Abstract
Background
Coronary artery disease (CAD) is the most common heart disease. Several studies have shown association between some polymorphism in different genes with CAD. Finding this association can be used in order to early diagnosis and prevention of CAD.
Method
101 CAD patients with ≥ 50% luminal stenosis of any coronary vessel as case group and 111 healthy individuals as control group were selected. the polymorphisms were evaluated by ARMS-PCR and RFLP-PCR methods.
Result
The results of this study show that there is no significant association between rs17228212, rs17465637, and rs708272 and risk of CAD. But there is significant association between risk of CAD and rs5355 (p-value = 0.022) and rs3917406 (p-value = 0.006) in total cases, and rs5882 (p-value = 0.001) in male cases.
Conclusions
Our findings revealed a significant interaction between CETP SNPs and CETP activity for affecting HDL-C levels. The SELE gene is a known cell adhesion molecule with a significant role in inflammation. Studies about possible linkage between SELE gene polymorphisms and the development of CAD are conflicting. We have found a significant association between polymorphisms of SELE gene and risk of CAD.
Similar content being viewed by others
Background
Cardiovascular diseases are among the major causes of fatality worldwide. Coronary artery disease (CAD) is a cardiac disorder with the highest prevalence resulting from coronary artery stenosis and atheroma plaque development. CAD is caused by multiple factors as a result of genetic parameters and environmental impacts and their interplays. Traditionally, several risk factors can merely elucidate a limited number of cases found in reports [1]. Thus, genetic parameters play a critical role in disease development. Studies have focused on multiple CAD-related genes. The hypothesis that CAD results from the heredity of genes with high or low penetrance responsible for inflammation procedures are nowadays of great interest [2].
In a genome-wide association investigation [3], specific single nucleotide polymorphism (SNP) at chromosome 1q41, rs17465637, was reported to have an association with CAD and myocardial infarction (MI). The SNP exists in the melanoma inhibitory activity-3 gene (MIA3), also referred to as TANGO, ARNT, D320, ODCD2, TANGO1, and UNQ6077 with a wide-ranging expression pattern. Existing evidence shows that TANGO is a member of a gene family of four homologous proteins, TANGO, MIA, OTOR (FDP, MIAL), and MIA2. By joining TANGO with the homologous proteins, a gene family is defined that shares essential structural specifications, substantial homology at both nucleotide and protein levels, and analogous arrangement in the genome [4]. A 14-kDa protein with unidentified activity hitherto is encoded by the TANGO gene. The down-regulation or loss of TANGO was reported in all hepatocellular and colon cell lines in comparison to primary human hepatocytes or normal colon epithelial cells in respective order and in the majority of the tumor samples including malignant melanoma and colon and hepatocellular carcinoma in comparison to tumor-free tissues. Therefore, TANGO was proposed to possibly serve as a tumor-suppressive gene in malignant melanoma [5, 6]. Moreover, TANGO can supposedly influence an increase in the risk of CAD via inflammatory procedures. The critical function of the inflammatory modes of action in forming atheroma is supported by copious data. This process involves leukocyte mobilization and pivotal participation of proinflammatory cytokines in the primary phase of atherogenesis [7]. Endothelial cells are activated for inflammation, followed by the entry of monocytes and other leukocytes into the atheroma. The direct binding of TANGO protein occurs to the leukocyte-specific b2-integrin CD11c/CD18, being responsible for leukocyte adherent interplays with vascular endothelium. Experimentally, it is evidenced that the TANGO protein modulates CD11c/CD18 activity, thereby reducing the adhesion and promoting monocyte migration through the endothelium [8]. After trans-migration, monocytes are differentiated into macrophages, forming the fatty streak that primarily indicates atherosclerosis in the arterial intima. Thus, atherosclerosis is driven by inflammation, eventually triggering thrombotic plaque consequences, often causing myocardial infarction (MI) and CAD [7]. In some earlier reports, the rs17465637 and CAD were also reported to have an association [9].
As stated above, atherosclerosis develops and progresses by the mobilization and coupling of circulatory leukocytes to inflammation sites inside the vascular endothelium [10]. This progression is mainly under the modulation and regulation of wide-ranging adherence molecules, such as selectins, integrins, immunoglobins, and chemokines [11]. The selectins are a class of cell adherence molecules responsible for chronic and acute inflammation procedures. The expression of these single-chain transmembrane glycoproteins occurs on endothelial cells when they are stimulated by inflammatory cytokines. The adherence of pathological cells to endothelium is determined by the expression profile of selectins on the vascular wall because the adherence is facilitated when endothelial selectins are present. A connection between selectins and the incidence of CAD is supported by growing evidence [12]. The SELE gene is situated on chromosome 1q22-q25. P-selectin, E-selectin, and L-selectin form the family of selectins. These three selectins differ fundamentally in their distribution, activation, and expression. The E-selectin expression only happens on the actuated endothelium and mobilizes polymorphonuclear leukocytes, myeloid cells, and T-lymphocytes to the inflammation areas [13]. The P-selectin expression occurs on both the endothelial cells and platelets and has an instant migration to the plasma membrane after exposure to some free radicals, nitric oxide synthase suppressors, and other intermediaries [14]. The expression of L selectin is seen on most kinds of leukocytes, as well as on some neutrophils and myeloid cells [13]; it acts as a homing receptor for lymphocytes to peripheral lymph nodes. Similar to E-selectin, L-selectin mobilizes cells to inflammation lesions. Currently, the SELE gene is reported as a high-risk factor involved in atherosclerosis development. An association between variations in rs3917406 and rs5355 (G1839T) of the SELE gene with CAD was reported previously [15, 16].
The present study focused on the SMAD3 gene that plays a role in the TGFB signaling route and has a major contribution to the inflammation procedure. Eight Smad family members (Mad-homologues (MADH), MADH2, MADH4, and MADH7 map to chromosome 18q21-22, a tumor suppressor locus, MADH3 and MADH6 map to chromosome 15q21-22, and MADH5, MADH1, and MADH8 to chromosomes 15q31, 4 and 13, respectively) are encoded by the human genome [17]. Three functional subfamilies of Smads include receptor-activated Smads (R-Smads: Smad1, Smad2, Smad3, Smad5, and Smad8), the phosphorylation of which is done by type I receptors; common mediatory Smads (Co-Smads: Smad4), which oligomerizes with activated R-Smads; and inhibitive Smads (I-Smads: Smad6 and Smad7), which are stimulated by members of the TGF-β family. The last one applies a negative feedback impact via competition with R-Smads for receptor interplay and through labeling the receptors for disintegration [18]. TGF-β1/SMAD3-dependent routes have a major mediatory contribution to diverse biological impacts of TGF-β1, including cell multiplication, immunity repression, and inflammation [19]. A heteromeric complex with the type II receptor is formed by binding TGF-β1 to its type I receptors, which then phosphorylates and activates the TGF-β1 downstream signaling molecules SMAD2 and SMAD3. After the heterodimerization of activated SMAD2 or SMAD3 with SMAD4, the mentioned complex is introduced into the nucleus, wherein it is engaged in regulating its target gene expression [19, 20]. Marked activation of myocardial TGF-β1 expression occurs in the cardiovascular system of patients with hypertrophic or dilated cardiomyopathy and in experimental models of myocardial hypertrophy and MI [21]. In other investigations, TGF-β signaling is proposed to be critical for suppressing the expression of inflammatory genes in healing infarcts by mediating an inflammatory infiltrate [22]. TGF-β1 is critical in the pathogenicity of infarct healing, cardiac remodeling. Through a cascade of intricate reactions, TGF-β modulates the balance of pro-inflammatory and anti-inflammatory T-cells in the immune system. SMAD3 has a major contribution to the down-regulation of T-cells and the rise of FoxP3 expression, a key stage for the differentiation of regulative T-cells [23]. The imbalance of proinflammatory Th17 and regulative T-cells is reported in acute coronary syndrome [24]. In general, few studies are available clinically on TGF-β1/SMAD3 signals in sizable groups of patients. In cardiovascular physiological processes, another regulative system is the lipoprotein system, where the anti-atherosclerotic impacts of HDL are practiced via a route called reverse cholesterol transfer (RCT), which is a cholesterol transportation route from extrahepatic cells and tissues to the liver [25]. This process involves the removal of cholesterol and phospholipids from the cell by the adenosine triphosphate-dependent transporter (ABCA1) to A-I apolipoproteins to form non-esterified HDL. Eventually, cholesterol is esterified by the plasma enzyme lecithin-cholesterol-acyltransferase (LCAT) to form mature HDL. This cholesterol is then taken directly or indirectly to the liver to be excreted in the bile. Indirectly, esterified cholesterol is transported to VLDL / LDL lipoproteins by the action of cholesteryl ester transfer protein (CETP) and finally to endocytosis by liver cells. In the direct pathway, HDL binds to liver receptors (SR-BI) and fat enters the liver cells [26]. This study focused on the main CETP gene in the RCT procedure. The CETP gene is located on 16q13, encoding a hydrophobic glycoprotein. Via participation in the RCT route, the cholesterol homeostasis in the body is maintained by cholesteryl ester transfer protein (CETP) [27].
CETP protein is a key plasma glycoprotein that is primarily produced by the liver and affects circulating HDL levels by transporting esterified cholesterol from HDL to APOB-containing particles instead of receiving triglycerides. As a result of this transfer, APOB-containing particles that are atherogenic increase and HDL levels decrease [28]. There are reports of declined levels of high-density lipoprotein cholesterol (HDL-C) and raised risk of CAD caused by the elevated activity of the CETP gene in some studies [29]. The influences of the two polymorphisms, rs708272 (G277A) and rs5882 (I405V), and the risk of vascular diseases has also been demonstrated previously [30]. According to existing reports, multiple genetic variants in the CETP gene have associations with changes in the plasma concentrations and function of CETP, HDL-C plasma concentrations, LDL and HDL particle size, and possibly the risk of CAD. This research aims to investigate the relationship between rs17465637 in the MIA3 gene, rs3917406 and rs5355 in the SELE gene, rs17228212 in the SMAD3 gene, and rs708272 and rs5882 in the CETP gene, with the risk of CAD.
Materials and methods
Ethics statement
After the approval of this study by the Ethics Committee of Rasad Pathobiology and Genetic Laboratory (Tehran, Iran), all participants presented written informed consent forms before participating in this research.
Subject selection
From April 2015 to March 2016, two Iranian groups were enrolled at Baqiyatallah Hospital, Tehran. The research sample consisted of 101 case-patients and 111 age- and sex-frequency-matched controls. CAD populations with either (a) the presence of stenosis ≥ 50% in a minimum of one main segment of coronary arteries (the right coronary artery, left circumflex, or left anterior descending arteries) by coronary angiography, (b) symptoms representing angina pectoris, electrocardiographic changes, and elevations of cardiac enzymes based on the criteria of the World Health Organization, and (c) a certified record of coronary artery bypass graft or percutaneous coronary intervention were included in the study. Exclusion criteria were patients with congenital heart disease, cardiomyopathy, and valvular disease. The control groups resided in the same populations as the cases and were specified to be without CAD, cardiovascular and cerebrovascular diseases, and peripheral atherosclerotic arterial disease by medical records, examining clinically, and electrocardiography. Participants with acute liver and/or kidney disease were also subjected to exclusion. Data on demographic variables, medical records, using a medication, and lifestyle factors were collected using structured questionnaires by skilled interviewers. The participants were described as smokers and nonsmokers. Subjects with a smoking frequency of below 100 cigarettes in the previous lifespan were grouped as nonsmokers; otherwise, they were determined as smokers. Body mass index (BMI) was obtained via dividing the weight (kg) by the square of the height (m). Additionally, patients with hypertension were considered as those having a previous diagnosis of hypertension and consuming antihypertensive medicines, or patients with a systolic blood pressure of 140 mm Hg, or a diastolic blood pressure of 90 mm Hg. Plasma levels of glucose, total cholesterol concentrations were measured by enzymatic colorimetric method (240 or Higher than 240 mg/dl).
After obtaining informed consent forms from all subjects, samples of peripheral blood were collected in EDTA-containing tubes. DNA was extracted using a Gene All kit. The ARMS-PCR method was employed for genotyping SNPs rs5882 and rs708272 RFLP-PCR and SNPs rs3917406, rs5355, rs17228212, and rs17465637.
Genotyping by ARMS-PCR and RFLP-PCR methods
For genotyping SNPs rs3917406, rs5355, rs17228212, and rs17465637 by the ARMS-PCR method, three primers were made for each polymorphism. PCR was done in a 25-µl reaction volume comprising 2 µl of genomic DNA, 8 µl of ddH2O, 12 µl of Master Mix (Ampliqon, Odense, Denmark), and 1 µl of every specific primer (Table 1). Amplifying by PCR was started by initial denaturation at 95 °C (4 min), and then amplifying with 30 cycles of 95 °C (45 s), (rs3917406 [64 °C], rs5355 [66 °C], rs17228212 [58 °C], and rs17465637[59 °C] (45 s), 72 °C (45 s), finalized by extension at 72 °C (7 min). Then PCR product were subjected to electrophoresis on 2% agarose gel.
The RFLP-PCR technique using one pair of the primers and one specific restriction enzyme was employed for genotyping SNPs rs5882 and rs708272 (Table 2). A 25-µl reaction volume containing 2 µl of genomic DNA, 8 µl of ddH2O, 12 µl of Master Mix (Ampliqon, Odense, Denmark), and 1 µl of every specific primer was prepared for PCR. The following settings were considered for PCR. Denaturation was initiated at 95 °C (4 min) and then amplification was done for 30 cycles consisting of denaturation at 95 °C (45 s), annealing (rs5882 [60 °C] and rs708272 [62 °C] for 45 s), extension at 72 °C (45 s), and an endpoint extension at 72 °C (7 min). The restriction enzyme was used to digest 10 μl of the PCR product at 37and 65 °C for Rsa1 and Taq1B, respectively, for about 12 h at night. The PCR fragments were subjected to electrophoresis on 2% agarose gel. The following are the final three genotypes of rs5882: GG (308 bps), AG (40, 268, and 308 bps), and AA (40, 268 bps), with GG (535bps), AG (174, 361, and 535bps), and AA (174, 361bps) as the three rs708272 genotypes. The Sanger sequencing technique was also applied to confirm the low number of results determined by ARMS-PCR and RFLP-PCR (available upon request).
Statistics
Data were analyzed statistically using SPSS version 18.0. The different frequencies of alleles and genotypes between the case and control subjects were examined in terms of Hardy–Weinberg equilibrium by the Chi-square (X2) test. The relationship between the genotypes and CAD as well as between the clinical covariates (such as age, height, weight, and BMI) and CAD in the patient and healthy groups were compared using the independent samples test. A p-value of ≤ 0.05 was regarded as statistical significance. Moreover, the values for odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to evaluate the relationship between the genotypes and the risk of CAD.
Result
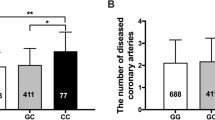
Table 3 summarizes the attributes of the CAD and non-CAD patients both clinically and biochemically. According to the test results, the control and patient subjects were statistically different concerning the height (p-value < 0.05), but they were almost similar in weight, age, and BMI (p-value > 0.05). As revealed by the results of the Chi-square test (× 2), the gender (OR = 2.36 (CI 1.36, 4.1); p-value = 0.002) and history of smoking (OR = 4.7(CI 1.0, 22.2); p-value = 0.035) were significantly different among control and patient groups, but no statistical differences were found for histories of hypercholesterolemia, diabetes, and hypertension (p-value > 0.05). The genotypes and allele frequencies of the MIA3, SELE, SMAD3, and CETP, polymorphisms are shown in Table 4. The TT genotype of the rs3917406 polymorphism was more frequent in the CAD patients than in the healthy group (p-value = 0.006). The CC genotype of the rs5355 polymorphism was more frequent in the CAD patients than in the healthy subjects (p-value = 0.022). A rising trend was noticed in the frequency of the GG genotype of the rs5882 polymorphism in male patients in comparison to that in healthy subjects (Table 5), which was different significantly (p-value = 0.001). The examined participants were not statistically different in terms of allele and genotype frequencies for the other polymorphisms, namely rs708272, rs17465637, and rs17228212.
Discussion
Cardiovascular diseases are one of the leading causes of death around the world. Coronary artery disease (CAD) is the most common heart disorder, caused by coronary artery stenosis and the formation of atheroma plaques [1]. Recent genome-wide association studies have found a link between elevated coronary artery disease susceptibility and particular SNPs within the genome that play a role in combination with other established CAD risk factors, but the exact mechanism remains unknown. Atherosclerosis is one of the key pathophysiological causes of CAD, according to clinical observation [31]. In the current research, the relationship of rs17465637 with susceptibility to CAD was examined among adults in Iran, and no significant association was detected between the rs17465637 in the MIA3 gene and CAD (OR = 0.57(CI 0.31, 1.05); p-value = 0.069). In a meta-analysis, on the other hand, Xiuchun Li et al. presented evidence of a case–control association in the Chinese Han population with 2503 CAD patients and 2920 controls. Their report suggested a significant association of the SNP rs17465637 in MIA3 with CAD (p-value = 0.01, OR = 1.11), with a rigorous confirmation by follow-up meta-analyses in five admixed Asian populations with 7263 CAD cases and 8347 controls for CAD. Highly significant relationships were reported between the SNP rs17465637 and CAD in the populations from Asia (p-value = 4.97 *10¯5, OR = 1.11). These data are strong supporters of the reality that the SNP rs17465637 in MIA3 of populations in Asia confers a substantive risk of CAD [32]. Unlike our findings, GWAS mostly denotes the C allele of this variant as the risky one. The rs17465637-A, the rapid development of carotid plaques, and the elevated risk of cardiovascular incidents tended to be associated among rheumatoid arthritis patients with dyslipidemia, though, the logistic regression analysis showed that this was not significant [33]. From another point of view, rs17465637-C presented 20% greater odds (OR = 1.20; CI (1.12, 1.30)) of CAD in a European cohort examined in a meta-analysis of the WTCC and the German MI Family Study [3]; other meta-analyses represented the same findings [34,35,36]. Similarly, Han Chinese showed such a relationship, with (OR = 1.11; CI (1.02, 1.21)) [32]. Additionally, rs17465637-C showed associations with myocardial infarction among Japanese (OR = 1.45, p-value = 0.006) and white Americans (P-adjusted = 0.0034) [9, 37]. In a meta-analysis of 11 investigations from Europe, China, the United States, and New Zealand, 1q41 polymorphisms and CAD showed relationships and the C allele was the risky allele for CAD rather than the A allele [38]. However, allele A presented a protecting impact against CAD in a study by Wang et al. [9]. Saleem Ullah Shahid et al. found no statistical differences between the CAD cases and controls, hence, rs17465637 cannot be regarded as a risk factor for CAD independently in the examined population [39]. Atherosclerosis is characterized by a chronic inflammatory procedure, in which E-selectin is a mediator and its expression occurs on the vascular endothelium cell in response to several inflammatory stimuli [40]. The concentration of E-selectin rises in patients with atherosclerosis and CAD [41]. founded a relationship between the E-selectin polymorphism and E-selectin levels (p-value = 0.031) in the Taiwanese population was reported by Wu et al. [16]. Hongwei Shan et al. [42] also detected that the E-selectin rs3917406 polymorphism and CAD risk were significantly associated in a Han population of China. The authors discovered that the T allele of rs3917406 comprised 47.8% in the CAD group, which was statistically greater than that in the control group (42.8%, p-value = 0.009). In the present study, an association between the T allele of the rs3917406 polymorphism and the elevated risk of CAD was found in comparison to the C allele (OR = 0.58(CI 0.397, 0.857); p-value = 0.006). Regarding the next polymorphism rs5355, this research revealed that the E-selectin rs5355 SNP was significantly linked to CAD (OR = 2.3 (CI 1.11, 4.79); p-value = 0.022), which corresponds to findings in Mexican people [43]. As a result, it increases the risk of CAD by almost 2.5 times. Contrary to the current findings, Issac et al. investigated the Leu575Phe C1880T (rs5355) polymorphism and claimed that this SNP had no association with the risk of developing carotid atherosclerosis in end-stage renal disease in a population in Egypt [44]. Similarly, the research on the Han population revealed that the rs5355 C/T polymorphism rate was not significantly different in hypertension and control groups [45]. Therefore, the two SNPs in the SELE gene can differentially interact with other confounding factors and have relationships with the pathogenic activity of vascular disorders. The relationship of TGF-β1 and SMAD3 concentrations with the risk factors of CAD indicates the possible contribution of this cytokine to the formation of CAD by the regulation of atherogenesis. According to the present findings, the rs17228212 polymorphism has no association with the risk of CAD (p-value = 1.0), which corresponds to that of Saleem Ullah Shahid et al. who reported no connection between rs17228212 in the SMAD3 gene and the risk of CAD [39]. Moreover, Vincent G Haver (2014) analyzed the link of seven SNPs, including rs17228212 at seven well-known CAD risk loci in 3,320 individuals with the diagnosis of systolic heart failure of ischemic etiology. All the seven loci showed no significant association with heart failure [46]. Samani et al. [3] on the contrary, identified several additional loci, namely 15q22.33 (rs17228212), which were significantly linked to CAD by a combinative analysis of both investigations. In susceptibility to CAD, the genetic variation of CETP mainly determines inter-individual variations. The contribution of the CETP rs708272 polymorphism to the recurring risk of CAD may be through the remodeling of anomalous HDL-C and by destroying the anti-atherogenic features of HDL-C. Therefore, it is possible to prevent and treat CAD by potentially targeting the genes involved in HDL. Our observations denote that the CETP rs708272 polymorphism is non-significantly associated with the risk of CAD in the Iranian population, which corresponds to those reported elsewhere indicating no association of the CETP rs708272 polymorphism with CAD [47, 48]. Contrary to the present findings, Bhanushali and Das [48], Rahimi et al. [49], Kaman et al. [50], and Iwanicka et al. [51] found positive relationships. Conflicting findings were observed in previous investigations on the impact of the next CETP rs5882 polymorphism on CAD [48, 52,53,54]. In a vast meta-analysis using 10,313 cases with coronary complications and 32,244 controls from 18 available investigations, rs5882 genotypes were associated with moderately inhibited CETP action (hence with the modest increase of HDL-C) and reversely with CAD [53]. Corresponding to this study, no relationship between the rs5882 polymorphism and the risk of coronary atherosclerosis has more recently been reported by some researchers [48, 54]. However, the association of rs5882 with CAD in healthy and diseased men groups was demonstrated by an additionally analyzed differential relationship (OR = 2.54(CI 1.44, 4.48); p-value = 0.001).
Conclusions
In conclusion, the results of current case–control studies show the importance of polymorphisms.
genetic effects on risk of CAD in the Iranian population. According to our findings, it can be concluded that rs17228212, rs17465637, and rs708272 are not associated significantly with the risk of CAD. However, the rs5355 (p-value = 0.022) and rs3917406 (p-value = 0.006) in total subjects, and rs5882 (p-value = 0.001) in men patients were associated significantly with the risk of CAD. The different observations in our research and other investigations can arise from different sizes of surveyed subjects, race differences, and ignoring SNPs-SNPs and gene-environment interplays.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the ClinVar. [SCV002515853–SCV002515858].
Abbreviations
- CAD:
-
Coronary artery disease
- MI:
-
Myocardial infarction
- MIA3:
-
Melanoma inhibitory activity 3 gene
- ABCA1:
-
Adenosine triphosphate-dependent transporter to A-I apolipoproteins
- LCAT:
-
Lecithin-cholesterol-acyltransferase
- RCT:
-
Reverse cholesterol transfer
- CETP:
-
Cholesteryl ester transfer protein
- SNP:
-
Single nucleotide polymorphism
- HDL-C:
-
High-density lipoprotein cholesterol
References
Lanktree MB, Hegele RA. Gene-gene and gene-environment interactions: new insights into the prevention, detection and management of coronary artery disease. Genome Med. 2009;1(2):1–1.
Ali M, Girgis S, Hassan A, Rudick S, Becker RC. Inflammation and coronary artery disease: from pathophysiology to Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Coronary Artery Dis. 2018;29(5):429–37.
Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357(5):443–53.
Bosserhoff AK, Moser M, Buettner R. Characterization and expression pattern of the novel MIA homolog TANGO. Gene Expr Patterns. 2004;4(4):473–9.
Arndt S, Bosserhoff AK. TANGO is a tumor suppressor of malignant melanoma. Int J Cancer. 2006;119(12):2812–20.
Arndt S, Bosserhoff AK. Reduced expression of TANGO in colon and hepatocellular carcinomas. Oncol Rep. 2007;18(4):885–91.
Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–51.
Arndt S, Melle C, Mondal K, et al. Interactions of TANGO and leukocyte integrin CD11c/CD18 regulate the migration of human monocytes. J Leukoc Biol. 2007;82:1466–72.
Wang AZ, Li L, Zhang B, Shen GQ, Wang QK. Association of SNP rs17465637 on Chromosome 1q41 and rs599839 on 1p13.3 with Myocardial Infarction in an American Caucasian Population. Ann Hum Genet. 2011;75(4):475–82.
Ellsworth DL, Bielak LF, Turner ST, Sheedy PF 2nd, Boerwinkle E, et al. Gender- and age-dependent relationships between the E-selectin S128R polymorphism and coronary artery calcification. J Mol Med. 2001;79:390–8.
Zhang J, Alcaide P, Liu L, Sun J, He A, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS ONE. 2011;6:e14525.
Wu Z, Lou Y, Lu L, Liu Y, Chen Q, Chen X, Jin W. Heterogeneous effect of two selectin gene polymorphisms on coronary artery disease risk: a meta-analysis. PLoS ONE. 2014;9(2):e88152.
Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213.
Lefer DJ. Pharmacology of selectin inhibitors in ischemia/reperfusion states. Ann Rev Pharmacol Toxicol. 2000;40:283.
Sakowicz A, Fendler W, Lelonek M, Pietrucha T. Genetic variability and the risk of myocardial infarction in Poles under 45 years of age. Arch Med Sci AMS. 2010;6(2):160.
Wu S, Hsu LA, Teng MS, Lin JF, Chang HH, Sun YC, Chen HP, Ko YL. Association of SELE genotypes/haplotypes with sE-selectin levels in Taiwanese individuals: interactive effect of MMP9 level. BMC Med Genet. 2012;13(1):1–8.
Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-β signal transduction. J Cell Sci. 2001;114(24):4359–69.
Grishin NV. Mh1 domain of Smad is a degraded homing endonuclease. J Mol Biol. 2001;307(1):31–7.
Xu F, Lin SH, Yang YZ, Guo R, Cao J, Liu Q. The effect of curcumin on sepsis-induced acute lung injury in a rat model through the inhibition of the TGF-β1/SMAD3 pathway. Int Immunopharmacol. 2013;16:1–6. https://doi.org/10.1016/j.intimp.2013.03.014.
Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF- superfamily signalling. Genes Cell. 2002;7:1191–204. https://doi.org/10.1046/j.1365-2443.2002.00599.x.
Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6. https://doi.org/10.1016/j.yjmcc.2010.10.033.
Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–95. https://doi.org/10.1016/j.cardiores.2006.10.002.
Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9(2):194–202.
Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, Yao R, Chen Y, Liao YH. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127(1):89–97.
Sikorski JA. Oral cholesteryl ester transfer protein (CETP) inhibitors: a potential new approach for treating coronary artery disease. J Med Chem. 2006;49(1):1–22.
Cuchel M, Rader DJ. Genetics of increased HDL cholesterol levels: insights into the relationship between HDL metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23(10):1710–2.
Guo SX, Yao MH, Ding YS, Zhang JY, Yan YZ, Liu JM, Zhang M, Rui DS, Niu Q, He J, Guo H. Associations of cholesteryl ester transfer protein TaqIB polymorphism with the composite ischemic cardiovascular disease risk and HDL-C concentrations: a meta-analysis. Int J Environ Res Public Health. 2016;13(9):882.
Carlquist J, Anderson JL. Inconsistencies in the genetic prediction of HDL cholesterol versus atherosclerosis. Curr Opin Cardiol. 2007;22(4):352–8.
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15.
Wang Q, Zhou SB, Wang LJ, Lei MM, Wang Y, Miao C, Jin YZ. Seven functional polymorphisms in the CETP gene and myocardial infarction risk: a meta-analysis and meta-regression. PLoS ONE. 2014;9(2):e88118.
Chen Z, Schunkert H. Genetics of coronary artery disease in the post-GWAS era. J Internal Med. 2021;290(5):980–92.
Li X, Huang Y, Yin D, Wang D, Xu C, Wang F, Yang Q, Wang X, Li S, Chen S, Xiong X. Meta-analysis identifies robust association between SNP rs17465637 in MIA3 on chromosome 1q41 and coronary artery disease. Atherosclerosis. 2013;231(1):136–40.
García-Bermúdez M, López-Mejías R, González-Juanatey C, Corrales A, Castaneda S, Miranda-Filloy JA, Gómez-Vaquero C, Fernández-Gutiérrez B, Balsa A, Pascual-Salcedo D, Blanco R. Association study of MIA3 rs17465637 polymorphism with cardiovascular disease in rheumatoid arthritis patients. DNA Cell Biol. 2012;31(8):1412–7.
Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, Kaplan L, Bennett D, Li Y, Tanaka T, Voight BF. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41(1):56–65.
Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–8.
van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. 2018;122(3):433–43.
Hiura Y, Fukushima Y, Yuno M, Sawamura H, Kokubo Y, Okamura T, Tomoike H, Goto Y, Nonogi H, Takahashi R, Iwai N. Validation of the association of genetic variants on chromosome 9p21 and 1q41 with myocardial infarction in a Japanese population. Circ J. 2008;72(8):1213–7.
He QC, Hu YY, Zhang QP, Tan LL, Liu YH, Liu T, Hu YQ, Qing L, Liang N. A meta-analysis of three identified single nucleotide polymorphisms at 1p13.3 and 1q41 and their associations with lipid levels and coronary artery disease. Kaohsiung J Med Sci. 2017;33(1):1–10.
Shahid SU, Shabana NA, Rehman A, Humphries S. GWAS implicated risk variants in different genes contribute additively to increase the risk of coronary artery disease (CAD) in the Pakistani subjects. Lipids Health Dis. 2018;17(1):1–7.
Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203.
Turhan H, Erbay AR, Yasar AS, Aksoy Y, Bicer A, Yetkin G, Yetkin E. Plasma soluble adhesion molecules; intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin levels in patients with isolated coronary artery ectasia. Coron Artery Dis. 2005;16(1):45–50.
Shan H, Zhang M, Zhang M, Liu X, Song X, Yin X, Lv S. Association of rs5368 and rs3917406 polymorphisms in E-selectin gene with premature coronary artery disease in Chinese Han population. Int J Clin Exp Med. 2015;8(3):4387.
Vargas-Alarcon G, Perez-Mendez O, Herrera-Maya G, Posadas-Romero C, Posadas-Sanchez R, Ramirez-Bello J, Escobedo G, Fragoso JM. The rs1805193, rs5361, and rs5355 single nucleotide polymorphisms in the E-selectin gene (SEL-E) are associated with subclinical atherosclerosis: The Genetics of Atherosclerotic Disease (GEA) Mexican study. Immunobiology. 2019;224(1):10–4.
Issac MS, Afif A, Gohar NA, Fayek NA, Zayed B, Sedrak H, El Din LA. Association of E-selectin gene polymorphism and serum PAPP-A with carotid atherosclerosis in end-stage renal disease. Mol Diagn Ther. 2014;18(2):243–52.
Wang Z, Xu Y, Chen S, Wang L, Ding H, Lu G, Wang D, Zhai Z, Duan J, Zhang W. A common missense single nucleotide polymorphism in the E-selectin gene is significantly associated with essential hypertension in the Han population but only weakly associated in the Uygur population. Hypertens Res. 2012;35(4):413–7.
Haver VG, Verweij N, Kjekshus J, Fox JC, Wedel H, Wikstrand J, van Gilst WH, de Boer RA, van Veldhuisen DJ, van der Harst P. The impact of coronary artery disease risk loci on ischemic heart failure severity and prognosis: association analysis in the COntrolled ROsuvastatin multiNAtional trial in heart failure (CORONA). BMC Med Genet. 2014;15(1):1–8.
Lu Y, Tayebi N, Li H, Saha N, Yang H, Heng CK. Association of CETP Taq1B and -629C > a polymorphisms with coronary artery disease and lipid levels in the multi-ethnic Singaporean population. Lipids Health Dis. 2013;12:85.
Bhanushali AA, Das BR. Genetic variants at the APOE, lipoprotein lipase (LpL), cholesteryl ester transfer protein (CETP), and endothelial nitric oxide (eNOS) genes and coronary artery disease (CAD): CETP Taq1 B2B2 associates with lower risk of CAD in Asian Indians. J Community Genet. 2010;1:55–62.
Rahimi Z, Nourozi-Rad R, Rahimi Z, Parsian A. Strong interaction between T allele of endothelial nitric oxide synthase with B1 allele of cholesteryl ester transfer protein TaqIB highly elevates the risk of coronary artery disease and type 2 diabetes mellitus. Hum Genomics. 2012;6(20):1–5.
Kaman D, Ilhan N, Ilhan N, Akbulut M. TaqIB and severity of coronary artery disease in the Turkish population: a pilot study. Bosn J Basic Med Sci. 2015;15(1):9–13.
Iwanicka J, Iwanicki T, Niemiec P, Balcerzyk A, Krauze J, Gorczyńska-Kosiorz S, et al. Relationship between CETP gene polymorphisms with coronary artery disease in polish population. Mol Biol Rep. 2018;45:1929–35.
Hassanzadeh T, Firoozrai M, Zonouz AE, Zavarehee A, Paoli M. Taq1B polymorphism of cholesteryl ester transfer protein (CETP) gene in primary combined hyperlipidaemia. Indian J Med Res. 2009;129:293–8.
Russell DW, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory: Cold Spring Harbor; 2001.
Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38(2):209–13.
Acknowledgements
The authors sincerely thank the patients for their participation in this study and the staff of Rasad Pathobiology and Genetic Laboratory. This work was supported by the Rasad Pathobiology and Genetic Laboratory and Science and Research Branch, Islamic Azad University, Tehran, Iran.
Funding
This study has no funding sources.
Author information
Authors and Affiliations
Contributions
SR, NR and NK that are responsible for the design of this study and acquisition of data. SM and MM and SR are responsible for analysis and interpretation of data for the work. SR and SZ drafted the work; SR and SM revised the draft critically for important intellectual content. MF and SR are responsible for the data analysis and interpretation and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by the Rasad Pathobiology and Genetic Laboratory ethics committee. Also, all methods in this study were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all individual participants included in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rayat, S., Ramezanidoraki, N., Kazemi, N. et al. Association study between polymorphisms in MIA3, SELE, SMAD3 and CETP genes and coronary artery disease in an Iranian population. BMC Cardiovasc Disord 22, 298 (2022). https://doi.org/10.1186/s12872-022-02695-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02695-6