Abstract
Introduction
Digital mental health interventions (DMHIs) overcome traditional barriers enabling wider access to mental health support and allowing individuals to manage their treatment. How individuals engage with DMHIs impacts the intervention effect. This review determined whether the impact of user engagement was assessed in the intervention effect in Randomised Controlled Trials (RCTs) evaluating DMHIs targeting common mental disorders (CMDs).
Methods
This systematic review was registered on Prospero (CRD42021249503). RCTs published between 01/01/2016 and 17/09/2021 were included if evaluated DMHIs were delivered by app or website; targeted patients with a CMD without non-CMD comorbidities (e.g., diabetes); and were self-guided. Databases searched: Medline; PsycInfo; Embase; and CENTRAL. All data was double extracted. A meta-analysis compared intervention effect estimates when accounting for engagement and when engagement was ignored.
Results
We identified 184 articles randomising 43,529 participants. Interventions were delivered predominantly via websites (145, 78.8%) and 140 (76.1%) articles reported engagement data. All primary analyses adopted treatment policy strategies, ignoring engagement levels. Only 19 (10.3%) articles provided additional intervention effect estimates accounting for user engagement: 2 (10.5%) conducted a complier-average-causal effect (CACE) analysis (principal stratum strategy) and 17 (89.5%) used a less-preferred per-protocol (PP) population excluding individuals failing to meet engagement criteria (estimand strategies unclear). Meta-analysis for PP estimates, when accounting for user engagement, changed the standardised effect to -0.18 95% CI (-0.32, -0.04) from − 0.14 95% CI (-0.24, -0.03) and sample sizes reduced by 33% decreasing precision, whereas meta-analysis for CACE estimates were − 0.19 95% CI (-0.42, 0.03) from − 0.16 95% CI (-0.38, 0.06) with no sample size decrease and less impact on precision.
Discussion
Many articles report user engagement metrics but few assessed the impact on the intervention effect missing opportunities to answer important patient centred questions for how well DMHIs work for engaged users. Defining engagement in this area is complex, more research is needed to obtain ways to categorise this into groups. However, the majority that considered engagement in analysis used approaches most likely to induce bias.
Similar content being viewed by others
Introduction
One in four people experience a mental health problem every year [1]. However, an estimated 70% with mental ill health are unable to access treatment [2]. App and web-based tools, collectively digital mental health interventions (DMHIs), are low cost, scalable [3], and have potential for overcoming traditional barriers to treatment access, such as physical access (flexibility in treatment location), confidentiality (providing anonymity), and stigma [4]. In recent years, the number of available DMHIs has rapidly increasd [5], the Apple App Store alone has over 10,000 behavioural apps [6]. This rapid increase combined with the complex nature of DMHIs has meant safety and effectiveness regulations have lagged behind [7]. Additionally, many DMHIs are developed for commercial purposes and marketed to the public without scientific evidence [8]. The current National Institute for Health and Care Excellence (NICE) guidelines [9] for digital health technologies, advocate for use of randomised controlled trials (RCTs) to evaluate the effectiveness of digital interventions in specific conditions such as mental health. Promisingly, the number of digital interventions evaluated in RCTs over the last decade has more than doubled [10].
Many DMHIs are developed through the digitalisations of existing services, such as online self-led formats of conventional therapist-delivered treatments. However, in contrary to conventional therapist-led treatments, DMHIs offer flexible anytime access for individuals [11]. This change in delivery means existing evidence of risk-benefit balance from structured therapist-delivered interventions is not translatable. DMHIs are potential solutions to provide more individuals with much needed treatment access, but they are not without challenges. In 2018 the James Lind Alliance (JLA) patient priority setting group for DMHIs set out the top 10 challenges to address [12]. Overcoming these challenges is essential for DMHIs to successfully improve treatment access and health outcomes in mental health [13, 14]. One theme that emerged from across the priorities was the importance of improving methods for evaluating DMHIs including the impact of user engagement.
The impact user engagement has on DMHIs efficacy is poorly understood [6, 15, 16]. Although DMHIs are widely available, user engagement with DMHIs is typically low [17]. For multi-component DMHIs (commonly including psychoeducation, cognitive exercises, self-monitoring diary), a minimally sufficient engagement in DMHIs is often crucial for establishing behavioural changes and thus improved health outcomes [18]. However, achieved sustained behavioural changes by engaging with DMHIs is a multidimensional construct that is both challenging to assess and the pathway for patients to achieve this is complex [19, 20]. Unlike other interventions, DMHIs are unique in that web-based or app-based interventions can capture interactions from individuals. User engagement can be measured and recorded using automatically captured indicators (e.g., pageviews, proportion of content/modules completed, or number of logins). However, the large variety in measurable indicators across different DMHIs [16, 21] further compounds challenges to understanding pathways to sustained behaviour changes.
For RCTS, the latest estimand framework in the ICH E9 R1 addendum [22] provides guidance on defining different estimands, which enables trialists to ensure the most important research questions of interest are evaluated. This includes guidance on handling post-randomisation events, such as user engagement with the DMHI, in efficacy analysis. For example, policy makers are likely to be most interested in a treatment policy estimand which provides an assessment of the benefit received on average under the new policy of prescribing the DMHI regardless of how it’s engaged with. For DMHIs typically engagement is poor, which means treatment policy estimands may underestimate the true intervention efficacy for those who engaged [23], so alternative estimands that address this may also be of interest to target. For example, the benefit received on average for individuals who would actively engage with the DMHI (a principal stratification estimand). However, to utilise available methods post-randomisation variables need to be clearly defined, but this is difficult for engagement with DMHIs because it is multifaceted with many different engagement indicators available to use.
This systematic review aimed to assess the current landscape of how RCTs for DMHIs are reported and analysed. The review primarily assessed how user engagement is described, what engagement indicators are reported and how, if at all, researchers assessed the impact of user engagement on efficacy. As the number of DMHIs evaluated in RCTs is ever increasing, this review is essential to identify current practice in trial reporting to inform further research to improve the quality of future trials. The specific research aims of interest were to: (1) examine trial design and characteristics of DMHIs; (2) summarise how user engagement had been defined and measured in RCTs of DMHIs; and (3) assess how often intervention efficacy was adjusted for user engagement and the impact of user engagement on efficacy estimates.
Methods
The protocol for this systematic review was prospectively published in Prospero [24], and PRISMA guidance was followed in reporting of this review.
Study selection
We included RCTs examining the efficacy of DMHIs, excluding pilot and feasibility studies [25]. Search terms for RCT designs followed guidance from Glanville et al. [26]. We included trials of participants with common mental disorders (CMD) defined by Cochrane [27] excluding populations with non-CMD comorbidities, such as patients with depression and comorbid diabetes. Populations with multiple CMDs were not excluded as there were many transdiagnostic interventions targeting overlapping symptoms of different conditions. Both trials requiring a confirmed clinical diagnosis and trials where participants self-referred were included. For consistency in DMHIs included interventions must meet any criteria from items 1.1 (targeted communication on health information), 1.3 (client to client communication, e.g., peer forums), 1.4 (health tracking or self-monitoring) or 1.6 (access to own health information) from the WHO Classification of Digital Health Interventions [28]. DMHIs must have been delivered on a mobile app or through a web-browser and where the intervention was self-guided by participants, defined as an intervention where participants have full autonomy over how this is used. Search terms for interventions followed guidance from Ayiku et al. [29]. All publications must have been reported in English.
The search was performed on the 17th September 2021 and included trials published between 1st January 2016 to 17th September 2021. Search terms were adapted for each database: MEDLINE, Embase, PsycINFO and Cochrane CENTRAL (see supplemental table S1 for search strategy). Title and abstracts were independently screened by two reviewers (JE, RB, SO, LB, LM & VH), and again at the full text review stage. Covidence [30] was used to manage all stages, remove duplicates and resolve disagreements.
Quality
As a methodology review to examine how user engagement was described and analysed a risk of bias tool to assess trial quality was not undertaken [31]. However, key CONSORT items [32] were extracted to determine adherence to reporting guidance, including reporting of a protocol or trial registration (item 23/24), planned sample size (item 7a) and amendments to the primary analysis (item 3b). For all items self-reported data from articles was extracted.
Data extraction
A data extraction form was developed by the lead author (JE) and reviewed by VC, SC and JS. Summary data extracted covered: trial characteristics (e.g., design and sample size); intervention and comparator descriptions (e.g., delivery method or primary function); participant demographics (e.g., age or gender); reporting of user engagement (e.g., indicators reported); and point estimates, confidence intervals and P-values of analysis results unadjusted and adjusted for user engagement. In trials with multiple arms the first active arm mentioned was included. No restriction was applied to the control arm in the trial. The full extraction sheet, including CONSORT items, is in the table S2 of the supplementary material.
Analysis
The analysis was predominantly descriptive and used mean and standard deviations, or medians and interquartile ranges (IQRs) to describe continuous variables. Frequencies and percentages summarized categorical variables. User engagement captured through engagement indicators (e.g., pageviews and total logins) and methods to encourage user engagement (e.g., automatic notifications) were summarised descriptively. Indicator data was summarised in four categories: duration of use (e.g., length of session), frequency of use (e.g., number of logins), milestone achieved (e.g., modules completed) and communication (e.g., messages to therapist). Descriptive summaries also assessed both the recommended user engagement definitions, the pre-specified minimum engagement level investigators told participants to use DMHIs, and active user definitions, the pre-specified engagement level of most interest to investigators for intervention effects accounting for user engagement. Both were summarised by indicators used in definitions.
To determine the impact of user engagement on intervention efficacy, restricted maximum likelihood random effects meta-analyses were conducted for articles that reported both intervention effect when user engagement was accounted for and when it wasn’t. Standardised effects were used due to outcomes and measures varying between articles. These were taken directly, where reported, otherwise calculated using guidance from Cochrane [33], and Cohen’s d formula for the standard deviation [34]. Articles were grouped by outcome domains (e.g., depression, anxiety or eating disorders) based on the reported primary clinical outcome used to evaluate efficacy. Analyses also group articles based on the analytical approach used for adjustment, those using statistical methods that retained all participants formed one group (recommend approaches) and those using statistical methods only retaining conventional per-protocol populations, i.e., exclude the data from those who did not comply, formed the other group (per-protocol approaches). All analysis was performed using Stata 17.
Results
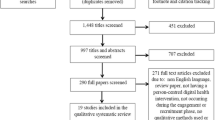
From a total of 6,042 articles identified, 184 were eligible and included in this review (see Fig. 1) randomising 43,529 participants. The most evaluated outcome domain was Depression, 74 (40.2%) articles, followed by Anxiety, 29 (15.8%) articles, and PTSD, 12 (6.5%) articles, see supplementary table S3 for full list. At least 123 unique interventions were assessed, however some interventions (n = 39) were only described in general terms, such as internet delivered cognitive behaviour therapy for depression, so could not be distinguished as separate interventions and are excluded from the count. On average 30.7 (SD 7.7) articles were published each year, a more detailed breakdown by outcome domain is in supplementary figures s1 and s2.
Extracted CONSORT items assessed trial reporting quality, 51 articles (27.7%) did not report their planned sample size and 36 articles (19.7%) did not clearly reference a trial protocol or trial registration number. For the 133 articles that reported both the planned and actual sample size, 43 (32.3%) failed to recruit to their target. The planned analysis approach was reportedly changed in 3 (1.6%) articles, one due to changes in the intervention [35] and the others due to high attrition [36, 37].
Most articles used “traditional” trial designs with 170 (92.4%) opting for a parallel arm design and the majority assessed only one new intervention (n = 134, 78.8%). Four articles (2.2%) used a factorial design allowing for the simultaneous evaluation of multiple treatments providing statistical efficiency by reducing the number of participants required in the trial. Two articles (1.1%) in the Body Dysmorphic Disorder outcome domain reported using a crossover design. However, the first had no wash-out period and instead those in the intervention arm were asked to stop engaging with the app after 16 days [38]. The second actually used a parallel arm design, where the control group received the intervention after 3 weeks [39]. Median delivery period for DMHIs was 56 days (IQR 42–84) post-randomisation and the median total follow-up time for primary outcome collection was 183 days post-randomisation (IQR 84–365).
Participants average age was 34.1 years (SD 11.1), and most participants were female (70.7%), see Table 1. Ethnicity data was not extractable in 133 (72.3%) articles. Most trials required a confirmed diagnosis of a CMD, such as through a structured interview, for inclusion (n = 110, 59.8%). Symptom severity could not be extracted in 97 (52.7%) trials, but where available the most common (49 trials, 56.3%) severity was a combination of both mild and moderate. Only 12 (6.5%) articles assessed participants with severe symptomatology in the depression domain (n = 7, 58.3%), anxiety (n = 1, 8.3%), psychological distress (n = 1, 8.3%), general fatigue (n = 1, 8.3%), post-traumatic stress disorder (n = 1, 8.3%), or psychosis (n = 1, 8.3%).
Most interventions were delivered through a website, 145 (78.8%), see Table 2. There were 76 (41.3%) trials that adapted interventions from existing in-person therapist led interventions, and 84 (45.7%) interventions were newly developed. App delivered interventions were more likely to be newly developed, 23 (71.9%), compared to website interventions, 57 (39.3%). Most common choice of control arm was usual care, 126 (68.5%). For articles with usual care as control, most opted to use wait-lists, 94 (74.6%), where intervention access was provided either immediately after the intervention period, 62/94 (66.0%), or after the total follow-up period, 32/94 (34.0%).
Most articles, 136 (73.9%), reported using at least one approach to encourage participants to engage with the intervention. Methods of encouragement were automatic notifications, n = 49/136 (32.5%), contacting participants by telephone or email, n = 68/136 (45.0%), or automated feedback on homework exercises, n = 76/136 (50.3%). Most used only one method of encouragement, n = 85 (62.5%), with 6 (4.4%) articles using all 3 methods of encouragement. Although many articles encouraged engagement, only 23.9% (n = 44) provided a recommended level of engagement to participants. Recommendations varied from using a rate to progress through content (e.g., one module per week or maximum of two modules per week), a specified duration to use the intervention (e.g., 1.5 h per week or 4 to 6 h per week), or specifying milestones to complete (e.g., complete one lesson every 1–2 weeks or complete daily homework assignments), a full list is in table s5 of the supplementary material.
User engagement data captured through indicators was reported in many articles, 76.1% (n = 140), Fig. 2. Typically, this included only reporting only one indicator (n = 41, 29.3%) ranging up to eight indicators for one (0.7%) trial [40]. Across the 140 studies reporting user engagement data, most commonly indicators described the frequency of use, 150 (40.7%), followed by indicators to capture milestones achieved, 124 (33.6%), further detail is found in table s4 of the supplemental. A total of 150 unique indicators were reported across the 140 articles, the most popular measure used was modules completed, 51.3% (n = 77), followed by the number of logins, 25.3% (n = 38). In website only delivered interventions there were 102 unique indicators compared to 41 unique indicators reported in app-based interventions, and 7 unique indicators in interventions delivered as both an app and website.
Proportion of trials describing user engagement in methods section (A) or in results section (B)
A) – How user engagement was reported in the methods section
Recommended – the participant was told how to use the intervention by the study team
Encouraged – reminders (e.g., notifications or emails) were sent to the participant
Active User – participants meeting a pre-specified engagement level set by the study team
B) – How user engagement data was reported in the results section
Reported – results describe activity for at least one engagement indicator
Analysis – results report an intervention effect where user engagement has been considered
Active user definitions, the engagement level of most interest to trial teams, was stated in the methods sections for 20.1% (n = 37) of articles. Digital components of active user definitions included setting a minimum number of modules completed (e.g., 4 out of 5 modules), a proportion of content accessed (e.g., at least 25% of pages viewed), or the total time accessed (e.g., used app for 30 min per week), a full list of active user definitions is in table s6 of the supplemental. From the 37 articles reporting active user definitions, 27 (14.7%) described statistical methods to perform an analysis accounting for user engagement but only 19 (10.3%) reported intervention effect estimates.
All articles reporting effects from the analysis accounting for user engagement also reported effects not accounting for engagement so were included a meta-analysis, Table 3. All articles used a treatment policy estimand (including all participants randomised regardless of the level of user engagement) for their primary outcome, where user engagement was not accounted for. In articles reporting an analysis accounting for user engagement, all outcome domains reported an increase in overall effect size favouring the intervention in comparison to estimates from analysis not accounting for user engagement. The largest increase in intervention efficacy was in the distress domain (n = 1) where the standardised mean effect size increased from − 0.61 (95% CI -0.86 to -0.36) to -0.88 (95% CI -1.17 to -0.59).
The results comparing changes in the intervention effect by the analysis approach used (recommended versus per-protocol) is in Table 4. From the 19 articles included in the analysis, 17 (89.5%) used a conventional per-protocol (i.e., exclude the data from those who did not comply) approach for the analysis accounting for user engagement [41]. A consequence of which is that the average sample size decreased to 76.9% (IQR 67.7–87.6%) of the original size, in the active arm the average size decreased by 61.8% (IQR 38.1–75.4%). The overall standardised intervention effect increased from − 0.14 (95% CI -0.24 to -0.03, n = 17), p = .01, to -0.18 (95% CI -0.32 to -0.04, n = 17), p = .01, but was also less precise. Two trials used a Complier Average Causal Effect (CACE) analysis [42], a recommended approach where assumptions hold, with all participants randomised included in the analysis. The overall standardised intervention effect increased in the meta-analysis with an overall change from − 0.16 (95% CI -0.38 to 0.06, n = 2), p = .16, to -0.19 (95%CI -0.42 to 0.03, n = 2), p = .09, with no decrease in sample size and slightly less impact on the precision of the estimate.
Discussion
This systematic review found that in trials of DMHIs for CMDs, promisingly many articles reported user engagement as summaries of automatically captured indicators, but the reported intervention effect rarely accounted for this. Overall, trials were not well reported, almost 30% did not reference a trial protocol and only 27% of articles had available data on ethnicity. The JLA patient priority group set user engagement as a research priority in 2018 and this review, including publications between 2016 and 2021, supports evidence that engagement data has been poorly utilised where only 10% (n = 19) of articles had available estimates to evaluate the impact of user engagement on intervention efficacy. Many (> 70%) articles reported summarised engagement data highlighting plenty of opportunities to better utilise this data and understand the relationship between user engagement and efficacy, a question of particular interest to the individual using DMHIs to know the true intervention efficacy.
Many articles reported at least one method used to encourage participants to engage with the intervention, however very few articles were able to specify what the recommended level of engagement should be for individuals. Additionally, only a small proportion of trials assessed the impact of user engagement on the intervention efficacy through active user definitions, but these were broad ranging and used a variety of different engagement indicators. This highlights the complex and challenging task to properly assess user engagement where currently there is little guidance available. This also shows how difficult it is for researchers to identify what the minimum required engagement to the intervention, active user definitions, should be due to the heterogeneity in both the individuals being treated and how the intervention is being delivered (e.g., timeliness and access to other support).
Most articles performing an analysis that accounted for engagement used a conventional per-protocol population. Although the per-protocol population can be unbiased under the strong assumption that user engagement is independent from treatment allocation [43], typically use of this population causes bias in the estimated intervention effect [44] and the underlying estimand cannot be determined, i.e. unclear precisely what is being estimated. User engagement is a post-randomisation variable and the estimand framework [22] suggests using more appropriate strategies for handling post-randomisation events. For example, conducting a complier average causal effect analysis [42] under the principal stratification strategy estimated using instrumental variable regression [45] with randomised treatment allocation used as the instrumental variable. Alternative statistical methods can also be used to implement the estimand framework [46], but due to large variation in the reported engagement indicators and therefore difficulties in how engagement as a post-randomisation variable should be defined comparisons between trials remain challenging.
Establishing better methods in how user groups are defined, based on all available engagement measures, for example by using clustering algorithms combining all engagement measures, are needed. Secondly, once groups are defined existing statistical methods available to implement the estimand framework need to be assessed to determine the optimal approach to analyse the impact of engagement on the efficacy analysis. This is now the focus of our future work.
Future implications
The JLA priority setting partnership occurred in 2018, meaning this review of publications between 2016 and 2021, includes very few trials recruiting after 2018. Therefore, implementation of the JLA priorities cannot be assessed. However, this review has shown user engagement data was available, showing potential for more trials to explore engagement in efficacy analysis. An update of this systematic review should be performed for the next 5 years (2021–2026) to assess whether issues identified in this review around user engagement have been improved. More trials exploring engagement in efficacy analysis will mean the pathway of sustained behaviour changes through engagement with DMHIs is better understood. Additionally, reporting of user engagement varied greatly, and although the CONSORT extension of e-health [47] outlines some detail on engagement reporting, more directed guidance is needed. Improvements should include reporting what and how many indicators were available and better guidance on how indicator data should be summarised. Additionally, trial publications varied greatly in quality of reported results and particularly for key demographic information such as ethnicity. CONSORT trial reporting guidance has been around since 1996 and more journals should enforce its implemented to ensure robust reporting of trials.
Finally, where data was available, participants were mostly female, white ethnicity and young, demographics consistent with another systematic review of DMHI trials [48] and the most recent 2014 Adult Psychiatric Morbidity Survey (APMS) for who is most likely to receive treatment [49]. However, the APMS 2014 also shows that individuals from black or mixed ethnicities are more likely to experience a CMD than those from white ethnicities. This supports other literature [50, 51] and highlights differences in those recruited into trials and those who experience a CMD and not represented in DMHI efficacy estimates.
Strengths and limitations of the review
This systematic review assessed a wide-ranging number of outcome domains, providing an overview for all current DMHIs evaluated, including articles from CMDs with lots of active research, such as anxiety and depression, to CMDs with very few published results. Additionally, this review collected detailed information on engagement indicators, how these were reported, and how they were utilised in the analysis of the intervention effect, providing a rich database of the typical indicators available across a wide range of DMHIs.
As the focus of this review was to assess user engagement the review does not analyse the temporal differences of when primary outcome data for the intervention effect were collected. This means the review ignores that differences of the intervention effects across articles could partly be due to temporal differences in when they were collected, assuming the intervention effect changes over time. However, comparisons of adjusted and unadjusted intervention effects are measured at the same timepoints within each article. Additionally, as very few studies reported analysis adjusted for user engagement there was limited data to assess the impact of user engagement on the intervention efficacy in most outcome domains. Further, as most studies assessing engagement used a similar approach, per-protocol population, a formal comparison of methods was not possible. Finally, as this review only focused on appraising how engagement was reported and statistical methods used to analyse engagement, we don’t consider the impact of loss to follow-up has on the efficacy of interventions but must acknowledge that DMHIs typically have high drop-out rates from studies with very low proportions of individuals completing the intervention [52].
Conclusion
This review assessed reporting of user engagement and how authors considered engagement in the efficacy analysis of digital mental health interventions. While many articles reported at least one measure of engagement, very few articles used the data to analyse how engagement affects intervention efficacy, making it difficult to draw conclusions on the impact of engagement. In the small proportion of articles that reported this analysis, nearly all used statistical methods at high risk of bias. There is a clear need to improve the methods used to define active users by using all available engagement measures. This will help ensure a more consistent approach to how user engagement as a post-randomisation variable is defined. Once these methods are established trialists can then utilise existing statistical methods to target alternative estimands, such as principal stratification, that mean the impact of user engagement with the intervention efficacy can be explored.
Data availability
The study protocol is already available on Prospero (CRD42021249503), datasets used are available from the corresponding author on reasonable request after the NIHR fellowship from which this project comes from is completed (April 2025). Any researchers interested in using the data extracted can contact the lead author using the shared correspondence information.
References
England N. The Five Year Forward View For Mental Health. 82NHS, NHS England, 2016.
Henderson C, Evans-Lacko S, Thornicroft G. Mental Illness Stigma, help seeking, and Public Health Programs. Am J Public Health. 2013;103:777–80.
Muñoz RF, et al. Massive Open Online interventions: a Novel Model for delivering behavioral-health services Worldwide. Clin Psychol Sci. 2015;4:194–205.
Ferwerda M, et al. What patients think about E-health: patients’ perspective on internet-based cognitive behavioral treatment for patients with rheumatoid arthritis and psoriasis. 2013.
Koh J, Tng GA-O, Hartanto A. Potential and Pitfalls of Mobile Mental Health Apps in traditional treatment: an Umbrella Review. LID – https://doi.org/10.3390/jpm12091376 LID – 1376. J Personalized Med. 2022;12.
Torous J, et al. Towards a consensus around standards for smartphone apps and digital mental health. World Psychiatry. 2019;18:97–8.
Capital FC. NICE and MHRA will review regulation of digital mental health tools. Volume 2023. Future Care Capital Website; 2022.
Torous J, Haim A. Dichotomies in the Development and Implementation of Digital Mental Health Tools. 2018.
NICE. NICE Evidience Standards For Digital Health. NICE, https://www.nice.org.uk/, 2019.
Koneska EA-O, Appelbe DA-O, Williamson PA-O, Dodd SA. -O. usage Metrics of web-based interventions evaluated in Randomized controlled trials. Systematic Review; 2020.
Patel SA-O, et al. The Acceptability and Usability of Digital Health Interventions for Adults With Depression, Anxiety, and Somatoform Disorders: Qualitative Systematic Review and Meta-Synthesis. 2020.
Hollis C, et al. Identifying research priorities for digital technology in mental health care: results of the James Lind Alliance Priority setting Partnership. Lancet Psychiatry. 2018;5:845–54.
Torous JB, et al. A hierarchical Framework for evaluation and informed decision making regarding smartphone apps for Clinical Care. Technol Mental Health. 2018;69:498–500.
Donker T, et al. Smartphones for smarter delivery of mental health programs: a systematic review. 2013.
Torous J, Nicholas J, Larsen ME, Firth J, Christensen H. Clinical review of user engagement with mental health smartphone apps: evidence, theory and improvements. Evid Based Mental Health. 2018;21:116.
Doherty K, Doherty G. Engagement in HCI: Conception, Theory and Measurement. ACM Comput Surv. 2018;51:99.
Lipschitz J, et al. Adoption of mobile apps for depression and Anxiety: cross-sectional survey study on patient interest and barriers to Engagement. JMIR Ment Health. 2019;6:e11334.
Michie SA-O, Yardley LA-OX, West RA-O, Patrick KA-O, Greaves FA-O. Developing and Evaluating Digital Interventions to Promote Behavior Change in Health and Health Care: Recommendations Resulting From an International Workshop. Journal of Medical Internet Research. 2017;19.
Haine-Schlagel R, Walsh NE. A review of parent participation engagement in child and family mental health treatment. Clin Child Fam Psychol Rev 2015.
Saleem MA-O et al. Understanding Engagement strategies in Digital Interventions for Mental Health Promotion: scoping review. JMIR Mental Health. 2021;8.
Perski O, Blandford A, West R, Michie S. Conceptualising engagement with digital behaviour change interventions: a systematic review using principles from critical interpretive synthesis. Transl Behav Med. 2017;7:254–67.
Use C. f.M.P.f.H. ICH E9 (R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials. European Medicines Agency; 2020.
Eysenbach G. The law of attrition. J Med Internet Res. 2005;7.
Elkes J. A systematic review to evaluate how user engagement is described and analysed in randomised controlled trials for digital mental health interventions. PROSPERO Int Prospective Register Syst Reviews. 2021.
Eldridge SM, et al. Defining feasibility and Pilot studies in Preparation for Randomised controlled trials: development of a conceptual Framework. PLoS ONE. 2016;11:e0150205.
Glanville J, et al. Translating the Cochrane EMBASE RCT filter from the Ovid interface to Embase.com: a case study. Health Inform Libr J. 2019;36:264–77.
Cochrane. Glossary of Cochrane Common Mental Disorders. Vol. 2021 A glossary of the definitions of common mental disorders. 2021.
World Health O. Classification of digital health interventions v1.0: a shared language to describe the uses of digital technology for health. Geneva: World Health Organization; 2018.
Ayiku L, et al. The NICE MEDLINE and Embase (Ovid) health apps search filters: development of validated filters to retrieve evidence about health apps. Int J Technol Assess Health Care. 2021;37:e16.
Innovation VH. Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. www.covidence.org. 2022.
Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139.
Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
Cochrane. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane. 2023.
Larry V, Hedges, Olkin I. Statistical methods for meta-analysis. 2014.
Fitzsimmons-Craft EE, et al. Effectiveness of a Digital Cognitive Behavior Therapy-guided self-help intervention for eating disorders in College Women. A Cluster Randomized Clinical Trial; 2020.
Salamanca-Sanabria AA-O, et al. A culturally adapted cognitive behavioral internet-delivered intervention for depressive symptoms. Randomized Controlled Trial; 2020.
Richards D, et al. Effectiveness of an internet-delivered intervention for generalized anxiety disorder in routine care: A randomised controlled trial in a student population. 2016.
Cerea S, et al. Cognitive behavioral training using a Mobile Application reduces body image-related symptoms in high-risk Female University students: a randomized controlled study. Behav Ther. 2021;52:170–82.
Glashouwer KA, Neimeijer RAM, de Koning ML, Vestjens M, Martijn C. Evaluative conditioning as a body image intervention for adolescents with eating disorders. 2018.
Milgrom JA-O, et al. Internet Cognitive Behavioral Therapy for Women With Postnatal Depression: A Randomized Controlled Trial of MumMoodBooster. 2016.
Hernán MA, Hernández-Díaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trails. 2011;9:48–55.
Dunn G, Maracy M, Fau - Tomenson B, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. 2005.
Kahan BC, White IR, Edwards M, Harhay MO. Using modified intention-to-treat as a principal stratum estimator for failure to initiate treatment. Clin Trails. 2023;20:269–75.
Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: Intention-to-treat versus per-protocol analysis. 2016.
Lipkovich I, et al. Using principal stratification in analysis of clinical trials. Stat Med. 2022;41:3837–77.
Parra CO, Rhian M, Daniel, Bartlett JW. Hypothetical estimands in clinical trials: a unification of causal inference and missing data methods. Arxiv - Stat Methodol. 2021.
Eysenbach G, Group C-E. CONSORT-EHEALTH: improving and standardizing evaluation reports of web-based and mobile health interventions. J Med Internet Res. 2011;13:e126.
Sin J, et al. Digital Interventions for Screening and Treating Common Mental disorders or symptoms of Common Mental illness in adults: systematic review and Meta-analysis. J Med Internet Res. 2020;22:e20581.
McManus S, Jenkins BP, Brugha R T, editors. Mental health and wellbeing in England: Adult Psychiatric Morbidity Survey 2014. in Adult Psychiatric Morbidity Survey 405 (Leeds, NHS Digital) 2016.
Iflaifel M, et al. Widening participation - recruitment methods in mental health randomised controlled trials: a qualitative study. 2023.
Coss NA, et al. Does clinical research account for diversity in deploying digital health technologies? Npj Digit Med. 2023;6:187.
Karyotaki E, et al. Predictors of treatment dropout in self-guided web-based interventions for depression: an ‘individual patient data’ meta-analysis. Psychol Med. 2015;45:2717–26.
Acknowledgements
This work was funded by the NIHR Doctoral Fellowship (NIHR301810). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The TMRP Health Informatics working group, which the lead author is a member of, was essential in finding members (SOC, LY, LB) to join this project and support the work.
Funding
This work was funded by the NIHR Doctoral Fellowship (NIHR301810).
Author information
Authors and Affiliations
Contributions
Conceptualization, JE, VC, SC and JS.; Methodology, JE, VC, SC and JS.; Software, JE.; Validation, JE, VC, SC, JS, SO, LB, RB, LY, and VH.; Formal Analysis, JE.; Investigation, JE, VC, SC, JS, SO, LB, RB, LY, and VH.; Resources, JE, VC, SC, and JS.; Data Curation, JE.; Writing – Original Draft, JE, VC, SC, and JS.; Writing – Reviewing & Editing, JE, VC, SC, JS, SO, LB, RB, LY, and VH.; Visualisation, JE, VC, SC, and JS.; Supervision, VC, SC, and JS.; Project Administration, JE, VC, and SC.; Funding Acquisition, JE, VC, SC, and JS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable as all data was publicly available.
Consent for publication
Not applicable as no participants were recruited for this research.
Competing interests
JE was recently a collaborator on a NIHR HTA grant (NIHR132896) for long term effectiveness of a video feedback intervention for parents. JE is also on the trial steering committee for a trial (NIHR302349) that is part of an NIHR Doctoral Fellowship called Restore-B. JE is also on the programme steering committee (NIHR204413) for a trial called ATTEND. SC was previously awarded funding for an NIHR advanced fellowship (NIHR300593) between Stepember 2020 and December 2023. VC was also involved in the NIHR HTA (NIHR132896) funded trial of long-term follow-up of the video feedback intervention for parents. VC is also on the trial steering committee for a problem solving intervention for adults with dementia and depression, a steering committee member for a trial called ADVANCE and the chair of a NIHR HTA funded data monitoring committee (NIHR132808) called BAY. No other competing interests are reported for all other authors (RB, SOC, LMY, LB, VH and JS).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elkes, J., Cro, S., Batchelor, R. et al. User engagement in clinical trials of digital mental health interventions: a systematic review. BMC Med Res Methodol 24, 184 (2024). https://doi.org/10.1186/s12874-024-02308-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12874-024-02308-0