of three AhLEAs under these stresses was further investigated in eukaryotic cells as described by Gao [26].
To assess the effect of AhLEA1, AhLEA3-1, and AhLEA3-3 on the growth of recombinant yeast under freezing, drought, heat, NaCl, and Al stresses, BY4741 yeast containing pYES2- AhLEA1, pYES2- AhLEA3-1, pYES2- AhLEA3-3 and pYES2 vectors were subjected to -20 ℃, mannitol, 50 ℃, NaCl, and Al treatment, respectively. As shown in Fig. 15, under normal conditions, there was no significant difference between BY4741 that carrying recombinant plasmid pYES2- AhLEAs and empty vector pYES2. However, under Al and freezing (-20 ℃) stresses, BY4741 harboring pYES2- AhLEA1, pYES2- AhLEA3-1, or pYES2- AhLEA3-3 showed high viability than the yeast containing empty vector pYES2. In addition, BY4741 harboring pYES2- AhLEA3-1 had higher viability under drought stress compared to empty vector pYES2, while the yeasts containing pYES2- AhLEA1 or pYES2- AhLEA3-3 exhibited similar viability with the empty vector control (Fig. 15). This implies that heterologous expression of AhLEA1, AhLEA3-1 and AhLEA3-3 enhanced tolerance to cold and Al stresses in yeast, and AhLEA3-1 could enhance the drought stress tolerance in yeast. Besides, under the high temperature condition of 50 ℃ and salt (NaCl) stresses, BY4741 harboring pYES2- AhLEA1, pYES2- AhLEA3-1, pYES2- AhLEA3-3 showed weaker viability with the yeast harboring empty vector pYES2 (Additional file 2: Fig. S2).
Growth of transformed yeast containing the pYES2-AhLEAs and pYES2 vectors under cold stress (A), drought stress (B), and Al stress (C). Note: Yeast cultures were grown in serial dilutions on SD-URA solid medium under control, -20 ℃ stress for 1 h, and 0.5 M Mannitol stress and 20 mM Al stress for 5 h
Discussion
Identification of the LEA gene family
In this study, 126 LEA genes were identified from whole peanut genome sequences. Based on the phylogenetic relationship with Arabidopsis, these 126 AhLEAs were distributed to eight groups. The number of peanut LEAs was twice that in Arabidopsis. According to the studies of the LEA family in other species, the number of LEAs may be related to the polyploidy of plants [27]. For example, many LEA genes were found in polyploids of upland cotton (Fang and Magwanga, 2018 [28]), Triticum aestivum [12], and Brassica napus [11]. The LEA2s had more members than other subfamilies in the majority of species so far studied [27]. The LEA2s were the largest LEA subfamily in Citrus sinensis, Oryza sativa, Populus trichocarpa [29], and upland cotton (Fang and Magwanga, 2018 [28]). Similarly, the AhLEAs mainly belong to the LEA2s, which accounted for 61.9% of the LEA genes. However, the LEA2s were not found as a large subfamily in the previous works in Arabidopsis [21], Oryza sativa [30], and Populus trichocarpa [5]. This result can be partly explained by the fact that improved annotation of higher plant genomes can be found on phytochrome (v10.2), and LEA2 is an unusual component of "a typical" LEA proteins because they are more hydrophobic. In addition, there were three pairs of tandem duplication in AhLEAs, which belong to the LEA2s. This result supported the view that tandem duplications have contributed significantly to the expansion and diversity of the LEA2s in most species [31].
Analysis of conserved domains and introns revealed that LEAs might be stress-response genes
Motif analysis of the AhLEAs showed that members of each LEA group contained specific conserved motifs. Most members of the same subfamily have similar motifs, indicating an important role of these conserved motifs in the evolution of the LEA gene family. Big differences were found in the structure of different clades. For example, LEA1s contained motifs 13 and 17, whereas LEA5s contained motifs 2 and 9, which indicated the complexity and group-specific of LEA protein function. The conserved motifs observed in each LEA group suggested that genes with the same motif might be amplified from genes within the same evolutionary clade or group. It has been reported that stress-responding genes usually contain fewer introns (Fang and Magwanga, 2018 [28]; [32]. Introns have harmful effects on gene expression by delaying transcription products [33]. Moreover, introns can extend the length of new transcripts, resulting in additional energy consumption for transcription [34]. Up to 85.7% of the AhLEAs had zero or only one intron, which further suggested that AhLEAs were stress-related genes.
Segmental duplication plays an important role in the evolution and amplification of AhLEAs
Gene duplication plays an important role in the evolution and amplification of gene families [35]. In this study, 93 pairs of segmental duplication and 5 pairs of tandem duplication were identified, it could be inferred that segmental duplication and tandem duplication contribute to the common expansion of the AhLEAs family, but the former played a predominant role. This finding was similar to previous studies on Brassica napus and upland cotton (Gossypium hirsutum) [11], Fang and Magwanga, 2018 [28]) and consistent with our previous study on receptor-like protein kinase (RLK) in peanut [36]. According to Ka/Ks estimation, 94.9% of the duplication gene pairs of AhLEAs were less than 1, indicating the results of the purification selection. The Ka/Ks ratios of two gene pairs (AH01G27080.1 and AH11G30560.1, AH05G16640.1 and AH15G06250.1) were more than 1, which indicated that these genes were in a state of positive selection in peanuts. We calculated the divergence time, and the results showed that many duplication events appeared to have occurred during relatively recent key periods. For example, all tandem duplication events occurred at 0–10 MYA, and 49.5% of segmental duplication occurred at 0–5 MYA. These results indicated that many AhLEAs were produced by the recent gene duplication events in peanuts. This may be related to the origin of cultivated peanut, through a single and recent polyploidization event, and then continuous selection in breeding work, forming a highly conserved genome [37].
The closer the species are, the greater the genome coverage of synteny fragments and the more genes they contain [38]. Syntenic analysis showed that more homologous gene pairs were found between peanut and soybean. There were 13 single peanut-to-Arabidopsis LEA gene correspondences and 5 single peanut-to-soybean LEA gene correspondences. These results indicated that these genes come from a common ancestor. Among these genes, two soybean genes (Glyma11g02290.1 and Glyma09g30400.1) did not belong to the LEA family. The rest genes showed one-to-more, more-to-one and more-to-more correspondence, and most of the genes included in these cases appeared more than once. However, 15 of the 23 orthologs of AhLEAs in soybean (Glyma09g38980.1, Glyma19g37350.1, Glyma08g22050.1, Glyma12g09590.1, Glyma12g32090.1, Glyma13g38380.1, Glyma03g34670.1, Glyma10g07360.1, Glyma13g21240.1, Glyma19g37340.1, Glyma06g01170.1, Glyma07g06960.1, Glyma13g43610.1, Glyma09g30400.1, and Glyma20g35880.1) were not LEA genes, which implied that their genomes underwent multiple rounds of chromosomal rearrangement and fusions. Allotetraploid‐cultivated peanut composed of A and B genomes and was generated from diploid A. duranesis (AA) and A. ipaensis (BB) [39]. Taking into account the divergence time of the duplications, we inferred that the divergence of many AhLEAs duplications occurred after the divergence of peanut and Arabidopsis/soybean from their last common ancestor. Combined with the results of phylogenetic tree analysis, there were nine orthologs including nineteen peanut LEA genes (AH12G35940.1-AT2G36640.1, AH17G19580.1-AT2G21490.1, AH12G35940.1-AT3G22500.1, AH02G22690.1/ AH12G24910.1-AT3G51810.1, AH05G04840.1/ AH15G00880.1-AT2G23110.1, AH04G10170.1/ AH14G12410.1-AT4G15910.1, AH06G03960.1/ AH12G32330.1/ AH12G37270.1/ AH16G06810.1-AT1G02820.1/ AT4G02380.3, AH02G06810.1/ AH12G08270.2-AT1G03120.1/ AT3G22490.1, AH02G02040.1/ AH04G26920.1/ AH12G02210.1/ AH14G31640.1-AT2G46140.1/ AT1G01470.1) that could be clustered together in the phylogenetic tree and were also contained in the syntenic map. We speculated that the functions of these AhLEAs were more similar to their Arabidopsis homologs than the other AhLEAs in the phylogenetic tree and syntenic map.
MYB and MYC recognition sites may be involved in the response of AhLEAs to abiotic stress
Many studies have shown that LEAs play an important role in abiotic stress. In this study, many cis-acting elements related to abiotic stress and plant hormones were identified, such as ABRE, ERE, MYB recognition sites, MYC recognition sites, and STRE. We found that the MYB and MYC recognition sites were presented in the most promoters of the AhLEAs.
It is reported that MYBs and MYCs are transcription factors that participate in ABA-dependent signaling pathways to cope with abiotic stresses such as drought, salt, and low-temperature [40], Boter, 2014). Consistently, the LEAs that contain MYB recognition sites and MYC recognition sites, including AH16G06810.1, and AH06G03960.1, were induced under ABA, salicylic acid, drought, and low-temperature stresses. Besides, most of the LEA1s, LEA5s, SMPs, and Dehydrins were highly expressed under aluminum stress, and these genes contained a large number of MYB and MYC recognition sites. Therefore, we speculated that the up-regulation of LEAs expression under aluminum stress might be regulated by MYB and MYC transcription factors. This provides a theoretical basis for further exploring the response regulation mechanism of LEAs containing cis-acting elements of MYB and MYC recognition sites under stress.
The plant hormone abscisic acid (ABA) accumulates mainly in leaves in response to drought stress, and ABA mediates various gene expression processes by stress responsive transcription factors (eg. ABREs, ABFs) [41]. Here, it was found that 28 AhLEAs contained ABRE cis-acting elements, of which 18 were in the LEA2 family and 4 belonged to the LEA3 family. Notably, seven genes had high transcription levels in response to drought stress. Previous studies found that the transcription levels of LEA genes were significantly up-regulated in root and shoot tissues after drought or ABA treatment [42]. These results suggested that AhLEAs responded to abiotic stresses such as drought, low-temperature, and Al stress might be activated directly or indirectly by ABA-dependent signaling pathways. Taken together, we proposed that most LEA genes have positive roles in coping with drought stress and that the seven genes containing ABRE cis-acting elements may be a direct target for ABA and have potential application value in improving drought tolerance in crops.
Expression analysis revealed AhLEAs respond to different abiotic stresses
It can obtain clues from gene expression patterns to explore the function of genes [43]. We investigated the transcription level of AhLEAs in different tissues, at different embryo development stages, under different abiotic stresses (drought, low-temperature, and Al treatment), and after different hormone treatments. In four different embryo development stages, there were sixty-eight differentially expressed genes. Consistent with previous studies [10] that LEAs were up-regulated as the embryo developed, most of the AhLEAs were expressed at a high level at stages III and IV. However, the majority of LEA3s were highly expressed at an early stage, suggesting the potential roles of LEA3s in the early embryo development stage. As shown in Fig. 9, subfamily LEA2 was the biggest subfamily, but the transcription levels of most LEA2s at four embryo development stages were stable, suggesting that LEA2s might not play important roles during embryo development.
The transcription level of most AhLEAs in the root, stem, leaf, and flower tissues was similar. The transcription level of many AhLEAs was low, while there were still several genes of subfamily LEA2, LEA3, and Dehydrin that exhibited a high transcription level in the four tissues. Two LEA3s (AH16G06810.1, AH06G03960.1) were very highly expressed in different peanut tissues (Fig. 10). It was reported that the LEA3s play an important role in plant growth, development, and response to abiotic stresses [44–46], and these two genes might be suitable candidates to understand the role of LEA3s in peanut.
Under drought stress, 50% of the AhLEAs were up-/down-regulated for more than twofold compared with control. Among them, LEA2s contributed most genes, containing 10 up-regulated genes and 24 down-regulated genes. This is consistent with the fact that LEA2s were the largest subfamily in peanuts. Among the genes that were down-regulated for more than twofold, most of them were LEA2s. Additionally, four AhLEA1s and three AhLEA3s were induced more than 60-fold by drought stress, implying their potential roles in enhancing drought stress tolerance in peanuts.
Under low-temperature stress, 36 AhLEAs were up-regulated more than twofold, while 18 genes were down-regulated more than twofold. LEA2s also contributed to most genes. Twenty-one AhLEA2s were up-regulated and eleven genes were down-regulated. Interestingly, the LEA2s that down-regulated under drought stress was also down-regulated under low-temperature stress, which suggested that there was a common mechanism to regulate LEA2s expression.
Many studies have been conducted to estimate the function of the LEA gene under abiotic stress in yeast [15, 26, 45–48]. For example, the overexpression of TaHVA1, tomato le4, ZmLEA3 and CpLEA5 improved the tolerance to low-temperature in yeast [26, 49]. Consistently, in our study, three AhLEAs (AhLEA1, AhLEA3-1, and AhLEA3-3) were found to enhance the cold stress tolerance in yeast. An important feature of LEA protein is its low molecular weight, which is a key factor in cell protection. Therefore, it is reasonable to speculate that the protective effect of AhLEA1, AhLEA3-1, and AhLEA3-3 on cells under cold stress may be closely related to its low molecular weight and highly hydrophilic properties [50].
Seventeen genes were up-regulated after 8 h of Al treatment in 99–1507, and two of their (AH16G20700.1 and AH06G16990.1) were also up-regulated after 24 h of Al treatment. In ZH2, only five AhLEAs were up-regulated after 8 h of Al treatment, while sixteen AhLEAs were up-regulated after 24 h of Al treatment. Interestingly, three SMPs (AH12G08270.1, AH12G08270.2, and AH02G06810.1) were up-regulated after 8 h of Al treatment in both cultivars, suggesting that these genes might play important roles in Al tolerance in peanuts. Together, the Al-tolerant cultivar 99–1507 exhibited a rapid response to Al treatment, and the LEAs that induced rapidly should be studied in future work.
As shown in Fig. S1, the majority of the 126 LEAs were induced under at least one stress condition. Among these genes, sixteen were induced only under drought stress, fourteen were induced only under low-temperature, and sixteen were induced only under Al stress (Additional file 2: Fig S1). These results implied that these genes play distinct roles in response to different abiotic stresses in peanuts.
Some AhLEAs were regulated by different stress conditions. Three genes including two LEA5s (AH12G24910.1 and AH12G24920.1) and one LEA1 (AH19G11740.1) were up-regulated greatly under both drought and Al stresses (Additional file 1: Table S6, Table S8). The expression of LEA1 (AH19G11740.1) was induced more than twofold by ABA treatment. Two LEA3s (AH01G27080.1 and AH11G30560.1) and one LEA4 (AH12G35940.1) were down-regulated under Al stress. The expression of that two LEA3s was significantly induced by ethylene, while LEA4 (AH12G35940.1) was down-regulated by ABA treatment. Two LEA2s (AH02G02040.1 and AH12G02210.1) were up-regulated under drought, low-temperature, and Al stresses, and they were also up-regulated by ABA. The genes that respond to many stress conditions suggested a common regulation mechanism that plants adopted to cope with environmental challenges.
Many AhLEAs that were regulated more than twofold by hormones such as abscisic acid, brassinolide, ethylene, and salicylic acid were found to be down-regulated. As revealed by table S7, these down-regulated genes showed no obvious subfamily preference. However, AhLEAs that were up-regulated more than twofold by ethylene and salicylic acid showed obvious subfamily preference. Seven AhLEA3s were induced by ethylene. Five AhLEA3s induced by ethylene were also involved in response to drought and low-temperature stresses. The transcription level of AH12G37280.1 was increased up to 8.45-fold under low-temperature stress. AH12G32330.1 was up-regulated 3.5-fold under drought stress. Moreover, three AhLEA3s (AH01G27080.1, AH01G27080.2, AH11G30560.1) were up-regulated greatly under both drought and low-temperature stresses. These results revealed the important roles of the AhLEA3 subfamily in the ethylene-mediated response under drought and low-temperature stresses. Additionally, all AhLEA4s were induced by salicylic acid, and all AhLEA4s were also regulated greatly under drought and low-temperature stresses. Among them, two genes (AH06G16990.1 and AH12G35940.1) were induced more than sixfold under drought and low-temperature stresses, and one gene (AH16G20700.1) was down-regulated 3.5-fold under low-temperature stress, which implied that subfamily AhLEA4 played important roles in SA-mediated response under drought and low-temperature stresses in peanut.
Taken together, these results suggested that common mechanisms might be initiated in peanuts to cope with different abiotic stresses. Hormones were involved in regulating LEA’s expression under abiotic stresses. The role of hormones in regulating gene expression had a preference among AhLEA gene families.
Conclusions
In this study, 126 LEA genes in Arachis hypogaea were identified. They were divided into eight groups according to homologous in Arabidopsis thaliana. AhLEAs are randomly distributed on the chromosome, and most of them may be segmental duplication. The exon–intron and motif structures indicated that the LEAs’ family functions were highly conserved. Some cis-elements of abiotic stress response were also found in the upstream sequences of most AhLEAs. The comprehensive analysis of AhLEAs gene expression profiles showed that the LEA3s, LEA4s, and SMPs played an important role in abiotic stress response, and also showed the functional differences among other subfamilies. Moreover, the functions of AhLEA1, AhLEA3-1 and AhLEA3-3 proteins were verified and found to enhance cold and aluminum tolerance in yeast, and AhLEA3-1 enhanced the drought tolerance in yeast. This study provided a reference for further exploring the mechanism of LEAs in response to abiotic stress in peanuts.
Materials and methods
Identification of LEAs in peanut
To identify the AhLEAs, we used 51 LEA genes [21] in Arabidopsis thaliana acquire Pfam ID (PF03760, PF03168, PF03242, PF02987, PF00477, PF10714, PF04927, PF00257) and InterPro ID (IPR005513, IPR004864, /IPR013990, IPR004926, IPR004238, IPR000389, IPR018930, IPR007011, IPR000167) from Peanut Base (https://www.peanutbase.org/). By acquiring LEA peanut protein sequences based on InterPro ID search of Peanut Genome Resource (PGR) (http://peanutgr.fafu.edu.cn/). NCBI’s Conserved Domains Database (https://www.ncbi.nlm.nih.gov/cdd) and PFAM (http://pfam.xfam.org/) database were used to verify the presence of the LEA domains and finally obtained 126 AhLEAs.
Phylogenetic relationships, gene structures, conserved motifs, and chromosomal locations of the AhLEAs
The phylogenetic tree was constructed by the maximum-likelihood method with 1000 bootstrap replicates in MEGA 7.0 software [51]. Multiple Expectation Maximization for Motif Elicitation (MEME) (http://meme-suite.org/tools/meme) [52] was used to identify the conserved protein motifs, with a maximum number of the different motif at 20. The exon–intron structures were identified using the TBtools software [53]. The physical location of each AhLEA is determined by identifying the starting position of all genes on each chromosome, searching the local database of Peanut Genome Resources by BLAST. Using TBtools of Gene location visualize from GFF/GFF3 to draw chromosome mapping and tandem duplication pairs.
Promoter cis-element analysis
Genomic data were obtained from Peanut Genome Resource (PGR) (http://peanutgr.fafu.edu.cn/), and TBtools software was used to extract all LEA upstream 2kd promoter sequences. Transcriptional response elements of LEA gene promoters were predicted using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [54].
Gene duplication and evolutionary analysis
We used Virtual Machine to construct the tandem and segmental of the putative duplication of the AhLEAs and calculate the ratio of the nonsynonymous substitution rate (Ka) to the synonymous substitution rate (Ks) by the Simple Ka/Ks calculator (NG) of TBtools [53]. LEAs clustered together within 100 kb, length of the alignable sequence covers > 75% of longer gene and similarity of aligned regions > 75% were regarded as tandem duplicated genes. The relationship between Ka/Ks ratio and value 1, Ka larger than Ks (or Ka/Ks > > 1), Ka equals Ks (Ka/Ks = 1), and Ka less than Ks (or Ka/Ks < < 1), which represent positive (or diversifying) selection, neutral evolution and negative (or purifying) selection, respectively. Divergence time was calculated with the formula T = Ks/2r, where r is 1.5 × 10−8 synonymous substitutions per site per year and it is the rate of divergence for nuclear genes from plants [55]. We used Multiple Synteny Plot software [53] to explore the collinear relationship between the AhLEA and LEA genes from Arabidopsis thaliana and Glycine max. All the soybean LEA domain-containing protein sequences were downloaded from the Soybase Glyma.Wm82.a2.v1 (http://www.soybase.org/). The NCBI’s Conserved Domains Database (https://www.ncbi.nlm.nih.gov/cdd) and PFAM (http://pfam.xfam.org/) database were used to verify the presence of the LEA domains. The GmLEAs that were identified in the previous study were also screened [56]. After eliminating the invalid sequence, a total of 132 GmLEAs were identified.
Expression analysis of AhLEAs
The blast was performed in the transcriptome of the PGR database using the protein sequences of 126 AhLEAs. RNA-Seq data were downloaded from PGR (http://peanutgr.fafu.edu.cn/Download.php) and used to generate the expression patterns of AhLEAs in different tissues (root, stem, leaf, and flower), different embryo development stages, and various abiotic stresses (cold, and drought), and different hormones treatment on leaves. Transcriptome data that were generated from peanut root tips under Al stress were used to generate the expression patterns of AhLEAs under Al stress. The data had been deposited in the database of the National Center for Biotechnology Information (NCBI) under accession number PRJNA525247 (https://www.ncbi.nlm.nih.gov/sra/PRJNA525247). TBtools were used to generate heat maps and combine phylogenetic tree, gene, and protein structure [53].
The expression of AhLEAs in Saccharomyces cerevisiae BY4741
According to the full-length coding sequence of the AhLEA1, AhLEA3-1, and AhLEA3-3 in the peanut genome resource, specific primers (Additional file 1: Table S9) were designed using CE Design software. Using this primer to amplify AhLEAs from cDNA, and the purified PCR products were cloned into the pMD19-T vector (TaKaRa, Dalian, China) for sequencing (Aoke, China). The correct sequence was inserted into the intracellular expression vector pYES2/CT for Saccharomyces cerevisiae.
The pYES2-AhLEAs fusion protein was expressed in Saccharomyces cerevisiae BY4741. Yeast harboring pYES2-AhLEAs and pYES2 were incubated in SD-URA (2% Glucose) medium to OD600 = 0.6, and the yeast solution was added to SG-URA (2% Galactose) medium at a ratio of 20: 1 induce protein at 30 °C for 48 h. And then, 1 mL yeast culture was treated at 0.5 M mannitol, 0.5 M NaCl, 20 mM AlCl3 for 5 h, respectively. Similarly, 1 mL yeast culture was treated at –20 °C and 50 °C for 1 h, respectively. In addition, 1 mL yeast culture was taken out as normal condition control. Then, 10 µL yeast culture from different treatments at different dilution ratios (10°, 101, 102, and 103) were dropped on SD-URA solid medium. After 48 h of culture at 30 °C, the growth situation of the yeast cells was observed and recorded as described in Gao’s (2020) report.
QRT-PCR analysis of the AhLEAs
The experiment was carried out with peanut root tips, and the treatment method was referred to as our previous report [57]. The gene specific primers of AhLEAs were designed, and the AhACTIN was used as the reference gene (Additional file 1: Table S9). The qRT-PCR was performed using SYBRⓇ Premix Ex Taq™ II (TaKaRa, Dalian, China). Three independent biological replicates were performed and the relative expression levels of AhLEA were calculated using the 2−ΔΔCT method.
Availability of data and materials
All raw data is downloaded from the public databases. The RNA-seq data of ZH2 and 99–1507 under Al treatment had been deposited in the database of the National Center for Biotechnology Information (NCBI) under accession number PRJNA525247 (https://www.ncbi.nlm.nih.gov/sra/PRJNA525247). The raw RNA-seq reads in different peanut tissues and after different treatments (including different hormones, drought and low temperature stresses) are available at Peanut Genome Resource (http://peanutgr.fafu.edu.cn/Download.php), and AhLEAs sequences are available at Peanut Genome Resource (http://peanutgr.fafu.edu.cn/Transcriptome.php). All data generated or analyzed in this study are included in this published article [Additional file 1. xlsx]. The plant and yeast materials used in the current study are available from the corresponding author on reasonable request.
Abbreviations
- Al:
-
Aluminum
- Al stress:
-
Aluminum stress
- At :
-
Arabidopsis thaliana
- Ah :
-
Arachis hypogaea. L
- ABRE:
-
ABA-responsive element
- ERE:
-
Ethylene response element
- WRE3:
-
Water response element
- MYB:
-
Transcription factor
- MYC:
-
Transcription factor
- TC-rich repeats:
-
Cis-acting element involved in defense and stress responsiveness
- MRE:
-
Metal responsive element
- STRE:
-
Stress response element
- DEGs:
-
Differentially expressed genes
References
Kentaro S, Kirilov CN, Sakae T, et al. Identification of a novel LEA protein involved in freezing tolerance in wheat. Plant and cell physiology. 2014;55(1):136–47. https://doi.org/10.1093/pcp/pct164.
Park JA, Cho SK, Kim JE, et al. Isolation of cDNAs differentially expressed in response to drought stress and characterization of the Ca-LEAL1 gene encoding a new family of atypical LEA-like protein homologue in hot pepper (Capsicum annuum L. cv. Pukang). Plant Science. 2003;165(3):471–81. https://doi.org/10.1016/S0168-9452(03)00165-1.
Zegzouti H, Jones B, Marty C, et al. Er5, a tomato cDNA encoding an ethylene-responsive LEA-like protein: characterization and expression in response to drought. ABA and wounding Plant Molecular Biology. 1997;35(6):847–54. https://doi.org/10.1023/A:1005860302313.
Battaglia M, Covarrubias AA. Late Embryogenesis Abundant (LEA) proteins in legumes. Frontiers in Plant Science. 2013;4(190):190. https://doi.org/10.3389/fpls.2013.00190.
Lan T, Jie G, Zeng QY. Genome-wide analysis of the lea (late embryogenesis abundant) protein gene family in Populus trichocarpa. Tree Genetics & Genomes. 2013;9(1):253–64. https://doi.org/10.1007/s11295-019-1364-3.
Adrien C., Gal P., Martine N. et al. The Ubiquitous Distribution of Late Embryogenesis Abundant Proteins across Cell Compartments in Arabidopsis Offers Tailored Protection against Abiotic Stress. [J]. The Plant Cell, 2014(7): 7. https://doi.org/10.1105/tpc.114.127316
Shao HB, Liang ZS, Shao MA. LEA proteins in higher plants: structure, function, gene expression and regulation. Colloids and Surfaces B Biointerfaces. 2005;45(3–4):131–5. https://doi.org/10.1016/j.colsurfb.2005.07.017.
Bies-Ethève N, Gaubier-Comella P, Debures A, et al. Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant Molecular Biology. 2008;67(1–2):107–24. https://doi.org/10.1007/s11103-008-9304-x.
Jin XF, Cao D, Wang ZJ, et al. Genome-wide identification and expression analyses of the LEA protein gene family in tea plant reveal their involvement in seed development and abiotic stress responses. Scientific Reports. 2019;9(1):14123–14123. https://doi.org/10.1038/s41598-019-50645-8.
Liang Y, Xiong Z, Zheng J, et al. Genome-wide identification, structural analysis and new insights into late embryogenesis abundant (LEA) gene family formation pattern in Brassica napus. Scientific Reports. 2016;6:24265. https://doi.org/10.1038/srep24265.
Liu H, Xing M, Yang W, et al. Genome-wide identification of and functional insights into the late embryogenesis abundant (LEA) gene family in bread wheat (Triticum aestivum). Scientific Reports. 2019;9(1):1–11. https://doi.org/10.1038/s41598-019-49759-w.
Shi HF, He XY, Zhao YJ, et al. Constitutive expression of a group 3 LEA protein from Medicago falcata (MfLEA3) increases cold and drought tolerance in transgenic tobacco. Plant Cell Reports. 2020;39(7):851–60. https://doi.org/10.1007/s00299-020-02534-y.
Sivamani E, Bahieldin A, Wraith JM, et al. Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Science. 2000;155(1):1–9. https://doi.org/10.1016/S0168-9452(99)00247-2.
Xu D, Duan X, Wang B, et al. Expression of a Late Embryogenesis Abundant Protein Gene, HVA1, from Barley Confers Tolerance to Water Deficit and Salt Stress in Transgenic Rice. Plant Physiology. 1996;110(1):249–57. https://doi.org/10.1104/pp.110.1.249.
Mowla SB, Cuypers A, Driscoll SP, et al. Yeast complementation reveals a role for an Arabidopsis thaliana late embryogenesis abundant (LEA)-like protein in oxidative stress tolerance. Plant Journal. 2010;48(5):743–56. https://doi.org/10.1111/j.1365-313X.2006.02911.x.
Yun L, Zheng Y. PM2, a group 3 LEA protein from soybean, and its 22-mer repeating region confer salt tolerance in Escherichia coli. Biochemical and Biophysical Research Communications. 2005;331(1):325–32. https://doi.org/10.1016/j.bbrc.2005.03.165.
Yun L, Zheng Y, Zhang Y. et al. 2010. Soybean PM2 Protein (LEA3) Confers the Tolerance of Escherichia. 60(5): 373–378. https://doi.org/10.1007/s00284-009-9552-2
Zamora-Briseño JA, de Jiménez ES. A LEA 4 protein up-regulated by ABA is involved in drought response in maize roots. Molecular Biology Reports. 2016;43(4):221–8. https://doi.org/10.1007/s11033-016-3963-5.
Bhogireddy S., Xavier A., Garg V. et al. 2020. Genome-wide transcriptome and physiological analyses provide new insights into peanut drought response mechanisms. Scientific Reports. 10(1). https://doi.org/10.1038/s41598-020-60187-z
Qiu W, Wang N, Dai J, et al. AhFRDL1-mediated citrate secretion contributes to adaptation to iron deficiency and aluminum stress in peanuts. Journal of Experimental Botany. 2019;70(10):2873–86. https://doi.org/10.1093/jxb/erz089.
Hundertmark M, Hincha DK. LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics. 2008;9(1):118. https://doi.org/10.1186/1471-2164-9-118.
Kent WJ, Baertsch R, Hinrichs A, et al. Evolution’s cauldron: Duplication, deletion, and rearrangement in the mouse and human genomes. Proceedings of the National Academy of Sciences. 2003;100(20):11484–9. https://doi.org/10.1073/pnas.1932072100.
Mehan MR, Freimer NB, Ophoff RA. A genome-wide survey of segmental duplications that mediate common human genetic variation of chromosomal architecture. Human Genomics1, 5(2004-08-01). 2004;1(5):335–44. https://doi.org/10.1186/1479-7364-1-5-335.
Xiao D, Li X, Zhou Y. Y. et al. Transcriptome analysis reveals significant difference in gene expression and pathways between two peanut cultivars under Al stress. Gene. 2021, 781(145535). https://doi.org/10.1016/J.GENE.2021.145535
Zhan J, Kou R J, He L F. et al. 2008. Effects of aluminum on morphological structure of peanut root tips. Chinese Journal of Oil Crop Sciences. (01):79–83. https://doi.org/10.3724/SP.J.1011.2008.00534
Gao T, Mo Y, Huang H, et al. Heterologous Expression of Camellia sinensis Late Embryogenesis Abundant Protein Gene 1 (CsLEA1) Confers Cold Stress Tolerance in Escherichia coli and Yeast. Horticultural Plant Journal. 2020;7(1):8. https://doi.org/10.1016/j.hpj.2020.09.005.
Ibrahime M, Kibar U, Kazan K, et al. Genome-wide identification of the LEA protein gene family in grapevine (Vitis vinifera L.). Tree Genetics and Genomes. 2019;15(4):55.1-55.14. https://doi.org/10.1007/s11295-019-1364-3.
Fang L, Magwanga RO. Characterization of the late embryogenesis abundant (lea) proteins family and their role in drought stress tolerance in upland cotton. BMC Genetics. 2018;19:6. https://doi.org/10.1186/s12863-017-0596-1.
Pedrosa AM, Martins C, Gonçalves LP, et al. Late Embryogenesis Abundant (LEA) Constitutes a Large and Diverse Family of Proteins Involved in Development and Abiotic Stress Responses in Sweet Orange (Citrus sinensis L. Osb.). Plos One. 2015;10(12):e0145785. https://doi.org/10.1371/journal.pone.0145785.
Wang XS, Zhu HB, Jin GL, et al. Genome-scale identification and analysis of LEA genes in rice (Oryza sativa L). Plant Science. 2007;172(2):414–20. https://doi.org/10.1016/j.plantsci.2006.10.004.
Artur M A S, Zhao T, Ligterink W, et al. Dissecting the Genomic Diversification of Late Embryogenesis Abundant (LEA) Protein Gene Families in Plants). Genome Biology and Evolution. 2019;11(2):459–71. https://doi.org/10.1093/gbe/evy248.
Xie DW, Wang XN, Fu LS, et al. Identification of the trehalose-6-phosphate synthase gene family in winter wheat and expression analysis under conditions of freezing stress. Journal of Genetics. 2015;94(1):55–65. https://doi.org/10.1007/s12041-015-0495-z.
Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467(7318):929–34. https://doi.org/10.1038/nature09486.
Jeffares DC, Penkett CJ, Bahler J. Rapidly regulated genes are intron poor. Trends in Genetics. 2008;24(8):375–8. https://doi.org/10.1016/j.tig.2008.05.006.
Flagel LE, Wendel JF. Gene duplication and evolutionary novelty in plants. New Phytologist. 2009;183(3):557–64. https://doi.org/10.1111/j.1469-8137.2009.
Wang X, Wu M H, Xiao D. et al. 2021. Genome-wide identification and evolutionary analysis of RLKs involved in the response to aluminium stress in peanut. BMC Plant Biology. 21(1). https://doi.org/10.1186/s12870-021-03031-4
Cunha F, Nobile PM, Hoshino AA, et al. Genetic relationships among Arachis hypogaea L. (AABB) and diploid Arachis species with AA and BB genomes. Genetic Resources and Crop Evolution. 2008;55(1):15–20. https://doi.org/10.1007/s00425-014-2215-y.
Ye J, Yang X, Hu G. et al. Genome-Wide Investigation of Heat Shock Transcription Factor Family in Wheat (Triticum aestivum L.) and Possible Roles in Anther Development. International Journal of Molecular Sciences, 2020, 21(2). https://doi.org/10.3390/ijms21020608
Zhuang WJ, Chen H, Yang M, et al. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution, and crop domestication. Nature Genetics. 2019;51(5):865–76. https://doi.org/10.1038/s41588-019-0402-2.
Li C, Ng KY, Fan LM. MYB transcription factors, active players in abiotic stress signaling. Environmental and Experimental Botany. 2015;114:80–91. https://doi.org/10.1016/j.envexpbot.2014.06.014.
Soma F, Takahashi F, Yamaguchi-Shinozaki K, et al. Cellular Phosphorylation Signaling and Gene Expression in Drought Stress Responses: ABA-Dependent and ABA-Independent Regulatory Systems. Plants. 2021;10(4):756. https://doi.org/10.3390/plants10040756.
Blaise, Pascal, Muvunyi, et al. 2018. Mining Late Embryogenesis Abundant (LEA) Family Genes in Cleistogenes songorica, a Xerophyte Perennial Desert Plant. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms19113430
Chen Q, Chen QJ, Sun GQ, et al. Genome-Wide Identification of Cyclophilin Gene Family in Cotton and Expression Analysis of the Fibre Development in Gossypium barbadense. International Journal of Molecular Sciences. 2019;20(2):349. https://doi.org/10.3390/ijms20020349.
Koubaa S, Brini F. Functional analysis of a wheat group 3 late embryogenesis abundant protein (TdLEA3) in Arabidopsis thaliana under abiotic and biotic stresses. Plant Physiology and Biochemistry. 2020;156:396–406. https://doi.org/10.1016/j.plaphy.2020.09.028.
Yu L, Kai K, Lu G, et al. Drought-responsive genes, late embryogenesis abundant group 3 (LEA3) and vicinal oxygen chelate (VOC), function in lipid accumulation in Brassica napus and Arabidopsis mainly via enhancing photosynthetic efficiency and reducing ROS. Plant Biotechnology Journal. 2019;17(11):2123–42. https://doi.org/10.1111/pbi.13127.
Yu ZY, Wang X, Tian Y, et al. The functional analysis of a wheat group 3 late embryogenesis abundant protein in Escherichia coli and Arabidopsis under abiotic stresses. Plant signaling & behavior. 2019;14(11):1667207. https://doi.org/10.1080/15592324.2019.1667207.
Dang NX, Popova AV, Hundert mark M, et al. Functional characterization of selected LEA proteins from Arabidopsis thaliana in yeast and in vitro. Planta. 2014;240(2):325–36. https://doi.org/10.1007/s00425-014-2089-z.
Liu Y, Wang L, Jiang S, et al. Group 5 LEA protein, ZmLEA5C, enhance tolerance to osmotic and low temperature stresses in transgenic tobacco and yeast. Plant Physiology & Biochemistry. 2014;84:22–31. https://doi.org/10.1016/j.plaphy.2014.08.016.
Zhang L, Akinori O, Masamichi T. et al. 2000. Expression of Plant Group 2 and Group 3 lea Genes in Saccharomyces cerevisiae Revealed Functional Divergence among LEA Proteins. Journal of Biochemistry. (4):611–6. https://doi.org/10.1093/oxfordjournals.jbchem.a022648
Wang Y. D., Chen G. J., Lei J. J. et al. Identification and characterization of a LEA-like gene, CaMF5, specifically expressed in the anthers of male-fertile Capsicum annuum. [J] Horticultural Plant Journal. 6 (2019)39–48. https://doi.org/10.1016/j.hpj.2019.07.004
Sudhir K, Glen S, Koichiro T. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution. 2016;33(7):1870–4. https://doi.org/10.1093/molbev/msw054.
Bailey TL, Boden M, Buske FA, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research. 2009;37(Web server):W202–8. https://doi.org/10.1093/nar/gkp335.
Chen C. J., Chen H., Zhang Y. et al. 2020. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Molecular Plant. 13(8). DOI: https://doi.org/10.1016/j.molp.2020.06.009
Higo K, Ugawa Y, Iwamoto M, et al. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Research. 1999;27(1):297–300. https://doi.org/10.1093/nar/27.1.297.
Koch M A, Bernhard H, Thomas M O. 2000. Comparative Evolutionary Analysis of Chalcone Synthase and Alcohol Dehydrogenase Loci in Arabidopsis, Arabis, and Related Genera (Brassicaceae). Molecular Biology and Evolution. (10):1483–98. https://doi.org/10.1161/01.STR.0000221702.75002.66
Li L, Xu H. L, Yang X L. et al. Genome-Wide Identification, Classification and Expression Analysis of LEA Gene Family in Soybean. Scientia Agricultura Sinica, 2011. https://doi.org/10.3864/j.issn.0578-1752
Xiao D, He H Y, Huang W J. et al. 2018. Analysis of Mitochondrial Markers of Programmed Cell Death. Methods Molecular Biology. 1743: 65–71. 10.1007 / 978–1–4939–7668–3_6
Anne R, Auer P L, Marc L, et al. The fate of duplicated genes in a polyploid plant genome. Plant Journal. 2013;73(1):143–53. https://doi.org/10.1111/tpj.12026.
Blanc G, Wolfe KH. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. The Plant Cell Online. 2004;16(7):1667–78. https://doi.org/10.1016/j.livsci.2009.01.009.
Boter M. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes and Development. 2004;18(13):1577–91. https://doi.org/10.1101/gad.297704.
Cao J, Li X. Identification and phylogenetic analysis of late embryogenesis abundant proteins family in tomato (Solanum lycopersicum). Planta. 2015;241(3):757–72. https://doi.org/10.1007/s00425-014-2215-y.
Du D., Zhang Q., Cheng T. et al. Genome-wide identification and analysis of late embryogenesis abundant (LEA) genes in Prunus mume. [J]. Molecular Biology Reports, 2013, 40(2): 1937–1946. DOI: https://doi.org/10.1007/s11033-012-2250-3Fang L., Magwanga R. O. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. [J]. BMC Genetics, 2018. DOI: https://doi.org/10.1186/s12863-017-0596-1
Feng K. W., Cui L. C., Wang L. et al. The improved assembly of 7DL chromosome provides insight into the structure and evolution of bread wheat. [J]. Plant Biotechnology Journal, 2020, 18(3). https://doi.org/10.1111/pbi.13240
Hernandez-Garcia CM, Finer JJ. A novel cis-acting element in the GmERF3 promoter contributes to inducible gene expression in soybean and tobacco after wounding. Plant Cell Reports. 2016;35(2):303–16. https://doi.org/10.1007/s00299-015-1885-7.
Mota A, Oliveira T N, Vinson C C. et al. Contrasting Effects of Wild Arachis Dehydrin Under Abiotic and Biotic Stresses. Frontiers in Plant Science, 2019, 10. https://doi.org/10.3389/fpls.2019.00497
Yamaguchi-Shinozaki K, Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends in Plant Science. 2005;10(2):88–94. https://doi.org/10.1016/j.tplants.2004.12.012.
Mota APZ, Oliveira TN, Vinson CC, et al. Contrasting Effects of Wild Arachis Dehydrin Under Abiotic and Biotic Stresses. Frontiers in plant science. 2019;10:497. https://doi.org/10.3389/fpls.2019.00497.
Boter M, Omar RR, Ashraf A, et al. Conserved myc transcription factors play a key role in jasmonate signaling both in tomato and arabidopsis. Genes & Development. 2004;18(13):1577-91. https://doi.org/10.1101/gad.297704.
Acknowledgements
We thank Mr Yun Xiong Zhao and Miss Yi Shen for providing the software needed for drawing and data collection.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31701356, 32060419) and College Students’ Innovative Entrepreneurial Training Plan Program (201910593083). Apart from providing financial support, funding bodies were not involved in the study design, data analyses, and interpretation of results or manuscript preparation. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RLH: Writing Original Draft, Data analysis, Conceptualization; XW: Data analysis; LFH, AQW, and JZ: manuscript review; DX: Conceptualization, supervision, Writing—Review & Editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare no conflicting interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 2: Fig S1.
Venn diagram showing the number of AhLEAs that responded to drought, and lowtemperature, Al stresses. Fig S2. Growth of transformed yeast containing the pYES2-AhLEAs and pYES2 vectors under heat stress (A) and salt stress (B).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, R., Xiao, D., Wang, X. et al. Genome-wide identification, evolutionary and expression analyses of LEA gene family in peanut (Arachis hypogaea L.). BMC Plant Biol 22, 155 (2022). https://doi.org/10.1186/s12870-022-03462-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03462-7