Abstract
Background
Few data are available on the long-term mortality and functional status of geriatric patients surviving after hospitalization for COVID-19. We compared the mortality and functional status 18 months after hospitalization for geriatric patients who were hospitalized for COVID-19 or another diagnosis.
Methods
This was a multicentric cohort study in Paris from January to June 2021. We included patients aged 75 years and over who were hospitalized with COVID-19 or not during this period and compared their vital and functional status 18 months after hospitalization.
Results
We included 254 patients (63 hospitalized for COVID-19). As compared with patients hospitalized for other reasons, those hospitalized for COVID-19 were younger (mean [SD] age 86 [6.47] vs. 88 [6.41] years, p = 0.03), less frail (median Clinical Frailty Scale score 5 [4–6] vs. 6 [4–6], p 0.007) and more independent at baseline (median activities of daily living score 5.5 [4–6] vs. 5 [3.5–6], p 0.03; instrumental activities of daily living score 3 [1–4] vs. 2 [0–3], p 0.04). At 18 months, 50.8% (n = 32/63) of COVID-19 patients had died versus 66% (n = 126/191) of non-COVID-19 patients (p 0.03). On multivariate analysis, COVID-19 positivity was not significantly associated with 18-month mortality (adjusted hazard ratio 0.67, 95% confidence interval 0.40 to 1.13). At 18 months, the two groups did not differ in activities of daily living or frailty scores.
Conclusions
In this multicenter study of long-term mortality in geriatric patients discharged alive after hospitalization, positive COVID-19 status was not associated with excess mortality.
Similar content being viewed by others
Introduction
In December 2019, a novel coronavirus was detected and described in patients with severe pneumonia in Wuhan, China [1]. Quickly, SARS-Cov-2 infection spread worldwide, and in January 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic. More than 775 million confirmed cases and about 7 million deaths were reported by May 2024 [2]. The pathophysiology and clinical characteristics are now well known [3]. This pandemic evolved in “waves”, punctuated by increased number of cases and mutations in the virus leading to new variants [4]. Over time, vaccines and treatments were developed [3, 5], which changed the mortality and prognosis of infected patients.
The geriatric population is characterized by atypical symptoms of COVID-19, and various clinical symptoms are observed [6]. Patients with COVID-19 aged 70 years and over have the highest in-hospital mortality [7]. The in-hospital mortality ranged from 30% in patients aged 70 to 79 years to 60% in those aged 80 years and over during the first wave as compared with 5% in patients less than 40 years old [7, 8]. This outcome is extremely high as compared with the 6% in-hospital mortality of a similar population out of the COVID-19 context [9].
Only few data are available on the long-term prognosis of older patients after hospitalization for COVID-19 [10, 11]. Among geriatric patients discharged alive from the hospital, the 6-month mortality ranged from 6 to 13% [12, 13], but no data are available at 18 months. Several pathologies such as sarcopenia, malnutrition or cardiovascular, respiratory or cognitive disease, induced or aggravated by COVID-19 [14,15,16,17], suggest that the long-term prognosis could be poor.
The objective of this cohort study was to compare the mortality and functional prognosis of geriatric patients hospitalized for COVID-19 or another reason at 18 months after hospitalization.
Materials and methods
Study design and setting
This was a multicentric cohort study of patients in three acute geriatric units (AGUs) in Paris, France from Assistance Publique Hôpitaux de Paris - Sorbonne Université (APHP-SU) hospitals. We included patients aged 75 years and over who were hospitalized in these AGUs from January 1 to June 15, 2021 for COVID-19 as main diagnosis or another medical reason and who were discharged from hospital alive. Eligible patients were identified retrospectively from medical records. The follow-up was 18 months after hospitalization, from September 2022 to February 2023. This report follows the STROBE recommendations (Supplementary Methods 1).
Ethical support
This study was approved by the ethics committee (CPP Ile de France, Paris, France, no. 107–2021). All included patients or their close relatives received an information letter specifying their rights and the terms of use of their medical data. Non-objection was collected by the physicians in charge of the patients.
Participants
Patients included were aged 75 years and over. They had been transferred to an AGU from an emergency department or intensive care unit. COVID-19 positivity had to be diagnosed by RT-PCR for SARS-CoV-2 and/or chest CT according to the WHO interim guidance [18]. Patients were excluded if they died during the hospitalization, if they were under legal protection or if they refused the use of their medical data.
Data collection
One physician per ward (MC, LB, AR) retrospectively collected medical data from computer medical records. Data included sociodemographic information (age, sex, place of living), clinical data such as comorbidities with the Charlson Comorbidity Index (CCI) [19], frailty with the Clinical Frailty Scale (CFS) [20], the functional independence scores activities of daily living (ADL) [21] and instrumental activities of daily living (IADL) [22] and polypharmacy defined by taking five or more chronic medications per day [23]. We recorded the descriptive data for the hospitalization including the main diagnosis for the hospitalization, the clinical severity at admission (quick Sepsis-related Organ Failure Assessment [qSOFA] score [24]) and laboratory data (hemoglobin level, lymphocyte and neutrophil count, and C-reactive protein [CRP], creatinine and albumin levels). We also collected information on where patients were discharged from hospital (at home, in rehabilitation service, other hospital departments or admission to nursing home).
One physician per ward (MC, LB, AR) followed up participants at 18 months by phone call. When the patient did not answer on several occasions, the close relatives or the general practitioner were contacted. Patients were considered lost to follow-up when there was no contact data available or no answer despite three phone calls. Data collected at 18 months included the vital status of the patient, new admission to a nursing home, hospital readmission, frailty, and functional independence (CFS, ADL, IADL scores) and quality of life with the EQ-5D-5 L (mobility, independence, daily activities, pain, and anxiety/depression scores from 0 to 4 and global evaluation of health scores from 0 to 100) [25].
Outcomes
The primary outcome was 18-month mortality and associated factors. Secondary outcomes were functional autonomy with the ADL and IADL, frailty with the CFS, quality of life with the EQ-5D-5 L and readmission rate at 18 months.
Statistical analysis
Demographic data and baseline characteristics are described for all patients according to COVID-19 status. Missing values are specified only if they were present. Data are presented as mean (SD) or median (interquartile range [IQR]) for continuous variables and number (percentage) for categorical variables. Comparison of quantitative variables between patients with and without in-hospital COVID-19 involved unpaired Student t test or Mann-Whitney test for non-normally distributed data. Normality was assessed by a graphical representation of the data distribution. Comparison of categorical variables involved the chi-squared or Fisher’s exact test as appropriate.
Our primary endpoint, death at 18 months, was described as a censured variable, with Kaplan-Meier curves. All included patients with available 18-month follow-up data were analyzed. Comparison between two groups involved the log-rank test. For adjusted analysis, we used a Cox regression model studying the association between COVID-19 positivity and mortality at 18 months, estimating adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs). Adjusting factors were selected if they were clinically significant or were significant at p < 0.2 on univariate analysis [26]. The proportional risk hypothesis was respected.
The secondary endpoints of rehospitalization, loss of autonomy (ADL and IADL scores at 18 months and ratio between 18 months and baseline), quality of life (EQ-5D-5 L score at 18 months) and frailty scores (at 18 months and ratio between 18 months and baseline) were compared with univariate statistical methods (Wilcoxon-Mann Whitney test for quantitative variables, chi-squared or Fisher’s exact test for categorical variables).
Statistical analyses were performed with RStudio 2023.06.0 + 421. All p-values were two-tailed and p < 0.05 was considered statistically significant.
Results
Characteristics of patients
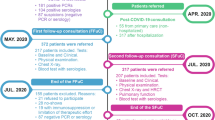
In total, 528 patients were hospitalized during the inclusion period: 311 met the inclusion criterion and 254 were finally included (63 with COVID-19, 191 other reasons) (Fig. 1). The mean (SD) age was 88 (6) years; 98 (39%) patients were male; and the median (IQR) CCI was 7 (5–9), median ADL score 5 (3.5-6) and median CFS score 5 (4–6). Baseline characteristics by COVID-19 status are summarized in Table 1. At baseline, as compared with patients without COVID-19, those with COVID-19 were significantly younger (mean (SD) 86 (6) vs. 88 (6) years; p = 0.03), less frail (median CFS score 5 [4–6] vs. 6 [4–6]; p = 0.007) and more independent (ADL score 5.5 [4–6] vs. 5 [3.5-6], p = 0.03; IADL score 3 [1–4] vs. 2 [0–3], p = 0.04). They were also more obese (19% vs. 7.3%; p = 0.02) but had less atrial fibrillation (25% vs. 44%; p = 0.009). Non-COVID-19 patients were mainly admitted in AGUs with a diagnosis of a fall (24%), acute heart failure (18%) and pulmonary infection (9.9%) (Table 1). They had significantly higher albumin level (mean 31 [5] vs. 29 [4] g/L; p 0.04) and less severe lymphopenia (mean lymphocyte count 1.05 [0.48] vs. 0.81 [0.52] G/L; p 0.001) as well as more impaired renal function (mean glomerular filtration rate estimated by Cockroft: 41 [21] vs. 50 [28] ml/min/m2; p 0.01). The two groups did not differ in clinical severity at admission (median qSOFA 0 [0–1] vs. 0 [0–1], p 0.48). (Table 1). Patients in the COVID 19 group were more likely to be admitted to rehabilitation department at discharge (57.1% vs. 25.1%, p < 0.001 (Table 1)).
Primary objective: 18-month mortality and associated factors
At 18 months, 50.8% (n = 32/63) of COVID-19 patients had died as compared with 66% (n = 126/191) of non-COVID-19 patients (p = 0.03) (Fig. 2).
COVID-19 positivity was not significantly associated with 18-month mortality (aHR 0.67, 95%CI 0.39 to 1.14) on multivariate Cox regression analysis adjusted on age; CFS score; presence of major cognitive disorder; history of coronary artery disease, atrial fibrillation, and cancer; previous SARS-Cov2-infection; ADL score, albumin level and lymphocyte count (Table 2). Factors associated with 18-month mortality were atrial fibrillation (aHR 1.51, 95%CI 1.01 to 2.25), cancer (aHR 1.84, 95%CI 1.15 to 2.94) and hypoalbuminemia (high-level albuminemia seemed protective: aHR 0.95, 95%CI 0.92 to 0.99) (Table 2). Admission to a rehabilitation department was not associated with mortality compared to patients discharges at home (aHr 1.14 95%CI 0.69 to 1.89) (Table 2).
Secondary objectives: Functional status, frailty, and quality of life at 18 months (Table 3)
At 18 months, the non-COVID-19 and COVID-19 groups did not differ in median ADL (4.0 [2.5–5.5] vs. 4.5 [3.0-5.5], p 0.50) or IADL (1 [0–3] for both groups, p 0.74). Patients lost a median of 0.5 (-1.5 to 0) ADL points and 1 (-2 to 0) IADL points as compared with baseline in these two measures, with no significant difference in loss of points between non-COVID-19 and COVID-19 patients (p 0.48 and p 0.35, respectively). The two groups did not differ in median CFS score at 18 months (6 [5,6,7] vs. 6 [5,6,7], p 0.89). The median difference in CFS score from baseline was 1 point (0–2) for both groups (p 0.32) (Table 3).
Although the two groups significantly differed in ADL, IADL and CFS scores at admission, this was no longer the case for the 18-month survivor population.
Among patients who were able to answer the EQ-5D-5 L questionnaire, the two groups did not significantly differ in any of the items (Table 3).
Discussion
To our knowledge, this is the first study to investigate such a long-term prognosis of COVID-19 in a geriatric population. Our cohort study evaluated the prognosis of geriatric patients 18 months after hospitalization for COVID-19 versus other reasons for hospital admission. The 18-month mortality rate was 51% in the COVID-19 group versus 66% in the non-COVID-19 group, COVID-19 positivity was not associated with excess mortality on multivariate analysis, and functional status and frailty at 18 months were similar between the two groups.
Some studies evaluated prognosis at 3 or 6 months after a hospital admission for COVID-19 in geriatric populations discharged alive. They reported a mortality rate of 13.5% at 3 month during the first two pandemic waves in Nantes, France [27], 8.5% at 3 month during first wave in Madrid, Spain [12]. Their included patients were less frail than in our study. A Norwegian study described 36% mortality at 6 months after hospitalization during the first wave [11]. The authors did not collect frailty status at admission nor CCI or functional status with ADL and IADL, so we cannot easily compare their results with our study.
Post-hospitalization mortality seems to vary widely. These studies took place during the first two waves of pandemic, when the wild-type SARS-CoV-2 virus was predominant (https://www.who.int/publications/m/item/historical-working-definitions-and-primary-actions-for-sars-cov-2-variants), (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports) whereas we were interested in patients hospitalized during the third wave in France, when the alpha variant was predominant (https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-chiffres-cles-et-evolution-de-la-covid-19-en-france-et-dans-le-monde). During our inclusion period, therapies were already developed and improved patient prognosis [5, 28]. Thrombotic complications were known to induce mortality, so prevention with anticoagulants was appropriate [29, 30]. Furthermore, vaccines were developed and were found effective in reducing the risk of severe forms of the disease [31, 32].
We did not find any study with a follow-up of more than 6 months in a geriatric population.
Mortality rate at 18 months in patients hospitalized for a medical reason other than COVID-19 seemed higher than that reported by Walter et al. in 2001 [33]. In a general geriatric population out of the COVID-19 context, a 2022 study reported 32% (n = 63/195) mortality at 20 months [34], whereas we found 66% (n = 126/191) mortality at 18 months. A part of the population in the previous study was admitted in general medicine, and patients were younger and less frail than in our study. The authors did not report autonomy and did not specify the medical history of included patients. The observed difference could be explained in part by a more frail and comorbid population in our study than in the previous one.
In our study, COVID-19 positivity was not associated with 18-month mortality on multivariate analysis. This result runs counter to our initial hypothesis. The limited sample size of the study may have limited the statistical power and affected these results. The COVID-19 population was younger, more independent, and less frail at hospital admission than the non-COVID-19 population, but our analysis was adjusted on these factors. Although CCI was not a predictor of mortality, some comorbidities such as cancer and atrial fibrillation were associated with excess mortality and occurred more in non-COVID-19 than COVID-19 patients.
Functional prognosis, frailty
The loss of functional independence in older people after hospitalization has been an assessed marker for many decades and is frequently reported in the literature [35, 36]. In our population, the mean loss of points was 0.5 points in ADL and 1 point in IADL, with no significant difference between the two groups. The studies mentioned aboved reported a mean loss of 1.5 points in ADL [27] or functional decline in 30% of the population [12] at 3-month. Although extended follow-up after hospitalization may increase the loss of functional independence, this delay also suggests a greater possibility of recovery for patients with few intercurrent acute events.
Patients’ frailty at 18 months after hospitalization did not significantly differ between the two groups, including the difference compared with CFS score at admission. The median increase in CFS score was 1 (0–2). This 1-point increase seemed to predict mortality on univariate analysis but not after multivariate adjustment. The literature previously demonstrated increased frailty after COVID-19 hospitalization [27, 37]. In 2023, Ferrara et al. reported an increase in frailty at 6 months in 34.5% of their patients [37]. The authors included patients aged 65 years and older who were discharged alive after hospitalization due to COVID-19during three pandemic waves in northern Italy. Prampart et al. reported a median increase in CFS score of 1 point (0–2) at 3-month follow-up [27].
Strengths and limitations
To our knowledge, this is the first study to compare a COVID-19 and non-COVID-19 population with such a long-term follow-up. Our multicentric study involved three AGUs in hospitals belonging to the same APHP university hospital group, where clinical practices are similar, particularly in terms of recommendations for the management of COVID-19 during our inclusion period. We did not select patients and included all those hospitalized in our units who met the inclusion criteria and consented to the study. Our population is representative of patients hospitalized in AGUs: 77% were aged 85 years and over and were comorbid. We studied health-related quality of life with a validated score based on patients’ feelings. This outcome is still unusual in geriatric population studies.
There are a few limitations. First, we did not have data for the COVID-19 variant for our patients. During the inclusion period, the predominant variant circulating in France was the alpha variant, with the gradual appearance of the beta variant. The omicron variant is currently responsible for most COVID-19 cases [38, 39]. In recent systematic reviews comparing long-term sequelae with different SARS-Cov-2 variants, patients infected with the historical variant seem more likely to develop long-COVID-19 symptoms [40]. Long-term prognosis does not seem to differ between alpha and omicron variant cases [41]. Our results seem applicable to current geriatric patients with COVID-19. Second, we did not know which treatments COVID-19 patients received, although recommendations for management and treatments for COVID-19 were similar in the three AGUs. Those treatments may have affected the long-term prognosis and mortality (https://apps.who.int/iris/bitstream/handle/10665/352027/WHO-2019-nCoV-therapeutics-2021.4-fre.pdf). We also do not know the vaccination status of all included patients. The national vaccination campaign began in January 2021 in France, improving patients’ prognosis even more (https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/vaccination-contre-la-covid-19). During our inclusion period, some patients may have received at least one vaccine. Third, the follow-up was by phone call, with no clinical evaluation of patients. The results reported depend on the declarations of patients or their relatives. Functional status could have been over- or underestimated. Some patients’ comprehension difficulties with the telephone method may have limited the results obtained (particularly EQ-5D-5 L results). Many patients were lost to follow-up. Finally, follow-up at 6, 12 and 18 months could have been considered to assess the variability over time of our secondary outcomes.
Conclusions
In this multicenter study of long-term mortality in geriatric patients, COVID-19 positivity was not associated with 18-month mortality. Functional status, frailty and quality of life were similar between COVID-19 and non-COVID-19 patients at 18 months.
Data availability
The data that support the findings of this study are not openly available for confidentiality reasons and are available from the corresponding author upon reasonable request.
References
Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. https://doi.org/10.1056/NEJMoa2001017.
WHO Coronavirus (COVID-19) Dashboard. May 2024. https://covid19.who.int/ Accessed June 1, 2024.
Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;324:782–93. https://doi.org/10.1001/jama.2020.12839.
Chavda VP, Patel AB, Vaghasiya DD. SARS-CoV‐2 variants and vulnerability at the global level. J Med Virol. 2022;94:2986–3005. https://doi.org/10.1002/jmv.27717.
The RECOVERY Collaborative Group. (2021). Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. https://doi.org/10.1056/NEJMoa2021436
Annweiler C, Sacco G, Salles N, et al. National French Survey of Coronavirus Disease (COVID-19) symptoms in people aged 70 and over. Clin Infect Dis. 2020;72:490–4. https://doi.org/10.1093/cid/ciaa792.
Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–9. https://doi.org/10.1001/jama.2020.6775.
Zerah L, Baudouin É, Pépin M, et al. Clinical characteristics and outcomes of 821 older patients with SARS-Cov-2 infection admitted to Acute Care Geriatric wards. J Gerontol Biol Sci Med Sci Glaa. 2020;210. https://doi.org/10.1093/gerona/glaa210.
Volpato S, Bazzano S, Fontana A, et al. Multidimensional Prognostic Index predicts mortality and length of Stay during hospitalization in the older patients: a Multicenter prospective study. J Gerontol Biol Sci Med Sci. 2015;70:323–9. https://doi.org/10.1093/gerona/glu167.
Chojnicki M, Neumann-Podczaska A, Seostianin M, et al. Long-term survival of older patients hospitalized for COVID-19. Do clinical characteristics upon Admission Matter? Int J Environ Res Public Health. 2021;18:10671. https://doi.org/10.3390/ijerph182010671.
Walle-Hansen MM, Ranhoff AH, Mellingsæter M, et al. Health-related quality of life, functional decline, and long-term mortality in older patients following hospitalisation due to COVID-19. BMC Geriatr. 2021;21:199. https://doi.org/10.1186/s12877-021-02140-x.
Carrillo-Garcia P, Garmendia-Prieto B, Cristofori G, et al. Health status in survivors older than 70 years after hospitalization with COVID-19: observational follow-up study at 3 months. Eur Geriatr Med. 2021;12:1091–4. https://doi.org/10.1007/s41999-021-00516-1.
Günster C, Busse R, Spoden M, et al. 6-month mortality and readmissions of hospitalized COVID-19 patients: a nationwide cohort study of 8,679 patients in Germany. PLoS ONE. 2021;16:e0255427. https://doi.org/10.1371/journal.pone.0255427.
Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–58. https://doi.org/10.1016/S0140-6736(21)01755-4.
Becker RC. COVID-19 and its sequelae: a platform for optimal patient care, discovery and training. J Thromb Thrombolysis. 2021;51:587–94. https://doi.org/10.1007/s11239-021-02375-w.
Składanek JA, Leśkiewicz M, Gumiężna K et al. (2023) Long COVID and its cardiovascular consequences: what is known? Adv Clin Exp Med. https://doi.org/10.17219/acem/167482
Baroni C, Potito J, Perticone ME, et al. How does Long-COVID Impact Prognosis and the long-term sequelae? Viruses. 2023;15:1173. https://doi.org/10.3390/v15051173.
WHO 2. COVID-19 Clinical management: living guidance. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-clinical-2021-1. Accessed November 13, 2021.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. https://doi.org/10.1016/0021-9681(87)90171-8.
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. https://doi.org/10.1503/cmaj.050051.
Katz S, Akpom CA. 12. Index of ADL. Med Care. 1976;14:116–8. https://doi.org/10.1097/00005650-197605001-00018.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–95. https://doi.org/10.1016/j.jclinepi.2012.02.018.
Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of Clinical Criteria for Sepsis: for the Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315:762–74. https://doi.org/10.1001/jama.2016.0288.
Bhadhuri A, Kind P, Salari P, et al. Measurement properties of EQ-5D-3L and EQ-5D-5L in recording self-reported health status in older patients with substantial multimorbidity and polypharmacy. Health Qual Life Outcomes. 2020;18:317. https://doi.org/10.1186/s12955-020-01564-0.
Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49:907–16. https://doi.org/10.1016/0895-4356(96)00025-x.
Prampart S, Le Gentil S, Bureau ML, et al. Functional decline, long term symptoms and course of frailty at 3-months follow-up in COVID-19 older survivors, a prospective observational cohort study. BMC Geriatr. 2022;22:542. https://doi.org/10.1186/s12877-022-03197-y.
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–45. https://doi.org/10.1016/S0140-6736(21)00676-0.
Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7. https://doi.org/10.1016/j.thromres.2020.04.013.
Flumignan RL, Civile VT, Tinôco JD de S, et al. Anticoagulants for people hospitalised with COVID-19. Cochrane Database Syst Rev. 2022;2022(CD013739). https://doi.org/10.1002/14651858.CD013739.pub2.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2034577. NEJMoa2034577.
Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. https://doi.org/10.1016/S0140-6736(20)32661-1.
Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a Prognostic Index for 1-Year mortality in older adults after hospitalization. JAMA. 2001;285:2987–94. https://doi.org/10.1001/jama.285.23.2987.
Engvig A, Wyller TB, Skovlund E, et al. Association between clinical frailty, illness severity and post-discharge survival: a prospective cohort study of older medical inpatients in Norway. Eur Geriatr Med. 2022;13:453–61. https://doi.org/10.1007/s41999-021-00555-8.
Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–8. https://doi.org/10.1046/j.1532-5415.2003.51152.x.
Boyd CM, Landefeld CS, Counsell SR, et al. Recovery in activities of Daily Living among older adults following hospitalization for Acute Medical Illness. J Am Geriatr Soc. 2008;56:2171–9. https://doi.org/10.1111/j.1532-5415.2008.02023.x.
Ferrara MC, Zarcone C, Tassistro E, et al. Frailty and long-COVID: is COVID-19 responsible for a transition in frailty status among older adults who survived hospitalization for COVID-19? Aging Clin Exp Res. 2023;35:455–61. https://doi.org/10.1007/s40520-022-02308-4.
Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–8. https://doi.org/10.1016/S0140-6736(21)02758-6.
Araf Y, Akter F, Tang Y, et al. Omicron variant of SARS-CoV‐2: Genomics, transmissibility, and responses to current COVID‐19 vaccines. J Med Virol. 2022;94:1825–32. https://doi.org/10.1002/jmv.27588.
Fernández-de-Las-Peñas C, Notarte KI, Peligro PJ, et al. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: a systematic review of the literature. Viruses. 2022;14:2629. https://doi.org/10.3390/v14122629.
Du M, Ma Y, Deng J, et al. Comparison of long COVID-19 caused by different SARS-CoV-2 strains: a systematic review and Meta-analysis. Int J Environ Res Public Health. 2022;19:16010. https://doi.org/10.3390/ijerph192316010.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Study concept and design: MC and HV. Acquisition of the data: MC, AR, LB. Data analysis: BG, LZ. Drafting of the manuscript: MC and HV. Patient recruitment: MC, AR, LB, PP, EH, CT, HV. Critical revision of the manuscript for important intellectual content: MC, BG, AR, LB, PP, EH, CT, LZ and HV. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the ethics committee (CPP Ile de France, Paris, France, no. 107–2021, 21/09/2022). All included patients or their close relatives received an information letter specifying their rights and the terms of use of their medical data. Informed consent was obtained from all subjects. Non-objection was collected by the physicians in charge of the patients. The protocol was registered in ClinicalTrials.gov (ID: NCT05261061, 28/02/2022).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Claes, M., Genet, B., Rouet, A. et al. Mortality and functional outcomes 18 months after hospitalization for COVID-19 in geriatric patients: a multicentric cohort study. BMC Geriatr 24, 763 (2024). https://doi.org/10.1186/s12877-024-05240-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05240-6