Abstract
Background
Adaptive interventions are often used in individualized health care to meet the unique needs of clients. Recently, more researchers have adopted the Sequential Multiple Assignment Randomized Trial (SMART), a type of research design, to build optimal adaptive interventions. SMART requires research participants to be randomized multiple times over time, depending upon their response to earlier interventions. Despite the increasing popularity of SMART designs, conducting a successful SMART study poses unique technological and logistical challenges (e.g., effectively concealing and masking allocation sequence to investigators, involved health care providers, and subjects) in addition to other challenges common to all study designs (e.g., study invitations, eligibility screening, consenting procedures, and data confidentiality protocols). Research Electronic Data Capture (REDCap) is a secure, browser-based web application widely used by researchers for data collection. REDCap offers unique features that support researchers’ ability to conduct rigorous SMARTs. This manuscript provides an effective strategy for performing automatic double randomization for SMARTs using REDCap.
Methods
Between January and March 2022, we conducted a SMART using a sample of adult (age 18 and older) New Jersey residents to optimize an adaptive intervention to increase COVID-19 testing uptake. In the current report, we discuss how we used REDCap for our SMART, which required double randomization. Further, we share our REDCap project XML file for future investigators to use when designing and conducting SMARTs.
Results
We report on the randomization feature that REDCap offers and describe how the study team automated an additional randomization that was required for our SMART. An application programming interface was used to automate the double randomizations in conjunction with the randomization feature provided by REDCap.
Conclusions
REDCap offers powerful tools to facilitate the implementation of longitudinal data collection and SMARTs. Investigators can make use of this electronic data capturing system to reduce errors and bias in the implementation of their SMARTs by automating double randomization.
Trial registration
The SMART study was prospectively registered at Clinicaltrials.gov; registration number: NCT04757298, date of registration: 17/02/2021.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Research Electronic Data Capture (REDCap) is a secure, browser-based web application available to approximately 2.3 million users in 151 countries. [1] The application is primarily used for developing, maintaining, and managing different types of surveys and securing online/offline data collection. REDCap also offers useful features like a randomization module that can be valuable for conducting randomized controlled trials (RCTs). The randomization module in REDCap allows researchers to implement specific randomization model appropriate for their projects, permitting them to randomize subjects in their study. This randomization module also functions to monitor the overall random subject allocation process and assignment.
Bio-behavioral interventions delivered by professionals in the real world setting normally involve a sequential and individualized approach in which interventions like prevention and treatment strategies are adapted and re-adapted over time in response to the specific needs and evolving status of the client. For instance, suppose a clinician starts a client who is suffering from depression in psychotherapy, an evidence-based intervention. After 30 days, the client has not experienced any improvement and has not been attending sessions consistently. The clinician needs to decide whether to continue the psychotherapy or add medication, another evidence-based intervention, to the treatment plan.
Most health intervention clinical trials have focused on testing individual level interventions such as psychotherapy or specific pharmacological therapy on their dosing and format which are then offered to the general population after the successful completion of the clinical trials. [2,3,4,5,6] With this approach, an intervention is provided to all consumers in the same format and dosage, regardless of individual responses to the intervention. [7] The limitation of this approach is that it does not offer evidence-based guidance on when and how to modify an intervention, which strategy works best for different subpopulations, or how to combine possible intervention strategies for improved outcome.
Recently, adaptive interventions have emerged as a new means of providing research-based prevention and treatment. [8, 9] Adaptive interventions recognize that the varying intervention needs of individuals may not be optimally met via a uniform composition and dosage. For this reason, an adaptive intervention assigns different dosages of certain program components to different individuals, and/or different dosages to the same individuals over time. Dosage varies in response to the intervention needs of clients, and dosages are assigned based on decision rules linking characteristics of the individual client with specific levels and types of program components. In some adaptive interventions, a dosage of zero is possible for a particular component, where some individuals do not receive certain components at all, and intervention components may be assigned to other individuals. [8, 9] One of the major advantages of this adaptive approach is its resemblance to clinical practices where treatment to individuals are tailored based on their unique needs, which may change over time.
Sequential Multiple Assignment Randomization Trial (SMART) was established as a research strategy for developing and testing adaptive interventions. It was initially introduced as “biased coin adaptive within-subject designs” by Lavori and Dawson, with the general framework proposed by Murphy. [10] Given the increasing interest in personalized medicine among general population and field of public health, SMARTs have gained more acceptance in the clinical trial landscape over the last decade. [11] The SMART approach is a randomized experimental design developed especially for building time-varying adaptive interventions. [12, 13] Developing such an adaptive intervention strategy requires addressing questions such as:
-
What is the best sequencing of intervention components?
-
Which tailoring variables should be used?
-
How frequently, and at what times, should tailoring variables be reassessed and an opportunity for changing amount and/or type of intervention be presented?
-
Is it better to assign treatments to individuals, or to allow them to choose from a menu of treatment options?
The SMART approach enables intervention scientists to address such questions holistically and rigorously by considering the order in which intervention components are presented rather than considering each intervention component in isolation. In this way, a SMART approach provides an empirical basis for selecting appropriate decision rules and tailoring variables, with the end goal of developing evidence-based adaptive intervention strategies to be evaluated in subsequent RCTs. [13].
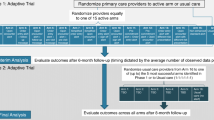
There are two common SMART designs: (a) all participants are re-randomized following initial treatment; and (b) only participants who failed to respond to their initial treatment are re-randomized and the rest continue their initially assigned treatment. In Fig. 1, a circled R indicates randomization, and a box indicates a particular treatment. Although more complex methods are required to estimate more deeply tailored regimen, simple regimen that tailor only in the variable used to determine second-stage randomizations can be easily conducted using intention-to-treat analysis, in which the intended treatment is determined by the outcome of the randomization.
COVID-19 Optimization Study Design Using SMART Method. Navigation: NS include assessment and support with service referrals. Each participant will meet with a peer navigator in person or on Zoom conferencing for 30 min to go over the results from their social and health needs assessment that is retrieved from the baseline survey. The navigator shares information about COVID-19, answers questions about testing, and makes. referrals to other needed services. Follow-up sessions occur as needed. *Brochure: A digital brochure will consist of a brochure containing CDC public health recommendations that is e-mailed or tested to participants immediately after randomization. The brochure includes information about COVID-19, testing, and vaccines. *Critical dialogue: CD includes three 1 h long, open-group sessions facilitated in person or online by a trained licensed facilitator. Group critical dialog is promoted by thematic images to foster a deeper understanding of how systematic stigma, feelings of rage as victims of discrimination, and/or apathy may impact participants’ beliefs and behaviors related to COVID-19 and empower participants to make critical choices to protect their health and the health of their communities. *Brief counseling: BC is a 15 min post-COVID-19 test session delivered by a trained licensed clinician in person or via Zoom conferencing. In the session, the clinician shares the test results and offers recommendations and information about COVID-19 treatment and prevention
In both designs, SMARTs are characterized by various treatment sequences. Automated randomizations can save time and reduce human error that can impede researchers’ ability to follow participants and engage them in research. However, automated implementation software solutions for SMARTs remain scarce. One significant challenge for SMART design study is the availability of affordable and accessible software capable of conducting automated randomization. [14] The current paper uses a case study [15] to address this gap by describing the development of a strategy that successfully automatically randomized participants multiple times to different interventions using Research Electronic Data Capture (REDCap) in conjunction with an application programming interface (API), based on a priori criteria regarding participants’ responses to interventions over time. We also provide statistical codes that future researchers can easily adapt to meet the unique needs of their individual projects.
Methods
Study case description
The rapid worldwide spread and impact of COVID-19 underlined the need for interventions that effectively increase adherence to public health recommendations (e.g., testing, vaccination, social distancing, and mask wearing). Returning safely to pre-pandemic routines and practices depends on governments’ and providers’ ability to streamline the delivery of these effective interventions to the individuals who need them. To this end, the National Institutes of Health (NIH) launched the Rapid Acceleration of Diagnostic-Underserved Populations (RADx-UP) program, designed to ensure that all Americans have access to COVID-19 testing, with a focus on communities disproportionately affected by the pandemic. Projects funded by RADx-UP include new applications of existing technologies that make tests easier to use, easier to access, and more accurate. As delineated in the study protocol document [15], this study case was a part of the RADx-UP network of projects.
This study case used a SMART with 670 people who are medically/socially vulnerable to COVID-19 to: (a) optimize an adaptive intervention to increase rates of COVID-19 testing among high-risk populations; and (b) identify predictors of COVID-19 testing uptake (ClinicalTrials.gov NCT05305443). The total analytic sample size of 582 had 85% to detect a difference of 15% in testing rates between the two interventions being tested for optimization. The study was not powered to detect changes in the second-stage interventions. Figure 1 shows the SMART design implemented of the study. The study took place at the North Jersey Community Research Initiative (NJCRI, www.njcri.org) located in Newark, Essex County, NJ. Clinical emergencies were handled by the NJCRI treatment team of experienced staff according to the study protocol. Appropriate assessments were conducted; treatment options were recommended and followed through as necessary.
The following were the inclusion criteria:
-
Over 18 years of age.
-
Having a high risk to contract COVID or develop related complications.
-
Able to speak English.
-
Able and willing to provide informed consent.
The following were the exclusion criteria:
-
Under 18 years of age.
-
Not at a high risk to contract COVID or develop related complications.
-
Unable to speak English.
-
Unable or unwilling to provide informed consent.
A hallmark of the SMART design is that it requires multiple randomizations at multiple timepoints. For instance, consider the current COVID-19 Optimization Trial study case, which seeks to increase COVID-19 testing in a marginalized community. Research questions in this trial include :
-
Will navigation services intervention lead to higher rates of COVID-19 testing compared to an electronic informational brochure intervention?
-
Among those who complete COVID-19 testing, will continuing with navigation services intervention lead to higher rates of adherence to CDC recommendations than changing to brief counseling treatment?
-
Among those who did not complete COVID-19 testing, will continuing with information brochure intervention lead to more people getting tested afterwards, compared to changing the intervention to critical dialogue intervention?
Participants are randomized for the first time to Navigation or Brochure treatments and then, at one week, randomized again to continue the original intervention or switch to a different approach. By randomizing each of the initial treatment groups, researchers can obtain a cohort of participants who followed each of the four possible treatment regimens (i.e., Navigation Only, Navigation with Brief Counseling, Brochure Only, Brochure with Brief Counseling), allowing a direct comparison of the effects of each regimen. The four possible treatment regimens are termed embedded regimen. More details about the trial itself is publicly available at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT05305443).
Randomization challenge
The REDCap software includes a platform for randomization. This module allows researchers to perform simple or stratified randomization, and to randomize participants by site in multi-site studies. Once the randomization model is established, the software also provides allocation table templates as an example for researchers to create their own allocation tables. Following a project’s move to production mode, REDCap locks the randomization model, ensuring it is not modified after a study becomes active. However, although the existing REDCap randomization model is robust, it cannot accommodate two randomization models within one project, making it difficult to perform a double randomization within one REDCap project. Concerningly, we could identify no other affordable software solutions capable of including multiple randomization models.
By consulting with REDCap administrators from other institutions on the REDCap Community Site – a message board where REDCap administrators from around the world can confer with one another to troubleshoot and develop innovative solutions – our research team identified one workaround to this limitation: namely, to create two separate REDCap projects, one for the first randomization and one for the second randomization. However, this model would require migrating data between projects, which increases the likelihood of accidental data loss or invalidation. The Illinois REDCap administrators provided two alternative suggestions. The first was to perform one randomization using the REDCap randomization module and the other randomization external to REDCap and manually enter the randomization result. The second solution was to write application programming interface (API) code for conducting one or both randomizations outside of REDCap. The research team decided to incorporate the REDCap API into the final suggestion, thus conducting both randomizations outside of REDCap and automating the entry of the randomization results generated outside of REDCap.
Implementing the double randomization
The technical implementation of the double randomization algorithm involved three services: the REDCap API, Amazon Web Services (AWS) Lambda, and AWS S3. The REDCap API is a programmatic interface that allows for the controlled movement of data between REDCap and other applications. The REDCap API can be reached using several different languages, but we chose to code in Python due to familiarity and ease of use. Lambda functions are short scripts that can run quickly and automatically within AWS when certain trigger conditions are met. As with the REDCap API, Lambda functions can be written in several different languages with Python being very common. The code can be written directly into a Lambda function through the AWS console or deployed through more technical means such as within a Docker container. Lambda functions are designed to immediately run this code when triggered by events like a certain time of day, delivery of a file to S3, and many other events. S3 is a file-storage system within AWS and has the ability to work in tandem with other AWS services (e.g., Lambda) to efficiently and securely provide and receive files in any format. All codes are made publicly available on the Github website (https://github.com/cdonelson/double-randomization) and also in the Appendix.
To accomplish the double randomization, two processes (described in the next paragraphs) automatically ran every morning. The processes operated similarly but had different inclusion criteria and randomization factors.
The first randomization began with a time-triggered Lambda function that called the REDCap project via the API and queried for a specific report. This REDCap report contained logic that generated a list of subjects who had consented, been approved by research staff, and had not yet received their first randomization. The REDCap API then returned this list of eligible subjects to the Lambda function. This list was narrowed to only include the record ID and the eligibility criteria. The function then queried a specific S3 bucket and fetched an allocation table in csv format. This table was created by the project’s statistician and contained a randomized list of first treatments. The entire table was fetched with each daily query. The list of subjects was then stepped through, and each subject was assigned the treatment at the top of the table. After a treatment was assigned, it was moved to the bottom of the table so that it would not be assigned again until the entire table had been exhausted (which was avoided by extremely over preparing the allocation cases in the table). Once every subject from that day had been assigned an treatment, the updated allocation table was pushed back to its location in S3 for use on the next day and the subject data were imported back into REDCap via the API. This new allocation table overwrote the previous table each day to help ensure accuracy and continuity.
The second randomization followed a similar process as the first but had more inclusion criteria. After the first randomization Lambda function was complete, a second Lambda function then queried the same REDCap project and fetched subjects who had been assigned a first randomization treatment and had reached their deadline for completing a COVID-19 test but had not yet received a second randomization treatment. As with the first randomization, this second query fetched a daily generated report in REDCap that captured subjects eligible for second randomization. The data contained only their record ID and their eligibility criteria. The Lambda function then queried the same S3 bucket but fetched a different allocation table than the first. The second allocation table contained treatments for the second randomization but was slightly more complex because there were four possible treatments with different inclusion criteria based on their first randomization status. As with the first allocation table, the entire second allocation table was fetched with each daily query. For each eligible participant, the table was read sequentially until a row was found that matched the participant’s criteria, including first randomization status and COVID-19 test status. This row’s treatment was attached to the subject’s data, and then moved to the bottom of the table in the same way as in the first allocation process. Participants in the same group based on first randomization and testing treatment had an equal likelihood of being randomized into the appropriate treatments at the second randomization. After all eligible participants had been assigned a second randomization, the allocation table was pushed back to the S3 bucket, and the subject data was imported back into REDCap via the API.
Our research team decided to write an API code for both randomizations to be conducted outside of REDCap and to automatically indicate the result of randomization for each participant in REDCap. The first randomization runs automatically at 3:00 a.m. It pulls in all records where:
approve_b variable equals “Yes”.
AND
consent_2 variable equals “Yes”.
AND
randomization1 variable is blank.
The second randomization runs automatically at 3:05 AM. It pulls in all records where:
randomization1 variable is not blank.
AND
randomization2 variable is blank.
AND
covid_test variable is not blank.
AND
covid_test_complete variable equals “Yes”.
The second randomization pulls anyone who has received the first randomization but not the second, has reported “Yes” or “No” on taking a COVID-19 test, and has a COVID-19 test instrument marked “complete.” The code checked every morning for records requiring randomization, and if it found none then no further action would be performed that day. We decided to run the code once daily, but it is entirely possible to schedule the code to run more or less frequently to fit the use case of a given study.
The main challenge of this type of programmatic solution was keeping the code in sync with the up-to-date REDCap project format. Changes in the project instruments, or certain variables within the instruments, required manually updating the code inside the Lambda functions to ensure that the data import would complete. Initially, another concern was that the growing size of the subject population created a longer Lambda function runtime. The early versions of the script queried all records from the project API and processed eligibility within the code itself. When the subject pool reached a few hundred, this full data import caused the Lambda function to take several minutes to complete. AWS Lambda has a maximum runtime of 15 min, and although the runtime never approached that timeout in the present study, we had to make occasional adjustments to ensure that the processes successfully ran every morning. Limitations on Lambda scaling means that studies with exceptionally large populations may cause the code to run past the allotted time limit under this design. We alleviated this by moving the eligibility check from the Lambda function to a REDCap report. This resulted in a dramatic drop in runtime because the REDCap API would now only return records eligible for randomization instead of the full data set. The Lambda function completed in 30–45 s rather than minutes.
Results of double randomizations
Overall, despite a few errors, the randomization strategy was very successful, saving the staff substantial time and minimizing randomization and data entry errors. In total, 675 participants met all the inclusion criteria and none of the exclusion criteria. All 675 participants have been successfully randomized to their first randomization assignment. Figure 2 shows the randomization results. A total of 333 (49.3%) were assigned to “Navigation Services” and 342 (50.7%) to “Brochure.” Participants needed to report whether they have completed a COVID-19 test in order to be included in a second round of randomization. Their covid-19 status is assessed by study staff 7 days after being enrolled either by phone or in-person, and responses are manually entered into the REDCap database. Only 22% (n = 151) of participants took the COVID-19 test, and 78% (n = 520) reported not taking the COVID-19 test. There were four participants with missing or no inputted COVID-19 test response.
Participants who were initially randomized to “Navigation Services” underwent second randomization as follows: among those who completed COVID-19 testing, 6% (n = 41) were randomized to “Navigation Services” and 5% (n = 35) were randomized to “Brief Counseling.” Among those who did not complete COVID-19 testing, 18% (n = 124) were randomized to “Navigation Services” and 19% (n = 130) were randomized to “Critical Dialogue.”
Participants who were initially randomized to “Brochure” underwent second randomization as follows: among those who completed COVID-19 testing, 5% (n = 36) were randomized to “Brochure” and 5% (n = 32) were randomized to “Brief Counseling.” Among those who did not complete COVID-19 testing, 19% (n = 131) were randomized to “Brochure” and 20% (n = 137) were randomized to “Critical Dialogue.”
The second randomization failed for nine participants (equivalent to 1.3% error): three participants were missing second randomization assignments, these participants dropped out before the second round of randomization occurred; four participants had no COVID-19 test information inputted, and therefore they were never assigned a second intervention; and two participants were inadvertently and manually assigned to a different intervention by a study staff member. The combination of these unexpected events led to slight difference in participant allocation ratio between the two intervention treatment arms. The first randomization sequence allocated and assigned interventions for 675 participants. The second randomization sequence allocated and assigned interventions for 666 participants, which is a difference of 9.
Because the randomization process occurred daily, in some instances the automatically assigned interventions were delivered two to three days after they were due to be delivered. These delays occurred when the randomization assignments were generated on weekends when study staff were not available to check the system and deliver the intervention within the required 24-hour window post-randomization. To mitigate this issue, we limited the REDCap entry of the two key variables (i.e., approve_b and consent_2) needed to start the randomization process limited from Mondays through Thursdays. This gave the study staff time within the work week to deliver the assigned interventions, avoiding potential delays.
Discussion
The current paper used a case study to demonstrate how SMART design researchers can conduct multiple randomizations despite the lack of accessible software capable of conducting automated multiple randomizations. Namely, we illustrated a strategy that our research team successfully used to automatically randomize participants multiple times to different interventions using REDCap.
As adaptive interventions have emerged as a new approach to research-based prevention and treatment, SMART designs have been gaining popularity. According to the NIH’s Research Portfolio Online Reporting Tools (RePORT) website, which allows users to search a repository of both intramural and extramural NIH-funded research projects and access publications and patents resulting from NIH funding, 109 NIH-funded projects used SMART design during the 2022 calendar year. The Fig. 3 shows a steady increase in NIH-funded projects employing a SMART design since 2009.
As SMART design gains increasing recognition and is increasingly implemented in practice, demand for software capable of performing the unique multiple randomization assignments required for SMARTs will also increase. Encouragingly, our strategy of utilizing API for the double randomization procedure was seamlessly implemented in conjunction with the REDCap platform. This strategy eliminated technological and logistical challenges by effectively concealing and masking intervention allocation sequences to investigators, involved health care providers, and subjects as they progressed through the complex SMART study procedures. At the same time, challenges common to all rigorous clinical trials (e.g., study invitations, eligibility screening, consenting procedure, data collection, and data confidentiality protocols) were addressed adequately through unique features of the REDCap platform. Additional benefits of using API in conjunction with REDCap for our SMART design project included: timely completion of complex intervention assignments, accurate assignments for participants at multiple time points, and the ability to reduce both the human resources required to implement the study and the likelihood of human error by automating most of the study procedures.
Although this strategy worked very well for our research team, some contextual factors might influence how other researchers adapt the strategy to their projects in different organizations. The global REDCap community may develop an add-on that accomplishes multiple randomizations within a single project. However, there may be a lag between its release and the ability of organizations to deploy it on different REDCap instances. For example, the University of Illinois at Urbana-Champaign’s REDCap instance, which housed our project, had a built-in Health Insurance Portability and Accountability Act (HIPAA)-compliant AWS account. The University also took steps to guarantee the security of the software. Most importantly, the Illinois REDCap instance undergoes an annual security review with the university’s Cybersecurity Governance, Risk, and Compliance (GRC) team. The review evaluates Illinois REDCap’s ability to meet a variety of security controls and measures in accordance with HIPAA and the University’s cybersecurity standards. In addition to the annual review, REDCap users are required to complete HIPAA training before accessing the system, system sign-on utilizes Shibboleth two-factor authentication, and projects are vetted by REDCap administration team for required protections before a project is moved to production to ensure security. Thus, due to the secure nature of Illinois REDCap, our process of integrating third-party solutions within REDCap was rigorous. The add-on was inspected for compliancy and potential vulnerabilities, then tested on a TEST server. Additionally, documentation and standard operating procedures for how the add-on will be upgraded and accessed by users were developed by the University.
The unique context of each organization must be taken in consideration before implementing this multiple randomization solution. Whereas our research team was able to successfully utilize the built-in HIPAA-compliant AWS account to implement our strategy in conjunction with rigorous REDCap team administration support for data security and protection, other research teams may not have the same resources and administrative support. Some of the ways in which research teams with limited resources to readily implement this strategy may consider consulting REDCap community that is very active in providing recommendations and support.
Conclusions
REDCap is a free, widely available data collection system that offers powerful tools for longitudinal data collection. Utilizing an online data collection system for SMART design studies can decrease biases within studies while requiring fewer human resources and decreasing opportunity for human error. Investigators can make use of this electronic data capturing system in conjunction with API to successfully complete their SMART design studies.
Data Availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request and with permission of University of Illinois at Urbana-Champaign.
References
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Dos Santos RG, Bouso JC, Alcázar-Córcoles M, Hallak JEC. Efficacy, tolerability, and safety of serotonergic psychedelics for the management of mood, anxiety, and substance-use disorders: a systematic review of systematic reviews. Expert Rev Clin Pharmacol. 2018;11(9):889–902. https://doi.org/10.1080/17512433.2018.1511424.
Glenn CR, Esposito EC, Porter AC, Robinson DJ. Evidence base update of psychosocial treatments for self-injurious thoughts and behaviors in youth. J Clin Child Adolesc Psychol. 2019;48(3):357–92. https://doi.org/10.1080/15374416.2019.1591281.
Jones MG, Rice SM, Cotton SM. Incorporating animal-assisted therapy in mental health treatments for adolescents: a systematic review of canine assisted psychotherapy. PLoS ONE. 2019;14(1):e0210761. https://doi.org/10.1371/journal.pone.0210761.
Larsen C, Shahinas J. Dosage, efficacy and safety of cannabidiol administration in adults: a systematic review of human trials. J Clin Med Res. 2020;12(3):129–41. https://doi.org/10.14740/jocmr4090.
Tomko RL, Jones JL, Gilmore AK, Brady KT, Back SE, Gray KM. N-acetylcysteine: a potential treatment for substance use disorders. Curr Psychiatr. 2018;17(6):30–9.
Sanson-Fisher RW, Bonevski B, Green LW, D’Este C. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med. 2007;33(2):155–61. https://doi.org/10.1016/J.AMEPRE.2007.04.007.
Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prev Sci. 2004;5(3):185–96. https://doi.org/10.1023/b:prev.0000037641.26017.00.
Rivera DE, Pew MD, Collins LM. Using engineering control principles to inform the design of adaptive interventions: a conceptual introduction. Drug Alcohol Depend. 2007;88(S2):31–S40. https://doi.org/10.1016/J.DRUGALCDEP.2006.10.020.
Lavori PW, Dawson R. A design for testing clinical strategies: biased adaptive within-subject randomization. J R Stat Soc Ser A Stat Soc. 2000;163(1):29–38. https://doi.org/10.1111/1467-985x.00154.
Wallace MP, Moodie EEM, Stephens DA. SMART thinking: a review of recent developments in sequential multiple assignment randomized trials. Curr Epidemiol Rep. 2016;3(3):225–32. https://doi.org/10.1007/s40471-016-0079-3.
Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment Randomized Trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5):112–S118. https://doi.org/10.1016/J.AMEPRE.2007.01.022.
Collins LM, Nahum-Shani I, Almirall D. Optimization of behavioral dynamic treatment regimens based on the sequential, multiple assignment, randomized trial (SMART). Clin Trials. 2014;11(4):426–34. https://doi.org/10.1177/1740774514536795.
Danielson L, Carlier J, Burzykowski T, Buyse M. Practical considerations for adaptive trial design and implementation. New York: Springer; 2014.
Windsor L, Benoit E, Pinto M, Sarol R. Optimization of a new adaptive intervention using the SMART Design to increase COVID-19 testing among people at high risk in an urban community. Trials. 2022;23(1). https://doi.org/10.1186/s13063-022-06216-w.
Acknowledgements
Not applicable.
Funding
National Institute on Minority Health and Health Disparities R01MD010629.
National Institute on Alcohol Abuse and Alcoholism T32AA007477.
Author information
Authors and Affiliations
Contributions
Carol Lee wrote the main manuscript text and prepared figures, reference list, and supplemental materials. Danilo Gamino, Michelle Lore, and Liliane Windsor provided written summary information for different sections of the main manuscript. Liliane Windsor provided supplemental materials. All authors reviewed, provided feedbacks, and checked information for accuracy.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All study methods were carried out in accordance with the Declaration of Helsinki and all experimental protocols were approved by the North Jersey Community Research Initiative (NJCRI) institutional review board (IRB) (NJCRI IRB FWA# 00001870). Informed consent to participate in the study were obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, C.A., Gamino, D., Lore, M. et al. Use of research electronic data capture (REDCap) in a sequential multiple assignment randomized trial (SMART): a practical example of automating double randomization. BMC Med Res Methodol 23, 162 (2023). https://doi.org/10.1186/s12874-023-01986-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12874-023-01986-6