Abstract
Background
A coronary artery aneurysm (CAA) is an abnormal dilation of a coronary artery segment often accompanied by coronary artery fistula (CAF), leading to communication between a coronary artery and a cardiac chamber or a part of the coronary venous system. Both CAAs and CAFs can present with symptoms and signs of myocardial ischemia and infarction.
Case presentation
We describe the case of a 46-year-old woman with non-ST-elevation myocardial infarction (NSTEMI) caused by a “giant” CAA. Various imaging modalities revealed a thrombus-containing aneurysm located at the right-posterior cardiac border, with established arteriovenous communication with the distal part of left circumflex artery (LCx). After initial treatment with dual antiplatelet therapy, a relapse of pain was reported along with a new increase in troponin levels, electrocardiographic abnormalities, reduced left ventricular ejection fraction (LVEF) and thrombus enlargement. Surgical excision of the aneurysm was favored, revealing its true size of 6 cm in diameter. Τhe aneurysm was excised without complications. The patient remained asymptomatic during follow-up.
Conclusions
Management of rare entities such as “giant” CAAs and CAFs can be challenging. Cases such as this can serve as precedents to facilitate treatment plans and develop consistent recommendations, emphasizing the importance of personalized strategies for future patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Both CAAs and CAFs are typically discovered incidentally during coronary angiography or noninvasive cardiac imaging. However, in this case, the CAA presented as an NSTEMI event.
A CAA is defined as an abnormal dilation of a coronary artery segment exceeding the diameter of normal adjacent segments or the patient’s largest coronary vessel by 1,5 times [1]. The most common cause of CAA is atherosclerosis, responsible for over 90% of the cases in adults, while other causes include congenital malformations (17%), Kawasaki vasculitis (17%) in children, Takayasu arteritis, connective tissue disorders and infections, with mycotic aneurysms being accountable for 11% of the cases [2]. CAA formation also seems to be the result of stent implantation during percutaneous coronary intervention (PCI) [3]. A “giant” CAA is defined by a diameter of more than 20 mm and is often accompanied by CAFs [4].
A CAF is a significant malformation of the coronary arteries defined as the abnormal communication between a coronary artery and a cardiac chamber or a part of the coronary venous system. The true incidence of CAF is speculative but ranges between 0,4 − 1,2% among different studies [5]. Approximately 10% of CAFs can cause a coronary artery aneurysm due to volume overload leading to remarkable dilatation of the coronary artery [6, 7]. While the majority of CAFs are congenital, they can also result from cardiac surgery, intracardiac device implantation, myocardial biopsy and direct trauma [8]. CAFs commonly originate from the right coronary artery (RCA) and drain into the right ventricle (RV) [9]. Fistulous communication with aneurysmal dilatation between the LCx and the coronary sinus is a rare phenomenon, with prevalence rates of 18.3% and 7% respectively [9].
Here we present a symptomatic case of a “giant” LCx artery aneurysm with a fistula draining into the coronary sinus. Despite its usual discovery being incidental, this case highlights the manifestation of “giant” CAA as an NSTEMI event. From our research, only 125 cases have been mentioned in the literature using the keywords “acute myocardial infarction AND giant coronary artery aneurysm” in the PubMed database. We highlight the dynamic presentation and not only a static morphology of the aneurysm while adding interesting histologic findings from the tissue excised. Due to their rarity and the general inability to conduct large clinical trials on cases like this, we strongly believe that our case contributes additional evidence, providing useful and educative insights into the personalized management of “giant” CAAs.
Case presentation
A 46-year-old female presented to the emergency department with intermittent chest pain radiating to the back and the left subclavian area starting 8 h prior. She had a smoking history of 20 pack years and a positive cardiac family history. The rest of the personal history was insignificant. The physical examination was unremarkable. The initial 12-lead electrocardiogram did not show any abnormal findings; however, laboratory results revealed an increased troponin value, indicating myocardial damage. Thus, the patient was admitted to the coronary care unit, with suspected NSTEMI and a coronary angiography was planned. The pharmacological treatment plan included dual antiplatelet therapy with aspirin and clopidogrel, atorvastatin and omeprazole. Interestingly, bedside echocardiography revealed a structurally and functionally normal heart, except for a cystic formation 25 mm in diameter and low flow gradient, located in the infero-lateral cardiac wall.
Coronary angiography performed the following day revealed a coronary aneurysm with arteriovenous communication and thrombus formation without stenosis in the periphery of the LCx in the absence of coronary artery disease (Fig. 1, supplementary movie 1, supplementary movie 2).
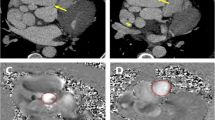
Cardiac computed tomography (CCT) revealed the presence of an aneurysm of 4,3 cm diameter located at the right-posterior cardiac border with thrombus formation (∼ 13 mm diameter) within the aneurysmal sac and an arteriovenous communication with the distal part of the LCx (Fig. 2).
The following day, the patient reported pain relapse accompanied by a new increase in troponin levels and electrocardiographic abnormalities in the inferolateral wall (negative T waves in leads II, III, aVF and V5-V6). Repeated echocardiography revealed a marginally reduced LVEF of 50–55% with hypokinesia of the posterior cardiac wall and right ventricle. Additionally, a hyperechoic area was observed in the aneurysmal space consistent with thrombus enlargement (Fig. 3, supplementary movie 3).
After consultation with the hospital’s cardiac surgeons, surgical excision of the aneurysm was favored. The aneurysm was macroscopically visible after elevation of the heart apex, measuring approximately 6 cm. The aneurysm was excised revealing the thrombus. Following the removal of the thrombus, the proximal and distal orifices were revealed and sutured. The aneurysm was ligated, and the patient was weaned off the Cardiopulmonary bypass (CPB) without complications.
Histopathological examination of the aneurysmatic tissue revealed an intima with mild myxomatoid changes, while the media was characterized by disarray of the smooth muscle and the elastic fibers. These findings seem to have resulted in the structural weakness of the vascular wall contributing to the formation of the sac [10]. There was evident separative destruction of the media wall with thrombus material found inside similar in constitution to the separate pieces of the large thrombus, with abundant erythrocytes, fibrin deposition and inflammatory cells. There were no findings of atherosclerotic lesions (Fig. 4). The histopathological examination did not show any specific findings towards vasculitis or connective tissue disorder. Therefore, the most probable cause of the CAA is supposed to be a congenital malformation.
The patient was discharged with the recommendation for 12 months dual antiplatelet therapy according to acute coronary syndrome (ACS) guidelines. During follow-up, the patient remained asymptomatic, with normalization of both the LVEF and the electrocardiogram.
Discussion
Both CAAs and CAFs can present with angina, dyspnoea, and signs of myocardial ischemia and infarction. Angina is thought to result from the coronary steal phenomenon, which is related to the diastolic pressure gradient caused by the runoff from a high-pressure coronary artery to a low resistance receiving cavity, thus making the myocardium distal to the origin of the fistula and prone to ischaemia. Moreover, the left-to-right shunt contributes to the development of angina and ischemia [9]. CAFs draining into the major coronary veins of the right heart chambers have similar effects in the circulation as an atrial septal defect [11]. Untreated CAFs can cause life-threatening complications, including thrombosis, cardiac tamponade and myocardial ischaemia and infarction [12]. Coronary aneurysms present with chest pain, dyspnea, paroxysmal nocturnal dyspnea, palpitations and fatigue [1]. Complications of CAA are rare but can be fatal and include thrombosis, distal embolization of the vessel, rupture, vasospasm, heart failure and cardiac compression or cardiac tamponade [13,14,15].
Coronary angiography is the imaging modality of choice. This technique provides information on the anatomy of CAAs and CAFs and the entire coronary artery structure. However, the true aneurysm size cannot be accurately measured in case of intramural thrombi. Additional imaging modalities supporting the diagnosis include multi-slice coronary artery computed tomography (MSCT-CA), transthoracic (TTE) and transesophageal echocardiography (TEE). A combination of multiple imaging modalities can be utilized to define the entire pathway of the CAF, which is crucial for preprocedural planning [16].
Treatment options for CAFs include transcatheter closure, cardiac surgery, or conservative treatment. The choice of intervention depends on various factors such as the size and anatomy of the fistula, its distal or proximal location, the presence of symptoms or complications related to the fistula, the patient’s age and their co-morbidities [11]. A heart team approach is crucial in decision-making and evaluating CAFs [17]. Conservative management is considered the best option in asymptomatic individuals, small fistulas, and patients with various comorbidities unable to undergo surgery, for whom regular-follow up is recommended. Small CAFs can close spontaneously over time [18], whereas medium or large sized CAFs tend to enlarge [19]. Indications for CAF closure include ischaemia in the territory of the affected coronary artery, arrhythmic events possibly related to the CAF, endarteritis, vessel rupture, cardiac chamber enlargement and ventricular dysfunction [20]. There are no consistent guideline recommendations due to variable size, anatomic and physiologic variants. The first transcatheter closure of a CAF was performed in 1983 by Reidy et al. [21]. Various techniques and approaches, including transarterial or transvenous approaches as well as the use of coils, vascular plugs or covered stents, have been developed [20]. The approach and materials depend on the criteria mentioned and the physician’s expertise. The goal is to eliminate the flow of the fistula and isolate all the feeders while preserving myocardial viability. Complications, such as device migration and occlusion, vascular trauma and residual flow have been reported in the literature [20].
Surgery is the usual treatment for CAFs due to their complex and often tortuous anatomy. It is indicated in large CAFs characterized by aneurysmal dilation and thrombus formation. Median sternotomy is used for access. CPB with or without cardioplegia is often employed for proper macroscopic visualization and safe dissection of the CAF. CAF closure can be achieved through the epicardium or the endocardium or with suture ligation [22]. A surgical closure of the CAF has a reported mortality rate of less than 1% [11]. Complications following surgery include periprocedural ST segment changes, myocardial infarction, stroke, pneumothorax and arrhythmias. Incomplete closure and residual flow have been reported in approximately 10% of the patients [11, 22]. Postprocedural anticoagulation is recommended for safe and good long-term outcomes.
Surgery options for treating CAA include coronary artery bypass grafting (CABG), resection or ligation of the CAA [23].
In our patient, surgical excision was favored due to local expertise and the concern that embolization coils might further migrate to the distal end of the fistulous aneurysm and finally land in the venous circulation due to the size of the aneurysm.
Conclusions
Despite their rarity, both CAAs and CAFs pose evident challenges for both interventional cardiologists and cardiac surgeons. The broad spectrum of clinical manifestations and the anatomical complexity of the malformations make the management of such cases highly personalized. Considering parameters such as the hemodynamics of the affected area and the various coexisting complications, detailed reports of the approach used for each specific case of CAA and CAF are imperative. Our case report highlights a “giant” CAA located at the right-posterior cardiac border, presenting as an NSTEMI event, which is not a typical presentation. Emphasizing the need for the bypass surgery pathway for thrombus-containing CAAs, characterized by an arteriovenous communication with the distal part of LCx we aim to ensure better outcomes for similar cases. Ultimately, we hope this report contributes to the enrichment of a database aimed at the development of consistent guidelines over the challenging and uncommon cardiological entities as this one.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- CAA:
-
Coronary Artery Aneurysm
- CAF:
-
Coronary Artery Fistula
- NSTEMI:
-
Non-ST-Elevation Myocardial Infarction
- LCx:
-
Left Circumflex Artery
- LVEF:
-
Left Ventricular Ejection Fraction
- PCI:
-
Percutaneous Coronary Intervention
- RCA:
-
Right Coronary Artery
- RV:
-
Right Ventricle
- CCT:
-
Cardiac Computed Tomography
- CPB:
-
Cardiopulmonary Bypass
- ACS:
-
Acute Coronary Syndrome
- MSCT-CA:
-
Multi-Slice Coronary Artery Computed Tomography
- TTE:
-
Transthoracic Echocardiography
- TEE:
-
Transesophageal Echocardiography
- CABG:
-
Coronary Artery Bypass Grafting
References
Swaye PS, Fisher LD, Litwin P, Vignola PA, Judkins MP, Kemp HG, et al. Aneurysmal coronary artery disease. Circulation. 1983;67(1):134–8.
Sheikh AS, Hailan A, Kinnaird T, Choudhury A, Smith D. Coronary artery aneurysm: evaluation, prognosis, and proposed treatment strategies. Heart Views. 2019;20(3):101–8.
Jha NK, Ouda HZ, Khan JA, Eising GP, Augustin N. Giant right coronary artery aneurysm- case report and literature review. J Cardiothorac Surg. 2009;4(1):18.
Li D, Wu Q, Sun L, Song Y, Wang W, Pan S, et al. Surgical treatment of giant coronary artery aneurysm. J Thorac Cardiovasc Surg. 2005;130(3):817–21.
Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21(1):28–40.
Hirose H, Amano A, Yoshida S, Nagao T, Sunami H, Takahashi A, Nagano N. Coronary artery aneurysm associated with fistula in adults: collective review and a case report. Ann Thorac Cardiovasc Surg. 1999;5(4):258–64.
Said SA, el Gamal MI. Coronary angiographic morphology of congenital coronary arteriovenous fistulas in adults: report of four new cases and review of angiograms of fifteen reported cases. Cathet Cardiovasc Diagn. 1995;35(1):29–35.
Mottin B, Baruteau A, Boudjemline Y, Piéchaud FJ, Godart F, Lusson JR, et al. Transcatheter closure of coronary artery fistulas in infants and children: a French multicenter study. Catheter Cardiovasc Interv. 2016;87(3):411–8.
Challoumas D, Pericleous A, Dimitrakaki IA, Danelatos C, Dimitrakakis G. Coronary arteriovenous fistulae: a review. Int J Angiol. 2014;23(1):1–10.
Toyoda Y, Takemura T, Shiratori K, Yazaki Y, Tachibana T, Niitsu H, Kunihara T. Giant aneurysm of left circumflex artery branch with fistula to the coronary sinus: a case report. J Cardiothorac Surg. 2022;17(1):197.
Latson LA. Coronary artery fistulas: how to manage them. Catheter Cardiovasc Interv. 2007;70(1):110–6.
Yoshino S, Minagoe S, Yu B, Kosedo I, Yamashita M, Ishizawa M, et al. Cardiac tamponade due to rupture of coronary artery fistula to the coronary sinus with giant aneurysm of coronary artery: usefulness of transthoracic echocardiography. Heart Vessels. 2013;28(4):536–40.
Pham V, Hemptinne Q, Grinda JM, Duboc D, Varenne O, Picard F. Giant coronary aneurysms, from diagnosis to treatment: a literature review. Arch Cardiovasc Dis. 2020;113(1):59–69.
Daneshvar DA, Czak S, Patil A, Wasserman PG, Coplan NL, Garratt KN. Spontaneous rupture of a left main coronary artery aneurysm. Circ Cardiovasc Interv. 2012;5(5):e63–5.
Libertini R, Wallbridge D, Jones HR, Gunning M, Satur CMR. Giant Circumflex Artery Aneurysm with a coronary sinus fistula. Ann Thorac Surg. 2018;106(5):e223–5.
Darwazah AK, Eida M, Batrawy M, Isleem I, Hanbali N. Surgical treatment of circumflex coronary aneurysm with fistulous connection to coronary sinus associated with persistent left superior vena cava. J Card Surg. 2011;26(6):608–12.
Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation. 2019;139(14):e637–97.
Sunder KR, Balakrishnan KG, Tharakan JA, Titus T, Pillai VR, Francis B, et al. Coronary artery fistula in children and adults: a review of 25 cases with long-term observations. Int J Cardiol. 1997;58(1):47–53.
Chaumont M, Unger P, Debbas N, Vandenbossche JL. Unoperated giant coronary arteriovenous fistula: a thirty-year follow-up. Eur Heart J. 2017;38(3):217.
Al-Hijji M, El Sabbagh A, El Hajj S, AlKhouli M, El Sabawi B, Cabalka A, et al. Coronary artery fistulas: indications, techniques, outcomes, and complications of Transcatheter Fistula Closure. JACC Cardiovasc Interv. 2021;14(13):1393–406.
Reidy JF, Sowton E, Ross DN. Transcatheter occlusion of coronary to bronchial anastomosis by detachable balloon combined with coronary angioplasty at same procedure. Br Heart J. 1983;49(3):284–7.
Hou B, Ma WG, Zhang J, Du M, Sun HS, Xu JP, Pan SW. Surgical management of left circumflex coronary artery fistula: a 25-year single-center experience in 29 patients. Ann Thorac Surg. 2014;97(2):530–6.
Beckmann E, Rustum S, Marquardt S, Merz C, Shrestha M, Martens A, et al. Surgical treatment of coronary artery aneurysms. J Card Surg. 2017;32(11):674–9.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
AV & MPX prepared the manuscript draft, KZ & APE contributed to the patient’s history, follow-up, electrocardiograph, APE, SM performed the coronary angiography, AS & GP performed the histological examination, PT did the surgical excision, VV & ST contributed to the writing and the revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vassilikou, A., Xenitopoulou, M., Ziampa, K. et al. Acute myocardial infarction due to giant coronary artery aneurysm and arteriovenous fistula: a challenging case report and review of the literature. BMC Cardiovasc Disord 24, 187 (2024). https://doi.org/10.1186/s12872-024-03851-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03851-w