Abstract
Background
General anesthesia is often necessary for dental treatment of outpatients with mental disabilities. Rapid recovery and effective management of postoperative nausea and vomiting (PONV) are critical for outpatients. This study aimed to investigate the effect of transitioning from propofol to remimazolam with flumazenil reversal administered toward the end of surgery during propofol-based total intravenous anesthesia (TIVA) on recovery.
Methods
Adults with mental disabilities scheduled to undergo dental treatment were randomly assigned to receive either propofol-based TIVA (Group P) or propofol-remimazolam-based TIVA with flumazenil reversal (Group PR). Propofol was replaced with remimazolam 1 h before the end of surgery in Group PR; moreover, 0.5 mg of flumazenil was administered after the neuromuscular blockade reversal agent. The primary outcome was the duration of stay in the post-anesthesia care unit (PACU). The secondary outcomes included time to eye-opening, time to extubation, occurrence of PONV, and quality of recovery.
Results
Fifty-four patients were included in this study. The duration of stay in the PACU in Group PR was significantly shorter than that in Group P (mean difference, 8.7 min; confidence interval [95% CI], 3.3–14.2; P = 0.002). Group PR exhibited a shorter time to eye opening (mean difference, 5.4 min; 95% CI, 3.3–8.1; P < 0.001) and time to extubation (mean difference, 5.5 min; 95% CI, 3.6–7.9; P < 0.001) than Group P. Neither group required the administration of rescue analgesics, and the incidence of PONV was not reported.
Conclusions
Transitioning from propofol to remimazolam 1 h before the end of surgery followed by flumazenil reversal reduced the duration of stay in the PACU and the time to eye opening and extubation without affecting the incidence of PONV and quality of recovery.
Trial registration number
Clinical Research Information Service (KCT0007794), Clinical trial first registration date: 12/10/2022.
Similar content being viewed by others
Background
General anesthesia is often the only feasible option for patients with mental disabilities who have difficulty cooperating during dental treatment [1, 2]. Since most dental treatments are typically conducted on an outpatient basis, it is crucial to ensure prompt recovery and effectively address postoperative nausea and vomiting (PONV) [2, 3]. However, patients with mental disabilities tend to awaken slowly from general anesthesia compared with those without mental disabilities. Moreover, this effect is exaggerated among patients receiving antiepileptic medications [4, 5].
Propofol is a short-acting intravenous anesthetic characterized by rapid anesthesia induction and recovery as well as a reduced incidence of PONV and postoperative pain, which makes it an appropriate anesthetic agent for outpatient settings [6,7,8]. However, it has no reversal agent, and its propofol infusion time is positively correlated with the context-sensitive half-time. This can result in a substantial delay in recovery and may even lead to respiratory depression in vulnerable patients [8,9,10].
Remimazolam is a benzodiazepine with ultrashort-acting properties, characterized by rapid onset and offset [11, 12]. It has a short context-sensitive half-time owing to its rapid plasma clearance mediated by nonspecific esterase, even when administered over extended periods [13]. Furthermore, its action can be reversed by flumazenil, and it has demonstrated hemodynamic stability in numerous clinical trials [14,15,16,17]. Specifically, it has demonstrated hemodynamic stability in clinically vulnerable patients, especially those classified as American Society of Anesthesiologists (ASA) physical status class III [18]. However, despite these advantages, some studies have reported delayed recovery of consciousness from remimazolam-based total intravenous anesthesia (TIVA) without flumazenil reversal compared with that from propofol-based TIVA [14]. Several randomized clinical trials have demonstrated that the additional use of flumazenil for reversal in remimazolam-based TIVA facilitates faster recovery [15, 17, 19] and a shorter stay in the post-anesthesia care unit (PACU) than those of propofol-based TIVA [19].
Nevertheless, remimazolam-based TIVA is associated with a longer time to loss of consciousness than propofol-based TIVA [14, 16, 19], which can impede anesthesia induction in uncooperative patients with disabilities who require rapid induction. In addition, remimazolam-based TIVA has a higher incidence of PONV than propofol-based TIVA [14, 20]. Furthermore, remimazolam is relatively expensive; thus, using remimazolam for the entire procedural time can lead to higher out-of-pocket expenses for patients than using propofol.
We hypothesized that by combining the rapid onset and low incidence of PONV associated with propofol with the swift recovery facilitated by flumazenil reversal of remimazolam-based anesthesia, we could administer more suitable anesthesia to outpatients with mental disabilities. Thus, this prospective, randomized controlled trial aimed to evaluate whether replacing propofol with remimazolam 1 h before the completion of dental treatment, followed by reversal with flumazenil, enhances postoperative recovery in patients undergoing dental procedures. Moreover, our study aimed to compare postoperative outcomes between propofol-based TIVA and propofol-remimazolam-based TIVA with flumazenil reversal.
Methods
Study design
This prospective, parallel-designed, single-center, randomized, single-blind, controlled study was conducted at the National Dental Care Center for Persons with Special Needs at Seoul National University Dental Hospital. The study protocol was approved by the Institutional Review Board of the Seoul National University Dental Hospital (IRB # CME22002; date of approval: 11/8/2022) and registered with the Clinical Research Information Service (number: KCT0007794; date of registration: 12/10/2022). This study adhered to the principles outlined in the Declaration of Helsinki, and written informed consent was obtained from the parents or legally authorized representatives of the patients before enrollment.
This study included mentally disabled adults with ASA physical status II–III scheduled to undergo dental treatment under general anesthesia. A mental disability is defined as a cognitive or psychological condition that limits significant life activities or require special care; such conditions include intellectual disabilities, developmental disabilities, autism, or dementia. The exclusion criteria were as follows: a history of an allergic reaction to any study medication; body mass index (BMI) ≥ 35 kg m− 2; an expected surgery duration of ≤ 1 h; previous participation in a study; and having undergone a tracheostomy. Further, we excluded patients who had received anxiolytics, hypnotics, or antipsychotics within 24 h before the administration of general anesthetics, except those who had been receiving a stable dose for ≥ 4 weeks prior to the study.
Randomization
The patients were randomly allocated to either the propofol group (Group P) or propofol-remimazolam group (Group PR) at a 1:1 ratio by an investigator blinded to the study using a computer-based random number sequence generator and the sealed envelope method. Propofol was replaced with remimazolam approximately 1 h before the end of surgery in Group P. Given the distinct properties of the two anesthetics, it was not feasible to blind the anesthesiologist to the group allocation. Therefore, only the patients, parents, and study investigators were unaware of the group allocation.
Anesthesia and perioperative management
All patients received standardized anesthetic care. Patients entered the operating room without premedication and underwent routine monitoring, including electrocardiography, pulse oximetry, non-invasive blood pressure, thermometry (3 M™ Bair Hugger™ Temperature Monitoring Patient Sensor, USA), acceleromyography (ToF scan®, Idmed, France), and patient state index (PSI, SedLine®, Masimo, USA).
In both groups, general anesthesia was induced by administering propofol through target-controlled infusion (TCI) (Injectomat TIVA Agilia® system, Fresenius Kabi, Germany) at an effect site concentration of 3.0–5.0 µg mL− 1. Following the confirmation of loss of consciousness, 0.6 mg kg− 1 of rocuronium and 3.0–5.0 ng mL− 1 Ce of remifentanil were administered. For propofol and remifentanil, the Schneider and Minto models were used as the pharmacokinetic models, respectively. Nasotracheal intubation was performed using a video laryngoscope (C-MAC® video laryngoscope; Karl Storz, Germany) after confirming sufficient muscle relaxation. Rocuronium was administered at an induction dose of 0.6 mg kg− 1 and a maintenance dose of 0.15 mg kg− 1 was administered to maintain a moderate neuromuscular block if the train of four (TOF) count was 4 or if spontaneous respiration occurred.
In Group P, anesthesia was maintained by adjusting the amount of propofol (1.5–5 µg mL− 1 Ce) using TCI while maintaining a PSI range of 25–50 until the end of surgery.
In Group PR, anesthesia induction and maintenance were performed in the same manner as in Group P, with propofol being replaced with remimazolam 1 h before the end of surgery; furthermore, the remimazolam maintenance dose was adjusted to a continuous infusion rate of 1–2 mg kg− 1 h− 1 at a PSI range of 25–50.
Remifentanil was administered via TCI in both groups, with the infusion rate being adjusted to 0.1–4 ng mL− 1 depending on the patient’s hemodynamic status. If the mean arterial blood pressure of the patient was < 65 mmHg despite being controlled with remifentanil, it was corrected via the administration of ephedrine or phenylephrine. All patients received 30 mg of ketorolac, 5 mg of dexamethasone, and 0.075 mg of palonosetron 1 h before the end of surgery. If the patient had a contraindication to ketorolac, 1 g of paracetamol was administered. Continuous infusion of general anesthetics was discontinued at the end of surgery, and sugammadex was administered at a dose of 2–4 mg kg− 1 to reverse neuromuscular blockade. Sugammadex was administered at a dose of 2 mg kg− 1 in patients with a moderate level of neuromuscular block indicated by a TOF count of 2 and 4 mg kg− 1 in those with a deep neuromuscular block indicated by a post-tetanic count of 1–2.
In Group PR, 0.5 mg of flumazenil was administered after neuromuscular blockade reversal. Tracheal extubation was performed after adequate spontaneous respiration, recovery of the airway reflex, and eye-opening, and the patients were transferred to the PACU.
In the PACU, 1 g of paracetamol was administered for pain relief if the numerical rating scale or Wong–Baker Faces pain scale score exceeded 6. However, if paracetamol had been intraoperatively administered due to contraindications to ketorolac, 20 mg of nefopam mixed in 100 mL normal saline was administered intravenously over a period of 30 min. If the patient experienced PONV, 10 mg of metoclopramide was administered intravenously as a rescue antiemetic. Patients were discharged from the hospital if they scored ≥ 9 on the Post Anesthetic Discharge Scoring System [21] or, as appropriate, according to the judgment of an anesthesiologist blinded to the group allocation.
Outcome assessment
Data regarding age, sex, height, weight, BMI, ASA physical status classification, underlying medical conditions, current medications, duration of anesthesia, duration of surgery, and total amount of anesthetic drugs were collected from the medical records of each patient. Furthermore, the Korean version of the Quality of Recovery-15 questionnaire (QoR-15 K) was administered to the patient’s parent or legal guardian 24 h after the patient was discharged from the PACU via telephone by an investigator blinded to the group allocation [22, 23]. We also checked for other complications and re-sedation. The primary study outcome was the duration of PACU stay, which was determined as the time from when a patient entered the PACU to when they met the appropriate discharge criteria. The secondary outcomes included the time between the end of general anesthesia and initial eye-opening, the time of extubation, the initial modified Aldrete score recorded in the PACU, the occurrence of PONV, and the QoR-15 K score at 24 h. Moreover, the use of intraoperative vasopressors, PSI values at the end of surgery, and the use of rescue analgesics and antiemetics in the PACU were also investigated. In addition, the mean arterial blood pressure and PSI were retrospectively obtained from electronic medical records. The stability of the intraoperative hemodynamic profile and anesthetic depths was compared based on the median performance error (MDPE, %), median absolute performance error (MADPE, %), and wobble (%) between the groups as well as before and after replacing propofol with remimazolam in Group PR [24]. Performance measurement (PM) is a quantitative method developed for use in pharmacokinetic studies to assess the difference between the measured and predicted concentrations of a drug [25]. The most frequently used PM variables are MDPE, MADPE, and wobble, which are used to measure bias, accuracy, and time-dependent variation in repeatedly measured values, respectively. In clinical practice, these variables can be used to evaluate hemodynamic instability by measuring significant deviations in blood pressure from the reference value [24,25,26]. A negative MDPE is indicative of relative hypotension, whereas a substantial wobble indicates unstable blood pressure characterized by fluctuations above or below the mean arterial blood pressure. The reference value for mean arterial blood pressure was based on blood pressure measured in a quiet place with a parent or legal guardian present prior to admission to the operating room. For uncooperative patients, blood pressure was determined based on blood pressure measured at the pre-anesthesia evaluation outpatient clinic. The reference value for the PSI was set as 38, based on the range recommended for general anesthesia.
Statistical analysis
In our preliminary study, the duration of PACU stay in Group PR was reduced by 13% compared with that in Group P, and the calculated effect size was 0.92. To achieve a power of 80% and a significance level of 5%, 21 patients had to be included in each group. Considering a dropout rate of 20%, 54 patients had to be included (27 patients per group).
Categorical variables were analyzed using the χ2 test or Fisher’s exact test and are presented as frequencies or numbers (percentages). Continuous variables were analyzed using Student’s t-test or the Mann–Whitney U test and are presented as mean ± standard deviation or median (interquartile range). A paired t-test or Wilcoxon signed-rank test was performed for within-group comparisons. The Shapiro–Wilk test was performed to evaluate the normality of data distribution. Statistical analyses were conducted using jamovi software, version 2.3.26 (The jamovi project, Sydney, Australia), and R software, version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria). P-values of < 0.05 were considered statistically significant.
Results
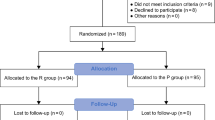
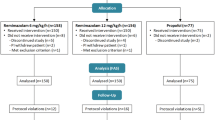
Between December 2022 and May 2023, 171 patients were assessed to determine their eligibility for inclusion in the study; among them, 54 were enrolled and randomized into Group P (n = 27) or Group PR (n = 27). No patients were excluded after enrollment, and there were no missing data. Thus, data from 54 patients were analyzed (Fig. 1). Table 1 summarizes the patient characteristics and intraoperative data, which were well balanced between the groups, except for variations in height and weight. Regarding the primary outcome, the duration of PACU stay was significantly shorter in Group PR than in Group P (41.9 ± 10.3 min vs. 50.6 ± 9.8 min, P = 0.002), with a mean difference of 8.7 min (95% confidence interval [CI], 3.3–14.2) (Fig. 2). As shown in Table 2, the time to eye-opening was significantly shorter in Group PR than in Group P (2.9 [1.6–5.0] min vs. 8.3 [5.9–12.6] min, P < 0.001), with a median difference of 5.4 min (95% CI, 3.3–8.1). Furthermore, the time to extubation was significantly shorter in Group PR than in Group P (4.8 [2.9–6.7] min vs. 11.2 [7.8–14.4] min, P < 0.001), with a median difference of 5.5 min (95% CI, 3.6–7.9).
None of the patients required rescue analgesics in the PACU, and the incidence of PONV was not reported. There were no significant between-group differences in the modified Aldrete and postoperative QoR-15 K scores (Table 2). Further, there were no significant between-group differences in the intraoperative hemodynamic and anesthetic depth stability based on PMs (Table 3). However, the mean arterial pressure MDPE (%) was significantly lower in Group P than in Group PR (-18.5 ± 11.5 vs. -10.7 ± 11.2, P = 0.021). In Group PR, no significant differences in the hemodynamic and anesthetic depth stability were observed before and after replacing propofol with remimazolam (Table 4). The PSI values were maintained within the 25–50 range after conversion to remimazolam in all but four patients. These four patients experienced a transient reduction in PSI values to ≤ 25, which subsequently returned to baseline levels within 15 min.
Discussion
This study explored the effects of replacing propofol with remifentanil 1 h before the completion of dental treatment, followed by flumazenil reversal. The results indicated that this approach led to faster recovery and shorter PACU stay than propofol-based TIVA alone.
Previous studies comparing the duration of PACU stay between propofol-based TIVA and remimazolam-based TIVA with the additional use of flumazenil have reported inconsistent findings. A previous study reported no significant difference in the duration of PACU stay [17], whereas another study reported a significantly shorter PACU stay using the latter approach [19]. The first study included patients with ASA I–II who underwent open thyroidectomy and received 0.2 mg of flumazenil, with patients who achieved a modified Aldrete score of ≥ 9 being discharged to the ward [17], whereas the other study included patients with ASA II–III who received 0.5 mg of flumazenil [19].
The administration of flumazenil (0.5 mg) following remimazolam has demonstrated efficacy in facilitating recovery from anesthesia or sedation [15, 19, 27, 28]. Although benzodiazepine premedication was not included in the present study, it is frequently required in patients with mental disabilities due to poor cooperation during anesthesia induction. In such cases, the administration of flumazenil may prove beneficial in facilitating recovery. However, caution must be exercised given the potential for re-sedation approximately 1–2 h postoperatively and the lack of evidence supporting the beneficial effects of excessive administration of flumazenil [29, 30]. In the current study, the patients recovered in the PACU and remained in the hospital for at least 2 h postoperatively. Although our study, did not include a case of re-sedation, it is imperative to remain vigilant for the occurrence of re-sedation.
The incidence of PONV was not reported in our study, suggesting that replacing propofol with remimazolam does not increase the incidence of PONV in our study. Propofol is known for its ability to prevent PONV, even when administered in small doses [31,32,33]. A recent study demonstrated that a small dose of propofol and dexamethasone in remimazolam-based TIVA effectively prevented PONV [34]. This was also evident in our empirical findings. In addition, we administered palonosetron to prevent PONV.
We observed no significant between-group differences in the postoperative QoR-15 K scores. Previous studies have compared postoperative QoR scores between propofol-based TIVA and remimazolam-based TIVA. Some studies have reported no significant between-group differences [16, 17], whereas one study reported a decrease in postoperative QoR scores in the remimazolam-based TIVA group [35]. Cessation of remimazolam administration may lead to undesirable desensitization effects and the incidence of rebound phenomena such as anxiety [13]. These phenomena may contribute to the decreased postoperative QoR scores in remimazolam-based TIVA [35]. However, none of the patients in Group PR showed a rebound phenomenon in our study.
Compared with propofol-based TIVA, remimazolam-based TIVA is associated with a relatively low incidence of hypotension [14, 19, 36]. Our findings indicated lower MDPE in Group P than that in Group PR. Nevertheless, this was within the normotensive range, and there was no significant between-group difference in the requirement for vasopressors. In addition, there was no significant between-group difference in intraoperative hemodynamic and anesthetic depth stability based on PMs, even after the replacement of propofol with remimazolam in Group PR.
Previous studies have demonstrated that remimazolam-based TIVA can lead to elevated PSI values compared with propofol-based TIVA, with some values exceeding the threshold of 50 [16, 37]. Additionally, there have been reports of chronic benzodiazepine users developing tolerance to remimazolam, necessitating the use of alternative anesthetics or higher remimazolam dosages [38, 39]. Although our study included chronic benzodiazepine users, we did not observe any such discrepancies. Several studies have reported that both remimazolam and propofol act on GABA-A receptors, leading to a synergistic effect [40, 41]. This effect may explain the presence of four cases in our study who experienced a transient reduction in PSI values to ≤ 25 after changing to remimazolam and why chronic benzodiazepine users included in our study did not show remimazolam tolerance and maintained their PSI effectively.
This study has several limitations. First, this study was performed at single center and had a limited sample size. Second, the assessment of QoR scores relied on responses from parents or legal guardians, which may have impeded accurate assessment of the patients’ recovery experiences. Third, the timing of anesthetic conversion to remimazolam varied among patients given the challenge of accurately predicting the duration of the surgical procedure. Lastly, our study focused on patients with mental disabilities, who often have comorbidities and receive various medications. Future research is required to validate the findings of this study.
Conclusions
In the context of outpatient general anesthesia for dental treatment in patients with mental disabilities, replacing propofol with remimazolam 1 h before the end of dental treatment and reversal with flumazenil improved recovery rates and reduced the duration of stay in the PACU, without the incidence of any adverse effects. Thus, this protocol can be considered a safe and effective anesthetic approach that prioritizes both patient safety and efficiency.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PONV:
-
Post postoperative nausea and vomiting
- ASA:
-
American Society of Anesthesiologists
- TIVA:
-
Total intravenous anesthesia
- PACU:
-
Post-anesthesia care unit
- BMI:
-
Body mass index
- Group P:
-
Propofol group
- Group PR:
-
Propofol-remimazolam group with flumazenil reversal
- PSI:
-
Patient state index
- TCI:
-
Target-controlled infusion
- QoR-15 K:
-
Korean version of the Quality of Recovery-15 questionnaire
- TOF:
-
Train of Four
- MDPE:
-
Median performance error
- MADPE:
-
Median absolute performance error
- PM:
-
Performance measurement
- CI:
-
Confidence interval
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
References
Haywood PT, Karalliedde LD. General anesthesia for disabled patients in dental practice. Anesth Prog. 1998;45:134–8.
Márquez-Arrico CF, Talaván-Serna J, Silvestre FJ, Viñoles J, Rodríguez-Martínez S, Silvestre-Rangil J. Dental treatment under general anesthesia in mentally disabled patients based on an ambulatory surgery model: a case-control study. J Clin Exp Dent. 2022;14:e192–8.
Mallineni SK, Yiu CKY. Dental treatment under general anesthesia for special-needs patients: analysis of the literature. J Investig Clin Dent. 2016;7:325–31.
Maeda S, Tomoyasu Y, Higuchi H, Ishii-Maruhama M, Egusa M, Miyawaki T. Independent predictors of delay in emergence from general anesthesia. Anesth Prog. 2015;62:8–13.
Choi J, Kim S. Delayed awakening time from general anesthesia for dental treatment of patients with disabilities. J Dent Anesth Pain Med. 2021;21:219–26.
Bergmann I, Göhner A, Crozier TA, Hesjedal B, Wiese CH, Popov AF, et al. Surgical pleth index-guided remifentanil administration reduces remifentanil and propofol consumption and shortens recovery times in outpatient anaesthesia. Br J Anaesth. 2013;110:622–8.
Gupta A, Stierer T, Zuckerman R, Sakima N, Parker SD, Fleisher LA. Comparison of recovery profile after ambulatory anesthesia with propofol, isoflurane, sevoflurane and desflurane: a systematic review. Anesth Analg. 2004;98:632 – 41, table of contents.
White PF. Propofol: its role in changing the practice of anesthesia. Anesthesiology. 2008;109:1132–6.
Bailey JM. Context-sensitive half-times: what are they and how valuable are they in anaesthesiology? Clin Pharmacokinet. 2002;41:793–9.
Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology. 1992;76:334–41.
Kim KM. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med (Seoul). 2022;17:1–11.
Sneyd JR, Gambus PL, Rigby-Jones AE. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth. 2021;127:41–55.
Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36:1021–7.
Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34:543–53.
Pan Y, Chen M, Gu F, Chen J, Zhang W, Huang Z, et al. Comparison of remimazolam-flumazenil versus propofol for rigid bronchoscopy: a prospective randomized controlled trial. J Clin Med. 2022;12:257.
Choi JY, Lee HS, Kim JY, Han DW, Yang JY, Kim MJ, et al. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: a randomized non-inferiority trial. J Clin Anesth. 2022;82:110955.
Lee HJ, Lee HB, Kim YJ, Cho HY, Kim WH, Seo JH. Comparison of the recovery profile of remimazolam with flumazenil and propofol anesthesia for open thyroidectomy. BMC Anesthesiol. 2023;23:147.
Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34:491–501.
Shi F, Chen Y, Li H, Zhang Y, Zhao T. Efficacy and safety of remimazolam tosilate versus propofol for general anesthesia in cirrhotic patients undergoing endoscopic variceal ligation. Int J Gen Med. 2022;15:583–91.
Suzuki Y, Kawashima S, Makino H, Doi M, Nakajima Y. Comparison of postoperative nausea and vomiting between remimazolam and propofol: a propensity score-matched, retrospective, observational, single-center cohort study. Korean J Anesthesiol. 2023;76:143–51.
Chung F. Discharge criteria–a new trend. Can J Anaesth. 1995;42:1056–8.
Yoon S, Joo H, Oh YM, Lee J, Bahk JH, Lee HJ. Validation and clinical utility of the Korean version of the quality of Recovery-15 with enhanced recovery after surgery: a prospective observational cohort study. Br J Anaesth. 2020;125:614–21.
Lee JH, Ki M, Choi S, Woo CJ, Kim D, Lim H, et al. Validity and reliability of the Korean version of the quality of Recovery-15 questionnaire. Korean J Anesthesiol. 2021;74:142–9.
Yoon S, Park JB, Lee J, Lee HC, Bahk JH, Cho YJ. Relationship between blood pressure stability and mortality in cardiac surgery patients: retrospective cohort study. J Clin Monit Comput. 2021;35:931–42.
Varvel JR, Donoho DL, Shafer SL. Measuring the predictive performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm. 1992;20:63–94.
Lee HC, Ryu HG, Jung CW. Performance measurement of intraoperative systolic arterial pressure to predict in-hospital mortality in adult liver transplantation. Sci Rep. 2017;7:7030.
Worthington MT, Antonik LJ, Goldwater DR, Lees JP, Wilhelm-Ogunbiyi K, Borkett KM, et al. A phase ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117:1093–100.
Chen X, Sang N, Song K, Zhong W, Wang H, Jiang J, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42:614–24.
Yamamoto T, Kurabe M, Kamiya Y. Re-sleeping after reversal of remimazolam by flumazenil. J Anesth. 2021;35:322.
Masui K. Caution!! Reappearance of remimazolam effect after a flumazenil bolus: a larger bolus of flumazenil and a lower total remimazolam clearance are higher risks. J Anesth. 2023;37:1–5.
Hvarfner A, Hammas B, Thörn SE, Wattwil M. The influence of propofol on vomiting induced by apomorphine. Anesth Analg. 1995;80:967–9.
McCollum JS, Milligan KR, Dundee JW. The antiemetic action of propofol. Anaesthesia. 1988;43:239–40.
Tramèr M, Moore A, McQuay H. Propofol anaesthesia and postoperative nausea and vomiting: quantitative systematic review of randomized controlled studies. Br J Anaesth. 1997;78:247–55.
Xiao H, Liu M, Man Y, Wei Y, Ji F. Effect of low-dose propofol combined with dexamethasone on the prevention of postoperative nausea and vomiting in gynaecological day surgery under remimazolam-based general anesthesia. Med (Baltim). 2023;102:e33249.
Mao Y, Guo J, Yuan J, Zhao E, Yang J. Quality of recovery after general anesthesia with remimazolam in patients’ undergoing urologic surgery: a randomized controlled trial comparing remimazolam with propofol. Drug Des Devel Ther. 2022;16:1199–209.
Ko CC, Hung KC, Illias AM, Chiu CC, Yu CH, Lin CM, et al. The use of remimazolam versus propofol for induction and maintenance of general anesthesia: a systematic review and meta-analysis. Front Pharmacol. 2023;14:1101728.
Shirozu K, Nobukuni K, Tsumura S, Imura K, Nakashima K, Takamori S, et al. Neurological sedative indicators during general anesthesia with remimazolam. J Anesth. 2022;36:194–200.
Miyanishi M, Yaguramaki T, Maehara Y, Nagata O. Three cases of difficulty in achieving definitive loss of consciousness with remimazolam. JA Clin Rep. 2022;8:4.
Yoshikawa H, Hosokawa M, Kashima Y, Oki S, Masui K. Remimazolam tolerance in long-term benzodiazepine users: a case report of 2 cases. Pract. 2021;15:e01460.
Lyu S, Deng Q, Lin W, Wu X. Randomized controlled trial for anesthesia during gastroscopy: interactions remimazolam and propofol in combination with sufentanil. Int J Clin Pharm. 2023;45:857–63.
Tan H, Lou AF, Wu JE, Chen SZ, Qian XW. Determination of the 50% and 95% effective dose of remimazolam combined with propofol for intravenous sedation during day-surgery hysteroscopy. Drug Des Devel Ther. 2023;17:1753–61.
Acknowledgements
None.
Funding
This work was supported by Hana Pharm, Seoul, Republic of Korea. (The grant number: 03-2022-0336).
Author information
Authors and Affiliations
Contributions
Study design, patient recruitment, data collection, data analysis, and preparation of the final manuscript draft were performed by SJ; study design, patient recruitment, and data collection were performed by JK and M-HK; Study design and preparation of the final manuscript draft were performed by J-TK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of the Seoul National University Dental Hospital (IRB # CME22002; date of approval: August 11, 2022) and registered with the Clinical Research Information Service (number: KCT0007794; date of registration: October 12, 2022). This study adhered to the principles outlined in the Declaration of Helsinki, and written informed consent was obtained from the parents or legally authorized representatives of the patients before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jeon, S., Kim, J., Karm, MH. et al. Effect of converting from propofol to remimazolam with flumazenil reversal on recovery from anesthesia in outpatients with mental disabilities: a randomized controlled trial. BMC Anesthesiol 24, 151 (2024). https://doi.org/10.1186/s12871-024-02526-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02526-5