Abstract
Purpose
Planning intraoperative fluid therapy in patients undergoing major abdominal surgery is important. It was aimed to define the difference between fluid therapy protocols for renal function, bleeding and postoperative service follow-ups.
Materials and methods
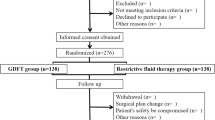
This is an observational case-controlled prospective study. Sixty patients aged 18–65 years who had undergone pancreatic surgery between December 2023– February 2023 were included in the study. Liberal (Group 1; n = 30) and targeted fluid therapies (Group 2; n = 30) were administered to the patients. Liberal fluid therapy was planned with 8–10 ml/kg/h crystalloid infusion. The targeted fluid therapy (TFT) group (Group 2; n = 30) began with a 2 ml/kg/h crystalloid infusion at the baseline. Additional fluid boluses were given in 250 ml of colloid infused over 10 min if PVI was > 13% for at least five minutes. The patients were staged using the KDIGO (Kidney Disease: Improving Global Outcomes) criteria. The amount of bleeding during surgery was recorded for both groups.
Results
No significant difference was observed in postoperative renal function. A significant difference was observed in the amount of intraoperative bleeding. The amount of bleeding was greater in patients managed with liberal fluid therapy. No significant difference was observed between the groups in the oral intake (hour), drain withdrawal (hour) mobilization (hour) and discharge (day) times and there isn’t any statistically significant differance between groups in cost effectivity (p>0.05).
Conclusion
Kidney function was preserved during individualized targeted fluid therapy using non-invasive haemodynamic monitoring parameters.
Similar content being viewed by others
Introduction
Planning ideal intraoperative fluid therapy in patients undergoing major abdominal surgery is critical because of the balance of oxygen supply consumption, fluid–electrolyte balance, adequate tissue perfusion, and prevention of excessive fluid overload [1]. Targeted fluid therapy (TFT) planned with PVI hemodynamic assessments has been associated with better outcomes, including survival [2].
Peroperative adverse effects such as pulmonary, cerebral, peripheral, and periorbital oedema, hypertension, bowel dysfunction, delayed wound healing, dilutional coagulopathy may occur because of excessive and uncontrolled fluid administration. Reduced cardiac output and impaired tissue perfusion, shock and multi-organ failure may result from inadequate fluid replacement [3].
Renal function is sensitive to volume changes, and hypovolemia may increase the risk of postoperative kidney damage [2]. TFT protocols are an effective method for preventing intraoperative fluid overload and insufficiency, as well as reducing postoperative complications in surgical patients [4].
The Pleth Variability Index (PVI) is a non-invasive cardiovascular parameter. It is a measurement based on respiratory changes and arterial pulse pressure. Using the PVI, a patient’s intravascular status can be evaluated. This is an effective method for evaluating perioperative fluid therapy in patients without vasoactive agents and vascular pathology. Effective cardiac stabilization can be achieved using less fluid. The PVI correlates with the SPV (systolic pressure variation) and PPV (pulse pressure variation) invasive parameters.
In this study it was aimed to define the difference between fluid therapy protocols for renal function and postoperative service follow-ups.
Methods
This study was initiated after Ankara City Hospital Clinical Research Ethics Committee No. 2 approved this study (Reference number E2-21-1078) and after receiving informed consent to participant was obtained from all participants. The clinical trial number is NCT06188299 (12.18.2023).
This is an observational case-controlled prospective study. Patients below the age of 18 years, presenting with cardiac arrhythmia, a cardiac ejection fraction of less than 30%, pulmonary pathology that precluded the generation of tidal volume in excess of 6 ml/kg, hepatic and renal dysfunction, and American Society of Anaesthesiologists (ASA) physical status ASA-3 were excluded from the study. Sixty patients aged 18–65 years who had undergone pancreatic surgery between December 2023– February 2023 were included in the study. Patients received either liberal (under MAP 65; Group 1; n = 30) or targeted fluid therapy (Group 2; n = 30) according to the clinician’s preference. Liberal fluid therapy was planned with 8–10 ml/kg/h crystalloid infusion. The goal-directed fluid therapy group (Group 2; n = 30) began with a 2 ml/kg/h crystalloid infusion at the baseline. PVI measurements were followed by Masimo SET version V7.1.1.5 pulse oximetry (Masimo Co., Irvine, California), which was used to continuously, automatically, and non-invasively measure plethysmographic variations throughout the respiratory cycle. Additional fluid boluses were given in 250 ml of colloid infused over 10 min if PVI was > 15% for at least five minutes. It was used 0.9% NaCl and succinylated gel (Gelofusine®, B. Braun). Patients whose mean arterial pressure could not be brought to 65 mmHg despite a PVI value of 13% or less were administered norepinephrine. Gray zone of PVI is 13 − 9. Values under 9 indicate fluid overload.
After providing 100% oxygen support with a mask for 3 min, lidocaine (1–1.5 mg/kg), propofol (2–3 mg/kg) (propofol 1%, Fresenius, Germany), remifentanil (1 µg/kg) (Ultiva®, GSK, USA), and rocuronium (0.6–1.2 mg/kg) (Esmeron®, MSD, Germany) were administered intravenously (iv) during induction. After waiting for sufficient myorelaxation, patients were intubated endotracheally. During maintenance anesthesia, 0.05–0.25 µg/ kg/ min remifentanil infusion and 2–2.5% sevoflurane (Sevorane®, liquid 100%, Abbvie, England) were administered with a 50:50% medical air: O2 mixture according to the IBW.
The basal ventilation tidal volume was set to 6–8 ml/kg, the frequency was 12, and the fraction of inspired oxygen (FiO2) was 50%. However, the ETCO2 (End-Tidal Carbon Dioxide) was maintained between 35 and 40 mmHg by changing the respiratory rate and inspiration expression ratio. The peak pressure remained below 38 cmH2O. At the end of surgery, 2 mg/kg of sugammadex (Bridion®, MSD, Netherlands) was used to reverse the residual effects of the intraoperative muscle relaxant. This was done according to the intravenous IBW.
The patients’ systolic arterial pressure (SAB), diastolic arterial pressure (DAP), mean arterial pressure (MAP), peak heart rate (PHR), oxygen saturation (SO2), and end–tidal CO2 (ETCO2) values were followed throughout the operation and recorded at five-minute intervals. Systolic and diastolic blood pressures were recorded for all patients before and after intubation and before and after extubation. All patients’ creatinine, BUN, and potassium values were recorded at 0, 1, 24, 48 and 72 h postoperatively. Patients were staged according to the KDIGO criteria. The amount of bleeding during the operation was recorded for both groups. In our study, lactate concentrations were followed by blood samples taken from patients.
All analyses were performed in SPSS v23 (SPSS Inc., Chicago, IL, USA). Compliance control of normally distributed numerical data was performed with the Shapiro‒Wilk test. None of the variables met the assumption of a normal distribution. Continuous numerical variables were analysed with the Mann‒Whitney U test. The mean, standard deviation, median, minimum, and maximum values of these variables are given. Chi-square analysis was performed for categorical variables. The frequency and percentage values of these variables are given and p values < 0.05 were considered to indicate statistical significance.
Results
There was no difference in demographic data between the groups. There was no significant difference between the groups in terms of the blood pressure measured for all patients. (p > 0.05). A significant difference was observed in the amount of intraoperative bleeding. The amount of bleeding was greater in patients managed with liberal fluid therapy (Table 1).
No significant difference was observed in postoperative renal function. The patients were staged according to the KDIGO criteria, and no significant difference was observed between the groups (p = 0.26) (Table 2).
The patients were analised according to the postoperative service follow-ups. No significant difference was observed between the groups in the oral intake (hour), drain withdrawal (hour) mobilization (hour) and discharge (day) times and there isn’t any statistically significant differance between groups in cost effectivity (p>0.05) (Table 3).
BUN and creatinine levels were not significantly different between the groups (p > 0.05). Group 2s’ urine output was significantly higher than the group1 (Figs. 1, 2 and Table 4).
Discussion
In this study, we concluded that there was no significant difference between different fluid therapies and postoperative renal functions.
A recent clinical trial, the RELIEF study, showed no significant difference in mortality between restrictive and free fluid therapy groups for patients following major abdominal surgery [5]. Another study suggested that administering more fluid is associated with biomarkers indicating endothelial damage and glycocalyx damage, and other factors may be responsible for the results of the RELIEF study [6].
Forget et al., observed no difference in the occurrence of postoperative kidney disease with targeted fluid therapy [7].
In a previous study, PVI-guided fluid management resulted in less perioperative administration of crystalloids and lower lactate levels during and after major abdominal surgery [7]. Hemodynamic parameters were stable, and lactate levels were lower in gynecological surgery patients who were followed up for PVI [8].
In a study by Çevikalp et al., the “pleth variability index” (PVI) provided more accurate results in evaluating the intravascular volume of the patient compared to fluid resuscitation based on central venous pressure monitoring of fluid therapy [9]. Delayed graft function has been associated with a PVI > 9 in renal transplantation patients [10].
In morbidly obese patients who underwent laparoscopic Roux-en-Y gastric bypass (RYGB) surgery, there was no difference between the inflow and outflow creatinine values in the group with PVI monitoring and those who underwent fluid resuscitation with mean arterial pressure monitoring [11].
In orthopedic surgery, no difference was found in acute postoperative renal failure in fluid treatment structured with PVI follow-up. (p = 0.808) [12]. In accordance with the literature, no difference in acute renal damage was found in this study.
In this study revealed that bleeding was greater in the group that received liberal fluid therapy. In a previous study by Gottin et al., targeted and restrictive fluid protocols were associated with a lower incidence of complications than liberal fluid protocols [13].
A study comparing liberal and restrictive fluid therapy revealed that a positive fluid balance might be associated with damage to the endothelium and glycocalyx layers [5]. The greater amount of bleeding in the liberal fluid protocol group was associated with dilution of coagulation factors and damage to the endothelium and glycocalyx layer.
It has been highlighted in the literature that the administration of substantial quantities of fluid may potentially result in the exacerbation of bleeding due to the development of secondary coagulation disorders. In individuals who are not pregnant and have experienced significant blood loss, the implementation of restrictive fluid management has been demonstrated to prevent the progression to a state of dilution coagulopathy [14].
Another investigation examined the impact of haemodilution on the results of global coagulation tests and clotting factors. The findings revealed a notable decline in the levels of dilution-dependent coagulation factors and aPTT, exhibiting a nearly linear reduction. The measurement of critically low activities for coagulation factors and a critically low level of fibrinogen at dilutions of between 60 and 75% indicates a notable decrease in the examined substances [15].
In their study, Bickell et al. compared the coagulation parameters in patients with penetrating torso injuries who were treated with either immediate or delayed fluid resuscitation. The patients who received immediate fluid administration exhibited diminished levels of haemoglobin, platelet count, PT and APTT in contrast to those who received delayed fluid administration [16].
On the contrary, the superiority of restrictive fluid resuscitation in women with mild postpartum haemorrhage has not been proven, it does not increase the need for blood transfusion, alter coagulation parameters or cause an increase in adverse events. It can be considered as an alternative treatment option to liberal fluid resuscitation [17].
However in a large systematik metaanalysis, the postoperative rebleeding did not differ in both groups: RR, 0.76 (95% CI, 0.28–2.06). The study concluded that a restrictive fluid policy in elective surgery, in comparison to a liberal fluid policy, resulted in a 35% reduction in patients with complications. It is therefore recommended that a restrictive fluid policy should be advised as the preferred fluid management policy [18]. In this study, the PVI-guided targeted fluid therapy protocol was associated with less bleeding.
Our study had several limitations. Although we included all patients who underwent pancreatic surgery in our hospital within a particular time period, our sample size was limited to 60 patients Four patients died during the course of the study, all of whom were undergoing liberal fluid therapy. The mortality rate was not significant since the sample size is small. In addition to these parameters, we looked at lactate follow-up in patients, but we primarily evaluated the parameters for the KDIGO criteria.
Conclusion
In conclusion, targeted fluid therapy does not impair postoperative renal functions, and not enhaced the recovery however optimization of tissue perfusion in high-risk surgical procedures should be based on an individualized targeted fluid therapy approach.
Data availability
Data is provided within the manuscript.
References
Messina A, Robba C, Calabrò L, Zambelli D, Iannuzzi F, Molinari E, Scarano S, Battaglini D, Baggiani M, De Mattei G, Saderi L, Sotgiu G, Pelosi P, Cecconi M. Perioperative liberal versus restrictive fluid strategies and postoperative outcomes: a systematic review and metanalysis on randomized-controlled trials in major abdominal elective surgery. Crit Care. 2021;25(1):205. https://doi.org/10.1186/s13054-021-03629-y.
Oh TK, Song IA, Do SH, Jheon S, Lim C. Association of perioperative weight-based fluid balance with 30-day mortality and acute kidney injury among patients in the surgical intensive care unit. J Anesth. 2019;33(3):354–63. https://doi.org/10.1007/s00540-019-02630-8.
Navarro e Lima LH, Papa FV, Amorim CG, et al. Perioperative fluid therapy: more questions than definitive answers. Braz J Anesthesiol. 2022;72(6):683–4.
Messina A, Robba C, Calabro L, et al. Association between periop- erative fluid administration and postoperative outcomes: a 20-year systematic review and a meta-analysis of randomized goal-directed trials in majör visceral/noncardiac surgery. Crit Care. 2021;25:43.
Bihari S, Dixon DL, Painter T, Myles P, Bersten AD. Understanding restrictive Versus Liberal Fluid Therapy for major abdominal surgery trial results: did Liberal fluids associate with increased endothelial Injury markers? Crit Care Explor. 2021;3(1):e0316. https://doi.org/10.1097/CCE.0000000000000316.
Mason SA, Nathens AB, Finnerty CC, et al. Hold the pendulum: rates of acute kidney injury are increased in patients who receive resuscitation volumes less than predicted by the Parkland equation. Ann Surg. 2016;264:1142–7.
Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010;111(4):910–4. https://doi.org/10.1213/ANE.0b013e3181eb624f.
Yilmaz G, Akca A, Kiyak H, Can E, Aydin A, Salihoglu Z. Pleth Variability Index-based goal-Directed Fluid Management in patients undergoing elective gynecologic surgery. Sisli Etfal Hastan Tip Bul. 2022;56(2):220–6. https://doi.org/10.14744/SEMB.2021.81073.
Çevikkalp E, et al. Efficacy of the Pleth Variability Index (PVI) for evaluating intraoperative Fluid Management during Orthopedic spinal surgery: a randomized controlled. Trial ARSS. 2020;28(1):18–25. https://doi.org/10.5222/jarss.2020.57441.
Collange O, Jazaerli L, Lejay A, et al. Intraoperative Pleth Variability Index is linked to delayed graft function after kidney transplantation. Transpl Proc. 2016;48(8):2615–21. https://doi.org/10.1016/j.transproceed.2016.06.046.
Demirel İ, Bolat E, Altun AY, Özdemir M, Beştaş A. Efficacy of goal-Directed Fluid Therapy via Pleth Variability Index during Laparoscopic Roux-en-Y gastric bypass surgery in morbidly obese patients. Obes Surg. 2018;28(2):358–63. https://doi.org/10.1007/s11695-017-2840-1.
Fischer MO, Lemoine S, Tavernier B, et al. Individualized Fluid Management using the Pleth Variability Index: a Randomized Clinical Trial. Anesthesiology. 2020;133(1):31–40. https://doi.org/10.1097/ALN.0000000000003260.
Gottin L, Martini A, Menestrina N, Schweiger V, Malleo G, Donadello K, Polati E. Perioperative Fluid Administration in pancreatic surgery: a comparison of three regimens. J Gastrointest Surg. 2020;24(3):569–77. https://doi.org/10.1007/s11605-019-04166-4.
Gillissen A, van den Akker T, Caram-Deelder C, et al. Association between fluid management and dilutional coagulopathy in severe postpartum haemorrhage: a nationwide retrospective cohort study. BMC Pregnancy Childbirth. 2018;18:398. https://doi.org/10.1186/s12884-018-2021-9.
Weiss G, Lison S, Spannagl M, Heindl B. Expressiveness of global coagulation parameters in dilutional coagulopathy. Br J Anaesth. 2010;105(4):429–36. https://doi.org/10.1093/bja/aeq199.
Bickell WH, Wall MJ Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–9. https://doi.org/10.1056/NEJM199410273311701.).
Schol PBB, de Lange NM, Woiski MD, Langenveld J, Smits LJM, Wassen MM, Henskens YM, Scheepers HCJ. Restrictive versus liberal fluid resuscitation strategy, influence on blood loss and hemostatic parameters in mild obstetric hemorrhage: an open label randomized controlled trial. (REFILL study). PLoS ONE. 2021;16(6):e0253765. https://doi.org/10.1371/journal.pone.0253765. PMID: 34170943; PMCID: PMC8232446.
Schol PB, Terink IM, Lancé MD, Scheepers HC. Liberal or restrictive fluid management during elective surgery: a systematic review and meta-analysis. J Clin Anesth. 2016;35:26–39. https://doi.org/10.1016/j.jclinane.2016.07.010. Epub 2016 Aug 4. PMID: 27871539.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ATDÖ and HG: reviewed the manuscript NT and UCE: collecting data. KY and YY: Prepared figures and tables. EÖ and CC: Writing main manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ankara City Hospital Clinical Research Ethics Committee No. 2 approved this study (Reference number E2-21-1078). All the authors declare their approval of the Helsinki Declaration.
Consent for publication
Informed consent to participant was obtained from all participant.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dumanlı Özcan, A., Taş, N., Ersoy, U.C. et al. Effects of intraoperative different fluid therapy protocols on postoperative renal functions. BMC Anesthesiol 24, 299 (2024). https://doi.org/10.1186/s12871-024-02679-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02679-3