Abstract
Background
The aging process induces neural and morphological changes in the human musculoskeletal system, leading to a decline in muscle mass, strength and quality. These alterations, coupled with shifts in muscle metabolism, underscore the essential role of physical exercise in maintaining and improving muscle quality in older adults. Muscle quality's morphological domain encompasses direct assessments of muscle microscopic and macroscopic aspects of muscle architecture and composition. Various tools exist to estimate muscle quality, each with specific technical requirements. However, due to the heterogeneity in both the studied population and study methodologies, there is a gap in the establishment of reference standards to determine which are the non-invasive and direct tools to assess muscle quality after exercise interventions. Therefore, the purpose of this review is to obtain an overview of the non-invasive tools used to measure muscle quality directly after exercise interventions in healthy older adults, as well as to assess the effects of exercise on muscle quality.
Main text
To address the imperative of understanding and optimizing muscle quality in aging individuals, this review provides an overview of non-invasive tools employed to measure muscle quality directly after exercise interventions in healthy older adults, along with an assessment of the effects of exercise on muscle quality.
Results
Thirty four studies were included. Several methods of direct muscle quality assessment were identified. Notably, 2 studies harnessed CT, 20 utilized US, 9 employed MRI, 2 opted for TMG, 2 adopted myotonometry, and 1 incorporated BIA, with several studies employing multiple tests. Exploring interventions, 26 studies focus on resistance exercise, 4 on aerobic training, and 5 on concurrent training.
Conclusions
There is significant diversity in the methods of direct assessment of muscle quality, mainly using ultrasound and magnetic resonance imaging; and a consistent positive trend in exercise interventions, indicating their efficacy in improving or preserving muscle quality. However, the lack of standardized assessment criteria poses a challenge given the diversity within the studied population and variations in methodologies.. These data emphasize the need to standardize assessment criteria and underscore the potential benefits of exercise interventions aimed at optimizing muscle quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The aging process generates neural [1] and morphological [2] changes in the human musculoskeletal system triggering a reduction in muscular parameters [3•]. Given the increase in life expectancy [4], it is imperative to promote active and healthy aging that improves the quality of life of older adults [5, 6]. Muscle quality is an important indicator of the overall health status of older adults [3, 7•]. The decline in muscle quantity and quality with age is a normal process that affects everyone [8, 9], which can lead to frailty, dependence, decreased quality of life and increased mortality [10, 11, 12•].
Maintaining and controlling muscle quality is of vital importance in the older adult, as it can help prevent the decline in muscle mass, strength and regenerative capacity, as well as slowing or preventing alterations in muscle metabolism [13]. Physical exercise interventions have been shown to be an effective means of prolonging average life expectancy [14, 15], as well as preventing and delaying the deterioration and loss of muscle quality inherent to aging [13, 14]. Research has shown that physical exercise not only enhances muscle quality [16, 17••,••] and function [18] but also improves functional fitness and metabolic health [12, 19•]. Additionally, it contributes to the stability and integrity of the cell membrane [20, 21, 22], which are key markers currently indicative of muscle quality. This scenario indicates that physical exercise plays a crucial role in mitigating the decline of muscle regeneration, boosting the number and activation of satellite cells, increasing myogenic potential, and reducing fibrosis formation. Furthermore, exercise effectively reduces the accumulation of age-related intermuscular fat and influences the composition of intramyocellular lipids [13]. Among exercise strategies, strength training, in its various forms, has proven to be a powerful tool to combat age-associated muscle decline [17, 23]. Firstly, moderate- to high-repetition strength training followed by high- and moderate-intensity aerobic exercise is a potential strategy to reverse the molecular features of skeletal muscle aging [24•], with power training being a preferred exercise modality in clinical populations [25]. Additionally, various strategies are explored, ranging from traditional strength training [23] to low-volume HIIT [26], as well as resistance methods such as plyometrics [27]. Equally important is recognizing the vital role of dietary interventions in promoting muscle health [28].
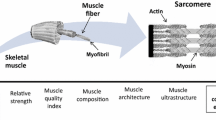
However, it is important to monitor, through systematic assessment, changes in muscle tissue after a physical exercise intervention in order to evaluate its effectiveness [29]. The European Working Group on Sarcopenia in Older People (EWGSOP) emphasizes the importance of assessing not only the quantity of muscle, but also its quality [30••]. Muscle quality is characterized by functional and morphological domains; the first one aligns with indirect measurements of muscle function relative to mass, while the second involves direct assessments of muscle architecture referring to the microscopic and macroscopic aspects of muscle architecture and composition [31•]. Despite the lack of a precise definition of muscle quality, it's crucial to analyze its construct and its relation to physical performance and muscle function [32•]. Our focus will be on analysing muscle quality through direct techniques that measure muscle architecture and composition.
Currently, there are several non-invasive techniques for monitoring muscle quality, but our focus will be on direct techniques measuring muscle architecture and composition [33,34,35]. These techniques are relatively easy to perform, do not require the insertion of invasive devices, and are an important tool for evaluating the effectiveness of physical exercise interventions in older adults [36].
Radiological imaging techniques allow the investigation of degenerative processes in individual muscle groups. These techniques can identify and quantify abnormalities, monitor patient progress and evaluate therapeutic interventions. Magnetic resonance imaging (MRI) and X-ray computed tomography (CT) stand as the current state-of-the-art in muscle quality assessment research [37,38,39]. CT, considered the gold standard for body composition analysis, excels in assessing muscle mass and quality, and diagnosing abnormal body composition phenotypes [40]. Notably, it offers exceptional visualization of intermuscular and intramuscular fat in tomographic sections [41]. Whereas, the development of new MRI sequences and tools has further increased the accuracy allowing for simultaneous assessment of body composition and identification of muscle quality issues such as disruption, edema, myosteatosis, and myofibrosis with the latter two tending to increase within muscles during aging [37, 42, 43]. In contrast, Dual-energy X-ray absorptiometry (DXA) is recommended as a reference in most EWGSOP guidelines to diagnose sarcopenia in clinical practice [30, 44••]. DXA provides a body composition model that includes fat, bone mineral density, and lean mass [45, 46], but even though it is a reference method for measuring total skeletal muscle mass, it cannot evaluate an individual muscle or assess muscle quality [7, 47].
In addition, ultrasound sonography (US) is a fast, non-invasive, and affordable imaging modality. The use of musculoskeletal ultrasound (MSK-US) for muscle quality assessment is rapidly gaining traction in clinical practice [40, 48]. A major advantage over other methods is that different muscle groups can be examined separately [49]. Common tissue characterization parameters measured include morphological measures of muscle thickness, pennation angle, cross-sectional area, echo intensity, and fascicle length [50•], which have shown correlations with muscle mass and strength [51]. Perkisas et al. [50•] standardized the use of ultrasound to assess muscle quality. In recent years, several qualitative tools aimed at identifying muscle quality loss have been developed in various care settings [52, 53]. Recent meta-analyses [54] underline the comparable and superior performance of MRI and CT in quantifying age-related morphological changes, highlighting their robustness in assessing muscle quality. In contrast, ultrasound, does not show a comparable level of accuracy in capturing age-related morphological changes [54, 55].
Another current non-invasive method is tensiomyography (TMG), a valuable tool for assessing neuromuscular function in older adults. The method is sensitive to muscle composition, architecture, and pre-atrophic changes in skeletal muscles, and may be sensitive to changes in muscle quality in aging and diseased populations [56, 57]. On the other hand, myotonometry is another tool that has been studied for the assessment of muscle viscoelastic properties [58]. Additionally, Bioelectrical Impedance Analysis (BIA) is a non-invasive, quick, and accessible technique that uses whole-body electrical conductivity to estimate body composition [59]. Notably, the Phase Angle (PhA) derived from BIA, a measure of cellular integrity and body water distribution, has become an important parameter for muscle quality assessment [60]. In fact, the European Working Group on Sarcopenia in Older People (EWGSOP) incorporates BIA-derived PhA in their criteria for muscle quality assessment, highlighting its potential for identifying sarcopenia [30••].
Thus, there is a need for a comprehensive review to compile and analyze the existing scientific evidence on the techniques mentioned in the evaluation of muscle architecture and composition in the field of promotion, intervention and design of physical exercise in the clinic of the older adult. Therefore, the aim of this systematic review is to obtain an overview of the non-invasive tools used to measure muscle quality directly after exercise interventions in healthy older adults. We aim to identify the different tools, measurement methods and their applicability in the direct assessment of muscle quality, providing a solid guide in the field of assessment and application of physical exercise interventions in older adults for future research in this area.
To achieve this goal, our research questions are as follows: (1) Which are the direct non-invasive tools used to measure muscle quality in older adults after exercise interventions?; (2) What are the effects of physical exercise programs on muscle quality in older adults measured by non-invasive tools?; (3) Which multisource objective parameters are predominantly utilized in the state of the art, and what priority have they shown in papers to measure muscle quality in older adults after exercise intervention?; and (4) What recent trends or advancements have been observed in the development of new non-invasive tools for quantifying muscle quality in older adults after exercise interventions?.
Methods
Registration
The systematic review was registered on the Open Science Framework (OSF) platform (https://osf.io/anjr4/?view_only=05969c336a0847028766e96f574eb63e), in October 24, 2023 (registration DOI: https://doi.org/https://doi.org/10.17605/OSF.IO/3GD6Y).
Procedures
The review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [61].
Eligibility criteria
Original, peer-reviewed, full-text studies were included/excluded using the PICOS method (participants, interventions, comparators, outcomes, and study design) [62]. The selection criteria are summarized in Table 1.
Literature search and screening process
Search strategy was developed by one reviewer (NV) specifically for PubMed (added below), and it was applied to the title, abstract, and keywords. This search strategy was later modified to align with the syntax and relevant subject headings of other databases. The literature search was performed in the electronic databases PubMed, Web of Science and Scopus, using the Boolean operators AND/OR, in combinations with the keywords (resistance OR strength* OR exercise* OR aerobic* OR multicomponent) AND (aged OR old OR elder* OR aging OR frail* OR older OR senior OR geriatric) AND ("contraction time" OR "reaction time" OR "contraction sustain time" OR "relaxation time" OR "muscle tone" OR stiffness OR "echo intensity" OR "pennation angle" OR "fat infiltration" OR "muscle lipid" OR "muscle hydration" OR "muscle microscopic fat" OR "macroscopic fatty infiltration" OR radiodensity OR "skeletal muscle radiodensity" OR "muscle density" OR "intermuscular adipose tissue" OR "extracellular water" OR "intracellular water" OR "phase angle" OR "muscle quality" OR "muscle composition") AND (muscle). The search was performed without date restriction and was updated until October 2023.
One author (NV) conducted the initial search, during which all the entries gathered from the databases were uploaded to the Rayyan QCRI website for the purpose of removing duplicates. Two reviewers (NV and XR) screened identified potentially eligible titles and abstracts, resolving disagreements together to mitigate interpretation bias. The full text of potentially eligible records was analyzed following the eligibility criteria for final inclusion. Reasons for exclusions were identified. When articles were not available we solicited authors by e-mail.
We decided not to include noninvasive imaging techniques in the search, given that our research question about the best noninvasive methods for assessing muscle quality could introduce bias by prejudging the results. Therefore, we chose to focus the search on relevant results related to muscle quality without explicitly incorporating the noninvasive techniques used. This decision was made to maintain impartiality in identifying the available evidence.
Data collection
Data from the included studies were collected and coded in Microsoft Excel (Microsoft Corp). The following information was extracted from each included study: (1) reference, author and year of publication, (2) participants characteristics (sample size; sex; age and health status), (3) intervention characteristics (frequency, type, duration), (4) muscle quality assessment procedures and outcomes, (5) group of muscles on which the measurement has been performed, and (6) results of the exercise intervention on muscle quality.
Risk of bias
To ensure the transparency and reliability of the results and findings, a Bias Risk Assessment has been performed for each study included in this review, using the Physiotherapy Evidence Database (PEDro) scale. The reliability of the PEDro scale in rating the quality of randomised controlled trials has been documented in a paper by Maher et al. [63].
To ascertain the overall risk of bias across the studies, the following convention was employed. The highest attainable score is ten, as the initial item is not included in the PEDro score computation. The methodological quality of the studies was classified as excellent when scores ranges from eight to ten, high with scores between six and seven, moderate with scores from four to five, and low with scores of three or below.
Results
Study selection
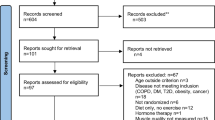
From scientific databases, potential studies were directly exported into Rayyan (https://www.rayyan.ai/) for removing duplicates and performing the screening applying inclusion and exclusion criteria previously determined. After the above procedure was completed, the following 6465 records were identified. A flow chart illustrating the study selection process is shown in Fig. 1. Duplicate records were removed (n = 3297). After titles and abstracts were screened, 2985 records were removed and 183 full texts were evaluated. An additional 143 studies were excluded after full text assessments for eligibility. Thereafter, 40 studies were considered eligible for the systematic review. After eliminating the original non-primary studies [64], a total of 34 studies were included.
Risk of bias of the included studies
The median score of the PEDro checklist (Table 2) was five (some risk of bias-moderate quality). 28 studies achieved four to fivepoints (some risk of bias-moderate quality) and six studies achieved six to seven points (low risk of bias-good quality).
Study characteristics
The characteristics of the included studies are detailed in Table 3. A total of 1,040 participants, with an age older than 60 years, were analyzed in this systematic review. Regarding participants sex, 21 studies reported a sample consisting of both male and females (n: 632, 61% of total participants). Five studies were composed of only men (n: 116, 11% of total participants) and nine groups involved only females (n: 292, 28% of total participants).
From the analyzed articles, two articles use CT, 18 use US, nine use MRI, two use TMG, two use myotonometry and one use BIA (there are articles with more than one test). Among the articles that analyze more than one test, one MRI + US and one tensiomyography + myotonometry.
As for the interventions performed in the studies analyzed, 25 studies conducted only resistance exercise, four studies only aerobic activity, five studies performed concurrent training. The frequency of the weekly sessions ranged from two to five sessions per week, with the majority of studies conducting two sessions per week (n: 14, 39%) and three sessions per week (n: 18, 50%). A total of 891 participants were enrolled in the intervention groups. This number exceeds that of the control groups, a discrepancy attributable to the inclusion of multiple studies evaluating diverse training regimens.
In our results, CT was predominantly used to evaluate cross-sectional areas, IMAT, thigh muscle density, total adipose tissue in the thigh, and muscle attenuation, focusing on the quadriceps and hamstrings muscles [71, 87]. MRI provided a wide range of muscle assessments, cross-sectional area analysis constituted 66.67% of the assessments, while IMAT and fat infiltration accounted for 22.22%, single assessments of muscle fat/water ratio, muscle mechanical quality, and intramuscular non-contractile tissue (IMNCT) comprised the remainder of the evaluations. The majority of MRI measurements (88.89%) targeted the quadriceps, except for one evaluation (11.11%) which assessed the BB [66, 68, 72, 74, 75, 88, 90,91,92].
US imaging revealed echo intensity as the most common measurement at 66.7%, predominantly analyzed in the RF (ten studies) and VL (seven studies). Pennation angle and fascicle length were assessed in 50% and 38.9% of studies, respectively, with the VL as the primary muscle of interest. CSA was examined in 22.2% of cases, focusing mainly on the RF and VL [43, 65,66,67, 69, 70, 73, 76, 77, 80,81,82,83,84,85,86, 89, 93,94,95]. Another muscle quality assessment tool highlighted in this review is the BIA, conducted in one study, to measure the PhA.
Building on this, the systematic review also reveals that TMG primarily assessed contraction time and displacement in the VL, along with the RF, BF, TA, GM, and GL [32, 96•]. Similarly, myotonometry [32, 78•] measured muscle tone and stiffness, focusing on the RF and TA, with additional tests on the BF, gluteus major, gastrocnemius, and VL. To conclude, a comprehensive summary outlining the specific outcomes measured to assess muscle quality using non-invasive tools is provided in Table 4.
For exercise effects on muscle quality in our systematic review, echo intensity decreased in eight studies [43, 73, 77, 80,81,82, 94, 95], while it remained unchanged in four [76, 85, 89, 93]. Within the review, seven articles reported improvements in pennation angle [65,66,67, 69, 70, 84, 86], while three articles observed no change [77, 83, 85]. In the systematic review we conducted, we observed that resistance exercise interventions improve CSA [66, 68, 75, 84, 85, 88, 90, 92] or maintain it [74, 77, 91, 95]. Also, our findings indicate improvements in fascicle length in two studies [65, 67], while five others reported no change [65, 70, 83,84,85].
Discussion
One of the key objectives of this systematic review was to collect and analyze studies focused on the use of non-invasive tools for direct assessment of muscle quality in older adults after exercise interventions. In addition, we aimed to understand the impact of these interventions on muscle quality.
Direct non-invasive muscle quality measurement tools
Non-invasive techniques provide a comprehensive assessment of muscle quality by evaluating factors such as muscle architecture, composition, fat infiltration, fibrosis, and neural activation [12•]. The following discussion will delineate the array of tools employed to directly measure muscle quality after physical exercise interventions. Direct methods for assessing muscle quality, involve the direct measurement of muscle architecture, addressing both microscopic and macroscopic aspects of muscle composition and structure [30••].
Among the non-invasive tools employed to directly assess muscular quality, the review of the literature revealed that US was utilized in 18 articles, MRI in nine, while CT, TMG, and myotonometry were each applied in two articles, and BIA was used in one, with some articles incorporating more than one diagnostic modality.
CT and MRI are essential for analyzing muscle composition, providing precise assessments of muscle quality through measures of intramuscular fat infiltration and cross-sectional area, both approved methodologies by EWGSOP2 for determining skeletal muscle quantity and quality [30, 39, 97,98,99••], these results are consistent with those obtained in this review. While CT offers rapid and cost-effective muscle quality analysis, it does generate radiation exposure. In contrast MRI ensures a radiation-free alternative at a higher cost. Notably both showed concordance in clinical muscle quality assessment [39, 98].
Ultrasonography is emerging as a fast, non-invasive, and accessible imaging modality for musculoskeletal assessment [100•]. Current B-mode ultrasound techniques enable detailed examination of muscle architecture, including cross-sectional area, echo intensity, fascicle length, and pennation angle, which are critical markers of muscle quality [97]. Our results highlight that the quadriceps is the most studied muscle due to its size and accessibility, corroborating what the scientific literature mentions [101]. Recent systematic reviews assessing the validity and reliability of ultrasonography for skeletal muscle evaluation have revealed strong interclass correlation coefficients and confirmed its comparative validity against other imaging modalities [35, 101, 102]. Although efforts to standardize these measurements are ongoing, these measurements are still highly dependent on operator expertise and do not provide definitive results for the early detection of muscle quality loss [50, 77•]. These findings are in line with the observations of the EWGSOP, which identifies ultrasound as a promising method for assessing skeletal muscle although it emphasizes the need for further research for its clinical application [30••].
BIA, through PhA analysis, emerges as an effective non-imaging method to characterize muscle quality components. BIA-derived PhA can be used to detect muscle quality and identify sarcopenia [60, 97]. Recent studies have started to recognize it as a significant predictor of muscle quality in older adults, associated with adverse clinical outcomes, including mortality [103, 104]. Also, the EWGSOP incorporates BIA-derived PhA in their criteria for muscle quality assessment [30••].
Expanding on these techniques TMG and myotonometry are non-invasive diagnostic tools that measure muscles mechanical properties. TMG utilizes electrodes to assess muscle contractile properties and tone in superficial muscles by quantifying radial deformation resulting from electrically induced contractions [105, 106•]. TMG has proven to be a valuable tool for assessing neuromuscular function in older adults, as it is sensitive to changes in muscle composition, architecture, and pre-atrophy of skeletal muscles [57]. A promising tool for the non-invasive assessment of muscle quality in aging and diseased populations [57]. Myotonometry measures muscle stiffness by monitoring radial tissue deformation in response to a perpendicular force applied through a hand-held device. It evaluates key muscle biomechanical and viscoelastic properties, including stiffness, compliance and elasticity [107]. Compared to elastography and TMG, myotonometry is fast, portable and cost-effective, displaying higher reliability and validity for differentiating muscle stiffness levels [107]. While existing studies affirm its reliability and validity within musculoskeletal diagnostics [108,109,110,111], further extensive validation is necessary for its routine clinical application. The research suggests that changes in muscle architecture, such as an increase in pennation angle, can impact tetanic tension and ultimately influence contractile properties [112]. This interplay between morphology, architecture, and contractile capacity in human pennate muscle is reflected in specific adaptation responses to intensive resistance training [112]. Additional studies emphasize the substantial influence of architectural parameters on muscular contractile dynamics, underscoring the relevance of architectural properties in the analysis of contractile behavior [113, 114].
Exercise effects on muscle quality
The heterogeneity in defining and assessing muscle architecture and composition contributes to the variance in results across different studies. This variation is further influenced by different training protocols and measurement techniques, which could explain the outcomes observed.
The mechanisms underlying the association between echo intensity and MQ are not fully elucidated, but it is hypothesized that intramuscular content alterations reflect performance outcomes [115]. Higher echo intensity usually denotes lower muscle quality and performance due to increased fibrous and adipose infiltration, conversely, reduced echo intensity tends to indicate enhanced performance [9, 115, 116]. The results of our systematic review, in which echo intensity decreased, aligns with findings from systematic reviews in which echo intensity is improved after exercise training [115]. Although ultrasound-based echo intensity is a common method for assessing the quadriceps femoris, its use raises questions in both research and clinical settings, particularly regarding the physiological interpretation of echo intensity changes and potential technical inconsistencies.
Also, exercise can influence the pennation angle of muscles, which is a potential indicator of muscle hypertrophy, the plasticity of muscle architecture, and the efficiency of force transmission [117]. Other reviews corroborate our findings, with seven studies noting improvements in pennation angle in older adults and others showing no change [118••]. These discrepancies may stem from the eccentric nature of resistance training or the short duration of certain studies [83, 85•].
During the aging process, there is a reduction in the size and number of muscles fibers, leading to atrophy and a reduction in cross-sectional area (CSA) [118, 119••]. In the systematic review we conducted, we observed that resistance exercise interventions improve CSA [66, 68, 75, 84, 85, 88, 90, 92] or maintain it [74, 77, 91, 95]. These improvements are attributed to muscle hypertrophy and myofibrillar protein turnover [85]. The results of our review are consistent with the results of other studies and reviews [118,119,120,121••], [119, 120].
Fascicle length is related to maximal shortening velocity and the force–length relationship. Such lengthening can result from an increase in serial sarcomere number or hypertrophy along the muscle fibers [115••]. As seen some studies resistance training increased it in older men [122, 123]. Yet, our results align with research showing some or no muscle architecture changes after certain training periods [124, 125].
When exploring the assessment of muscle quality, it allows us to unravel the implications that aging generates on it. Aging-associated fatty infiltration of skeletal muscle has been linked to negative health effects and functional deficits [74, 126]. There is a connection between fat infiltration in skeletal muscle and physical inactivity in elderly persons. Less is known about the idea that an exercise program can alter an older adult's IMAT level measured by MRI [126]. This justifies the improvement of fat infiltration and IMAT [66, 75] with some exercise interventions analyzed in the systematic review, while being maintained in others [68, 72, 74]. Prior research has looked at how resistance and multimodal exercise training affect older adults' muscle composition and has demonstrated the ability to reduce IMAT [74, 127, 128]. Whereas others cite no change in fat infiltration with exercise interventions [129, 130]. It has also been observed that physical exercise is capable of generating significant changes in CSA [130, 131]. In our systematic review CSA improves in three [88, 90, 92] studies and remains unchanged in one [91]. Other studies have shown positive changes in CSA with moderate intensity resistance training, but did not obtain improvements with low intensities [130]. Also, in another study only those with a high percentage of IMAT improved CSA [74].
While exploring the effects of age and exercise interventions on muscle composition, CT is crucial for assessing muscle quality by quantifying muscle attenuation and fat content, based on the specific attenuation of each tissue measured in Hounsfield units (HU). Increases in these areas are linked to poorer muscle quality and higher mortality risk [132]. Age-related increases in these fat deposits have been associated with metabolic and muscular dysfunction [126]. Our systematic review elucidates that physical exercise prevents the increase of intermuscular fat and the decrease of muscle density, compared to control [71], while another study shows that exercise improves muscle attenuation without increasing IMAT [133]. The findings align with existing research, a study with a similar population showed that while muscle CSA remained unchanged, there was a reduction in subcutaneous fat and IMAT [134]. In obese older adults, interventions including exercise and nutrition are proven to enhance subcutaneous and intermuscular fat, muscle CSA, and muscle attenuation [126, 132, 133, 135, 136•].
Moreover, aging leads to a decline in muscle contractile properties, often due to the loss of type II fibers [56]. TMG measured Tc has been found to correlate with muscle fiber composition, in muscles such as vastus lateralis [56], while Dm correlates with muscle atrophy [56]. As far as we have been able to observe the vast majority of interventions focus on young populations, where a regular decrease in dm is a common post-training response to strength training [57, 96, 137, 138]. Improvements in BB Dm and Tc have also been reported [139], although in some studies Dm has improved but Tc has remained unchanged [140]. These results agree with those obtained in the systematic review [32, 96•].
In the findings of our systematic review, it is observed that resistance training notably enhances muscle stiffness, whereas aerobic training maintains muscle tone and frequency [32, 78•] assessed by myotonometry. Comparable populations have shown improvements in muscle tone, stiffness, and elasticity following neck stabilization exercises [141], with muscle stiffness responding more noticeably than tone or elasticity to upper-extremity rehabilitation post-stroke [142].A field review reveals that resistance training effects on muscle are inconsistent, while plyometric training improves muscle stiffens also in pathological cases, exercise normalizes stiffness, but further study is needed [143•].
Furthermore, as evidenced by the results of the review, resistance exercise increases BIA-derived PhA [79], aligning with the literature linking resistance with improvements in strength and PhA in older adults [79, 104, 144]. Likewise, these studies associate PhA with changes in muscle strength [145]. To enhance PhA, a program of at least twelve weeks is recommended, with three weekly sessions of six to ten exercises, as applied in the intervention analyzed [104].
Primary multisource parameters in muscle quality assessment research
Among the articles incorporating multiple tests, some specifically combined different methodologies like MRI + US and tensiomyography + myotonometry [32, 66•]. MRI and US provide detailed images of muscle composition and structure, while tensiomyography and myotonometry assess muscle mechanical properties and stiffness, respectively.
Muscle quality is characterized by functional and morphological domains; the first one aligns with indirect measurements (Table 5) of muscle function relative to mass, while the second involves direct assessments of muscle architecture [31•]. Our review shows that studies often use both methods for a holistic understanding [17••]. Direct measurements offer precision for clinical research, yet are costly and require specialized skills. Indirect methods are prized for their speed and practicality [31•]. Employing both allows for cross-validation and a more comprehensive understanding of muscle quality, blending structural and functional insights.
Moreover, the inclusion of functional capacity tests in many articles of the systematic review [32, 68, 71, 72, 76, 79, 80, 82, 90, 91, 93, 94, 146, 147•,••] is justified by the association between muscle quality and functional capacity, especially in older adults [148]. Therefore, functional capacity tests provide valuable information on how muscle quality translates into daily practical performance.
Trends in direct muscle quality assessment tools
From the perspective of the EWGSOP, which emphasizes the importance of evaluating not just muscle quantity but also quality, our review reveals that a defined criterion for selecting one evaluation tool over another based on an individual's specific characteristics has not yet been established [30••]. This underscores the urgency of further researching the concept of muscle quality and how new technologies, combined with current physiological knowledge, can be appropriately applied to assess muscle quality depending on each individual's unique characteristics. Innovative technologies such as tensiomyography and myotonometry are emerging as important tools in this field [32•]. Phase angle measurements using BIA also show promise as a biomarker for monitoring muscle quality in older adults [103, 104]. In addition, recent advances in quantitative ultrasound techniques, such as echogenicity analysis, texture parameters, elastography and acoustic wave properties, are moving forward although so far, their clinical application has been limited [101, 149].
Conclusions
To our knowledge, this study represents one of the most comprehensive syntheses of evidence aimed at assessing muscle quality (microscopic and macroscopic aspects of muscle architecture and composition) in older adults through direct methods following physical exercise interventions. Key findings include: (1) the results of this review reflect that the most commonly used methods for the direct assessment of muscle quality after an exercise intervention are ultrasound (US) and magnetic resonance imaging (MRI). US imaging commonly reported outcomes such as echo intensity, pennation angle, fascicle length, and cross-sectional area (CSA) in the rectus femoris (RF) and vastus lateralis (VL). MRI, primarily assessed CSA, intramuscular adipose tissue (IMAT), and fat infiltration, with a predominant focus on the quadriceps. Exercise-induced reductions in echo intensity, improvements in pennation angle, and CSA enhancements were observed with ultrasound. MRI highlighted benefits in fat infiltration and IMAT; (2) a general tendency of exercise interventions to improve or maintain muscle quality; (3) the frequent combination of direct measures of muscle quality with indirect methods and functional capacity tests in current research. The majority of the reviewed articles employ both direct and indirect methods to assess muscle quality; and (4) an emerging development of technological innovation in the design of new tools for the direct detection of muscle quality, exemplified by tools such as US and phase angle measurement, although their clinical application remains limited in the target population.
Regarding the limitations of the study, the condition imposed to include studies, where muscle quality had to be measured directly in conjunction with exercise interventions, significantly limited the number of articles eligible for this review. Furthermore, a limitation has been observed in the inclusion of studies using phase angle as a parameter, this is because the studies did not perform a comparative analysis with muscle quality, which could have left out relevant articles. Likewise, no studies were found that employ all the direct measurement parameters of muscle quality together. The lack of a standardized protocol and the diversity in the evaluation methods used by different authors prevent an accurate and unified comparison of the results. The decision not to perform a meta-analysis on the effects of exercise on muscle quality is grounded in the notable diversity observed in the included studies. Variability in the tools used to measure muscle quality, differences in the muscles assessed, and the various aspects measured contribute to a significant level of methodological heterogeneity, compromising the necessary comparability for a robust meta-analysis.
A promising direction for future research is the development of personalized protocols for the selection of measurement tools, tailored to the specific conditions of each patient. This would include the identification of which tool is the most appropriate according to the individual profile and needs. In parallel, it is crucial to investigate advanced non-invasive techniques in sports medicine and rehabilitation to measure muscle quality with greater precision and sensitivity, which could lead to the creation of personalized physical exercise programs based on each person's specific muscle weaknesses. Furthermore, there is a need to explore the effectiveness of these tools in different muscles, determining the most effective one in which to perform the measurements in order to extrapolate the data to the diagnosis of muscle diseases in clinical settings.
Availability of data and materials
The systematic search queries and data charting methods employed in this study are available upon request. Additionally, our systematic review was registered on the Open Science Framework (OSF) platform on October 24, 2023, with the registration DOI [https://doi.org/https://doi.org/10.17605/OSF.IO/3GD6Y]. The authors are committed to fostering transparency in research, and for any inquiries or requests for specific methodological details, we welcome communication with the corresponding author.
Abbreviations
- BB:
-
Biceps Brachii
- BF:
-
Biceps Femoris
- BF:
-
Biceps Femoris
- BIA:
-
Bioimpedance
- BR:
-
Brachialis
- CSA:
-
Cross-Sectional Area
- CG:
-
Control Group
- CON:
-
Low-intensity + normal speed
- CT:
-
Computed Tomography
- Dm:
-
Maximal radial displacement
- DXA:
-
Dual Energy X-ray Absorptiometry
- EG:
-
Exercise Group
- EWGSOP:
-
The European Working Group on Sarcopenia in Older People
- GL:
-
Gastrocnemius Lateralis
- GM:
-
Gastrocnemius Medialis
- HITT:
-
High-Intensity Interval Training
- IMAT:
-
Intramuscular Adipose Tissue
- LST:
-
Low-intensity + slow movement
- MRI:
-
Magnetic Resonance Imaging
- MRI:
-
Magnetic Resonance Imaging
- MSK-US:
-
Musculoskeletal Ultrasound
- PA:
-
Pennation Angle
- PhA:
-
Phase Angle
- QF:
-
Quadriceps Femoris
- RF:
-
Rectus Femoris
- RT:
-
Resistance Training
- S/W:
-
Session/Week
- TA:
-
Tibialis Anterior
- Tc:
-
Time contraction
- TMG:
-
Tensiomyography
- US:
-
Ultrasound Sonography
- VI:
-
Vastus Intermedius
- VL:
-
Vastus Lateralis
- VM:
-
Vastus Medialis
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–34. https://doi.org/10.1093/gerona/63.8.829.
Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001;137. https://doi.org/10.1067/mlc.2001.113504.
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. https://doi.org/10.1093/gerona/61.10.1059.
Crimmins EM. Recent trends and increasing differences in life expectancy present opportunities for multidisciplinary research on aging. Nat Aging. 2021;1:12–3. https://doi.org/10.1038/s43587-020-00016-0.
Río X, González-Pérez A, Larrinaga-Undabarrena A, Coca A. Analysis of quality of life parameters in a health-promoting program for a population with cardiovascular risk factors: a preliminary study. SN Compr Clin Med. 2020;2:2221–9. https://doi.org/10.1007/s42399-020-00512-9.
Río X, Guerra-Balic M, González-Pérez A, Larrinaga-Undabarrena A, Coca A. Valores de referencia del SPPB en personas mayores de 60 años en el País Vasco. Atención Primaria. 2021;53:102075. https://doi.org/10.1016/j.aprim.2021.102075.
Koo BK. Assessment of muscle quantity, quality and function. J Obes Metab Syndr. 2022;31:9–16. https://doi.org/10.7570/jomes22025.
Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. 2019;99:427–511. https://doi.org/10.1152/physrev.00061.2017.
Ikezoe T. Age-related change in muscle characteristics and resistance training for older adults. Phys Ther Res. 2020;23:99–105. https://doi.org/10.1298/ptr.R0009.
McLeod M, Breen L, Hamilton DL, Philp A. Live strong and prosper: the importance of skeletal muscle strength for healthy ageing. Biogerontology. 2016;17:497–510. https://doi.org/10.1007/s10522-015-9631-7.
Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42:203–9. https://doi.org/10.1093/ageing/afs194.
McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longevity & Healthspan. 2014;3:9. https://doi.org/10.1186/2046-2395-3-9.
Distefano G, Goodpaster BH. Effects of exercise and aging on skeletal muscle. Cold Spring Harb Perspect Med. 2018;8:a029785. https://doi.org/10.1101/cshperspect.a029785.
Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016;23:1034–47. https://doi.org/10.1016/j.cmet.2016.05.007.
Gremeaux V, Gayda M, Lepers R, Sosner P, Juneau M, Nigam A. Exercise and longevity. Maturitas. 2012;73:312–7. https://doi.org/10.1016/j.maturitas.2012.09.012.
Hortobágyi T, Vetrovsky T, Brach JS, van Haren M, Volesky K, Radaelli R, et al. Effects of exercise training on muscle quality in older individuals: a systematic scoping review with meta-analyses. Sports Med Open. 2023;9:41. https://doi.org/10.1186/s40798-023-00585-5.
Radaelli R, Taaffe DR, Newton RU, Galvão DA, Lopez P. Exercise effects on muscle quality in older adults: a systematic review and meta-analysis. Sci Rep. 2021;11:21085. https://doi.org/10.1038/s41598-021-00600-3.
Kalache A, Kickbusch I. A global strategy for healthy ageing. World Health. 1997;50:4–5.
Seo M-W, Jung S-W, Kim S-W, Lee J-M, Jung HC, Song J-K. Effects of 16 weeks of resistance training on muscle quality and muscle growth factors in older adult women with sarcopenia: a randomized controlled trial. Int J Environ Res Public Health. 2021;18:6762. https://doi.org/10.3390/ijerph18136762.
Ward LC, Brantlov S. Bioimpedance basics and phase angle fundamentals. Rev Endocr Metab Disord. 2023;24:381–91. https://doi.org/10.1007/s11154-022-09780-3.
Kumar S, Dutt A, Hemraj S, Bhat S, Manipadybhima B. Phase angle measurement in healthy human subjects through bio-impedance analysis. Iran J Basic Med Sci. 2012;15:1180–4.
Cole KS. Electric phase angle of cell membranes. J Gen Physiol. 1932;15:641–9. https://doi.org/10.1085/jgp.15.6.641.
Lopez P, Pinto RS, Radaelli R, Rech A, Grazioli R, Izquierdo M, et al. Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin Exp Res. 2018;30:889–99. https://doi.org/10.1007/s40520-017-0863-z.
Harper C, Gopalan V, Goh J. Exercise rescues mitochondrial coupling in aged skeletal muscle: a comparison of different modalities in preventing sarcopenia. J Transl Med. 2021;19:71. https://doi.org/10.1186/s12967-021-02737-1.
Sklivas AB, Robinson LE, Uhl TL, Dupont-Versteegden EE, Mayer KP. Efficacy of power training to improve physical function in individuals diagnosed with frailty and chronic disease: A meta-analysis. Physiol Rep. 2022;10:e15339. https://doi.org/10.14814/phy2.15339.
Wu Z-J, Wang Z-Y, Gao H-E, Zhou X-F, Li F-H. Impact of high-intensity interval training on cardiorespiratory fitness, body composition, physical fitness, and metabolic parameters in older adults: A meta-analysis of randomized controlled trials. Exp Gerontol. 2021;150:111345. https://doi.org/10.1016/j.exger.2021.111345.
Vetrovsky T, Steffl M, Stastny P, Tufano JJ. The Efficacy and Safety of Lower-Limb Plyometric Training in Older Adults: A Systematic Review. Sports Med. 2019;49:113–31. https://doi.org/10.1007/s40279-018-1018-x.
Luo D, Lin Z, Li S, Liu S-J. Effect of nutritional supplement combined with exercise intervention on sarcopenia in the elderly: A meta-analysis. Int J Nurs Sci. 2017;4:389–401. https://doi.org/10.1016/j.ijnss.2017.09.004.
Chen J, Zhou R, Feng Y, Cheng L. Molecular mechanisms of exercise contributing to tissue regeneration. Sig Transduct Target Ther. 2022;7:1–24. https://doi.org/10.1038/s41392-022-01233-2.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. https://doi.org/10.1093/ageing/afy169.
de Lucena Alves CP, de Almeida SB, Lima DP, Neto PB, Miranda AL, Manini T, et al. Muscle quality in older adults: a scoping review. J Am Med Dir Assoc. 2023;24:462-467.e12. https://doi.org/10.1016/j.jamda.2023.02.012.
Labata-Lezaun N, Canet-Vintró M, López-de-Celis C, Rodríguez-Sanz J, Aiguadé R, Cuadra-Llopart L, et al. Effectiveness of a multicomponent training program on physical performance and muscle quality in older adults: a quasi-experimental study. Int J Environ Res Public Health. 2023;20:222. https://doi.org/10.3390/ijerph20010222.
Ackermans LLGC, Rabou J, Basrai M, Schweinlin A, Bischoff SC, Cussenot O, et al. Screening, diagnosis and monitoring of sarcopenia: When to use which tool? Clin Nutr ESPEN. 2022;48:36–44. https://doi.org/10.1016/j.clnesp.2022.01.027.
Sánchez Barrancos IM, Ruiz Serrano AL, González Santisteban R, Manso García S, Hernández Rodríguez T, Lozano Gago P, et al. Utilidad y fiabilidad de la ecografía clínica musculoesquelética en medicina familiar (1): rodilla, hombro y entesis. Aten Primaria. 2018;50:629–43. https://doi.org/10.1016/j.aprim.2018.07.010.
Price KL, Earthman CP. Update on body composition tools in clinical settings: computed tomography, ultrasound, and bioimpedance applications for assessment and monitoring. Eur J Clin Nutr. 2019;73:187–93. https://doi.org/10.1038/s41430-018-0360-2.
Calleja Gonzalez J, Marqués-Jiménez D, Jones M, Valdivielso F, Delextrat A, Mielgo-Ayuso J, et al. Muscle recovery after exercise, training and competition: physiological indicators and non-invasive monitoring techniques. 2020.
Chianca V, Albano D, Messina C, Gitto S, Ruffo G, Guarino S, et al. Sarcopenia: imaging assessment and clinical application. Abdom Radiol. 2022;47:3205–16. https://doi.org/10.1007/s00261-021-03294-3.
Giovannini S, Brau F, Forino R, Berti A, D’Ignazio F, Loreti C, et al. Sarcopenia: diagnosis and management, state of the art and contribution of ultrasound. J Clin Med. 2021;10:5552. https://doi.org/10.3390/jcm10235552.
Oba H, Matsui Y, Arai H, Watanabe T, Iida H, Mizuno T, et al. Evaluation of muscle quality and quantity for the assessment of sarcopenia using mid-thigh computed tomography: a cohort study. BMC Geriatr. 2021;21:239. https://doi.org/10.1186/s12877-021-02187-w.
Tagliafico AS, Bignotti B, Torri L, Rossi F. Sarcopenia: how to measure, when and why. Radiol Med. 2022;127:228–37. https://doi.org/10.1007/s11547-022-01450-3.
Huber FA, Grande FD, Rizzo S, Guglielmi G, Guggenberger R. MRI in the assessment of adipose tissues and muscle composition: how to use it. Quant Imaging Med Surg. 2020;10:1636649–1631649. https://doi.org/10.21037/qims.2020.02.06.
Engelke K, Chaudry O, Gast L, Eldib MAB, Wang L, Laredo J-D, et al. Magnetic resonance imaging techniques for the quantitative analysis of skeletal muscle: State of the art. J Orthopaed Transl. 2023;42:57–72. https://doi.org/10.1016/j.jot.2023.07.005.
Wilhelm E, Rech A, Minozzo F, Botton C, Radaelli R, Teixeira B, et al. Concurrent strength and endurance training exercise sequence does not affect neuromuscular adaptations in older men. Exp Gerontol. 2014;60:207–14. https://doi.org/10.1016/j.exger.2014.11.007.
Albano D, Messina C, Vitale J, Sconfienza LM. Imaging of sarcopenia: old evidence and new insights. Eur Radiol. 2020;30:2199–208. https://doi.org/10.1007/s00330-019-06573-2.
Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J. 2007;83:509–17. https://doi.org/10.1136/pgmj.2007.057505.
Jain RK, Vokes T. Dual-energy X-ray Absorptiometry. J Clin Densitom. 2017;20:291–303. https://doi.org/10.1016/j.jocd.2017.06.014.
Scafoglieri A, Clarys JP. Dual energy X-ray absorptiometry: gold standard for muscle mass? J Cachexia Sarcopenia Muscle. 2018;9:786–7. https://doi.org/10.1002/jcsm.12308.
Virto N, Río X, Angulo-Garay G, Molina RG, Céspedes AA, Zamora EBC, et al. Development of continuous assessment of muscle quality and frailty in older patients using multiparametric combinations of ultrasound and blood biomarkers: protocol for the ECOFRAIL study. JMIR Res Protoc. 2024;13:e50325. https://doi.org/10.2196/50325.
Xie W-Q, Xiao G-L, Hu P-W, He Y-Q, Lv S, Xiao W-F. Possible sarcopenia: early screening and intervention-narrative review. Ann Palliat Med. 2020;9:4283293–4293. https://doi.org/10.21037/apm-20-967.
Perkisas S, Baudry S, Bauer J, Beckwee D, De Cock A-M, Hobbelen H, et al. Application of ultrasound for muscle assessment in sarcopenia: towards standardized measurements. European Geriatric Medicine. 2018;9:739–57. https://doi.org/10.1007/s41999-018-0104-9.
Ramírez-Fuentes C, Mínguez-Blasco P, Ostiz F, Sánchez-Rodríguez D, Messaggi-Sartor M, Macías R, et al. Ultrasound assessment of rectus femoris muscle in rehabilitation patients with chronic obstructive pulmonary disease screened for sarcopenia: correlation of muscle size with quadriceps strength and fat-free mass. Eur Geriatr Med. 2019;10:89–97. https://doi.org/10.1007/s41999-018-0130-7.
Mourtzakis M, Parry S, Connolly B, Puthucheary Z. Skeletal muscle ultrasound in critical care: a tool in need of translation. Ann Am Thorac Soc. 2017;14:1495–503. https://doi.org/10.1513/AnnalsATS.201612-967PS.
Nagae M, Umegaki H, Yoshiko A, Fujita K. Muscle ultrasound and its application to point-of-care ultrasonography: a narrative review. Ann Med. 2023;55:190–7. https://doi.org/10.1080/07853890.2022.2157871.
Dallaway A, Kite C, Griffen C, Duncan M, Tallis J, Renshaw D, et al. Age-related degeneration of the lumbar paravertebral muscles: Systematic review and three-level meta-regression. Exp Gerontol. 2020;133: 110856. https://doi.org/10.1016/j.exger.2020.110856.
Dallaway A, Hattersley J, Diokno M, Tallis J, Renshaw D, Wilson A, et al. Age-related degeneration of lumbar muscle morphology in healthy younger versus older men. Aging Male. 2020;23:1583–97. https://doi.org/10.1080/13685538.2021.1878130.
Šimunič B, Pišot R, Rittweger J, Degens H. Age-related slowing of contractile properties differs between power, endurance, and nonathletes: a tensiomyographic assessment. J Gerontol: Ser A. 2018;73:1602–8. https://doi.org/10.1093/gerona/gly069.
Pus K, Paravlic AH, Šimunič B. The use of tensiomyography in older adults: a systematic review. Front Physiol. 2023;14:1213993. https://doi.org/10.3389/fphys.2023.1213993.
Garcia-Bernal M-I, Heredia-Rizo AM, Gonzalez-Garcia P, Cortés-Vega M-D, Casuso-Holgado MJ. Validity and reliability of myotonometry for assessing muscle viscoelastic properties in patients with stroke: a systematic review and meta-analysis. Sci Rep. 2021;11:5062. https://doi.org/10.1038/s41598-021-84656-1.
Sergi G, De Rui M, Veronese N, Bolzetta F, Berton L, Carraro S, et al. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin Nutr. 2015;34:667–73. https://doi.org/10.1016/j.clnu.2014.07.010.
Di Vincenzo O, Marra M, Di Gregorio A, Pasanisi F, Scalfi L. Bioelectrical impedance analysis (BIA) -derived phase angle in sarcopenia: a systematic review. Clin Nutr. 2021;40:3052–61. https://doi.org/10.1016/j.clnu.2020.10.048.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. https://doi.org/10.1016/j.jclinepi.2009.06.006.
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21. https://doi.org/10.1093/ptj/83.8.713.
Ferreira González I, Urrútia G, Alonso-Coello P. Revisiones sistemáticas y metaanálisis: bases conceptuales e interpretación. Rev Esp Cardiol. 2011;64:688–96. https://doi.org/10.1016/j.recesp.2011.03.029.
Baptista RR, Onzi E, Goulart N, Santos LD, Makarewicz G, Vaz M. Effects of concentric versus eccentric strength training on the Elderly’s Knee extensor structure and function. J Exer Physiology Online. 2016;19:120–32.
Bruseghini P, Capelli C, Calabria E, Rossi A, Tam E. Effects of High-Intensity Interval Training and isoinertial training on leg extensors muscle function, structure, and intermuscular adipose tissue in older adults. Frontiers in Physiology 2019;10. https://doi.org/10.3389/fphys.2019.01260.
Cepeda CCP, Lodovico A, Fowler N, Rodacki ALF. Effect of an eight-week ballroom dancing program on muscle architecture in older adult females. J Aging Phys Act. 2015;23:607–12. https://doi.org/10.1123/japa.2014-0101.
Da Boit M, Sibson R, Meakin J, Aspden R, Thies F, Mangoni A, et al. Sex differences in the response to resistance exercise training in older people. Physiol Rep 2016;4. https://doi.org/10.14814/phy2.12834.
Franchi MV, Monti E, Carter A, Quinlan JI, Herrod PJJ, Reeves ND, et al. Bouncing Back! Counteracting muscle aging with plyometric muscle loading. Front Physiol. 2019;10:178. https://doi.org/10.3389/fphys.2019.00178.
Gallo LH, Rodrigues EV, Filho JM, da Silva JB, Harris-Love MO, Gomes ARS. Effects of virtual dance exercise on skeletal muscle architecture and function of community dwelling older women. J Musculoskelet Neuronal Interact. 2019;19:50–61.
Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105:1498–503. https://doi.org/10.1152/japplphysiol.90425.2008.
Greig C, Gray C, Rankin D, Young A, Mann V, Noble B, et al. Blunting of adaptive responses to resistance exercise training in women over 75 y. Exp Gerontol. 2011;46:884–90. https://doi.org/10.1016/j.exger.2011.07.010.
Hill MW, Roberts M, Price MJ, Kay AD. Effects of flywheel training with eccentric overload on standing balance, mobility, physical function, muscle thickness, and muscle quality in older adults. J Strength Condition Res. 2022;36:3190. https://doi.org/10.1519/JSC.0000000000004006.
Jacobs JL, Marcus RL, Morrell G, LaStayo P. Resistance Exercise with Older Fallers: Its Impact on Intermuscular Adipose Tissue. Biomed Res Int. 2014;2014:e398960. https://doi.org/10.1155/2014/398960.
Konopka A, Wolff C, Suer M, Harber M. Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am J Physiol-Regul Integr Compar Physiol. 2018;315:R461–8. https://doi.org/10.1152/ajpregu.00030.2018.
Lopez P, Crosby B, Robetti B, Turella D, Weber T, de Oliveira M, et al. Effects of an 8-week resistance training intervention on plantar flexor muscle quality and functional capacity in older women: A randomised controlled trial. Exper Gerontol 2020;138. https://doi.org/10.1016/j.exger.2020.111003.
Lopez-Lopez S, Pareja-Galeano H, Almazan-Polo J, Cotteret C, Tellez-Gonzalez P, Calvo-Lobo C, et al. Quantitative ultrasound changes in echotexture and functional parameters after a multicomponent training program in pre-frailty individuals: a pilot randomized clinical trial. Healthcare 2021;9. https://doi.org/10.3390/healthcare9101279.
Mollà-Casanova S, Muñoz-Gómez E, Sempere-Rubio N, Inglés M, Aguilar-Rodríguez M, Page Á, et al. Effect of virtual running with exercise on functionality in pre-frail and frail elderly people: randomized clinical trial. Aging Clin Exp Res. 2023;35:1459–67. https://doi.org/10.1007/s40520-023-02414-x.
Nunes J, Ribeiro A, Silva A, Schoenfeld B, dos Santos L, Cunha P, et al. Improvements in phase angle are related with muscle quality index after resistance training in older women. J Aging Phys Act. 2019;27:515–20. https://doi.org/10.1123/japa.2018-0259.
Radaelli R, Botton CE, Wilhelm EN, Bottaro M, Lacerda F, Gaya A, et al. Low- and high-volume strength training induces similar neuromuscular improvements in muscle quality in elderly women. Exp Gerontol. 2013;48:710–6. https://doi.org/10.1016/j.exger.2013.04.003.
Radaelli R, Botton CE, Wilhelm EN, Bottaro M, Brown LE, Lacerda F, et al. Time course of low- and high-volume strength training on neuromuscular adaptations and muscle quality in older women. Age (Dordr). 2014;36:881–92. https://doi.org/10.1007/s11357-013-9611-2.
Radaelli R, Brusco CM, Lopez P, Rech A, Machado CLF, Grazioli R, et al. Muscle quality and functionality in older women improve similarly with muscle power training using one or three sets. Exp Gerontol. 2019;128:110745. https://doi.org/10.1016/j.exger.2019.110745.
Raj I, Bird S, Westfold B, Shield A. Effects of eccentrically biased versus conventional weight training in older adults. Med Sci Sports Exerc. 2012;44:1167–76. https://doi.org/10.1249/MSS.0b013e3182442ecd.
Rodriguez-Lopez C, Alcazar J, Sanchez-Martin C, Baltasar-Fernandez I, Ara I, Csapo R, et al. Neuromuscular adaptations after 12 weeks of light- vs heavy-load power-oriented resistance training in older adults. Scand J Med Sci Sports. 2022;32:324–37. https://doi.org/10.1111/sms.14073.
Scanlon TC, Fragala MS, Stout JR, Emerson NS, Beyer KS, Oliveira LP, et al. Muscle architecture and strength: Adaptations to short-term resistance training in older adults. Muscle Nerve. 2014;49:584–92. https://doi.org/10.1002/mus.23969.
Suetta C, Andersen J, Dalgas U, Berget J, Koskinen S, Aagaard P, et al. Resistance training induces qualitative changes in muscle morphology, muscle architecture, and muscle function in elderly postoperative patients. J Appl Physiol. 2008;105:180–6. https://doi.org/10.1152/japplphysiol.01354.2007.
Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. 2009. https://doi.org/10.1159/000182084
Tanton L, Cappaert T, Gordon P, Zoeller R, Angelopoulos T, Price T, et al. Strength, size, and muscle quality in the upper arm following unilateral training in younger and older males and females. Clin Med Insights-Arthr Musculoskelet Disord. 2009;2:9–18.
Teodoro J, Izquierdo M, da Silva L, Baroni B, Grazioli R, Lopez P, et al. Effects of long-term concurrent training to failure or not in muscle power output, muscle quality and cardiometabolic risk factors in older men: A secondary analysis of a randomized clinical trial. Exp Gerontol 2020;139. https://doi.org/10.1016/j.exger.2020.111023.
Tracy B, Ivey F, Hurlbut D, Martel G, Lemmer J, Siegel E, et al. Muscle quality. II. Effects of strength training in 65- to 75-yr-old men and women. J Appl Physiol. 1999;86:195–201. https://doi.org/10.1152/jappl.1999.86.1.195.
Vojciechowski AS, Silva CTDS, Rodrigues EV, Gallo LH, Melo Filho J, Gomes ARS. Does physical dance training with virtual games change muscle quality of community-dwelling older women? Games Health J. 2021;10:391–9. https://doi.org/10.1089/g4h.2020.0223.
Watanabe Y, Madarame H, Ogasawara R, Nakazato K, Ishii N. Effect of very low-intensity resistance training with slow movement on muscle size and strength in healthy older adults. Clin Physiol Functional Imaging. 2014;34:463–70. https://doi.org/10.1111/cpf.12117.
Yoshiko A, Kaji T, Sugiyama H, Koike T, Oshida Y, Akima H. Twenty-four months’ resistance and endurance training improves muscle size and physical functions but not muscle quality in older adults requiring long-term care. J Nutr Health Aging. 2019;23:564–70. https://doi.org/10.1007/s12603-019-1208-8.
Yoshiko A, Tomita A, Ando R, Ogawa M, Kondo S, Saito A, et al. Effects of 10-week walking and walking with home-based resistance training on muscle quality, muscle size, and physical functional tests in healthy older individuals. Eur Rev Aging Phys Act. 2018;15:13. https://doi.org/10.1186/s11556-018-0201-2.
Yoshiko A, Kaji T, Kozuka T, Sawazaki T, Akima H. Evaluation of rehabilitation exercise effects by using gradation-based skeletal muscle echo intensity in older individuals: a one-group before-and-after trial study. BMC Geriatr. 2021;21:485. https://doi.org/10.1186/s12877-021-02423-3.
Zubac D, Paravlic A, Koren K, Felicita U, Simunic B. Plyometric exercise improves jumping performance and skeletal muscle contractile properties in seniors. J Musculoskelet Neuronal Interact. 2019;19:38–49.
Correa-de-Araujo R, Harris-Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: a symposium report. Front Physiol. 2017;8:87. https://doi.org/10.3389/fphys.2017.00087.
Niklasson E, Borga M, Dahlqvist Leinhard O, Widholm P, Andersson DP, Wiik A, et al. Assessment of anterior thigh muscle size and fat infiltration using single-slice CT imaging versus automated MRI analysis in adults. BJR. 2022;95:20211094. https://doi.org/10.1259/bjr.20211094.
Faron A, Sprinkart AM, Kuetting DLR, Feisst A, Isaak A, Endler C, et al. Body composition analysis using CT and MRI: intra-individual intermodal comparison of muscle mass and myosteatosis. Sci Rep. 2020;10:11765. https://doi.org/10.1038/s41598-020-68797-3.
Sconfienza LM, Albano D, Allen G, Bazzocchi A, Bignotti B, Chianca V, et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol. 2018;28:5338–51. https://doi.org/10.1007/s00330-018-5474-3.
Casey P, Alasmar M, McLaughlin J, Ang Y, McPhee J, Heire P, et al. The current use of ultrasound to measure skeletal muscle and its ability to predict clinical outcomes: a systematic review. J Cachexia Sarcopenia Muscle. 2022;13:2298–309. https://doi.org/10.1002/jcsm.13041.
Nijholt W, Jager-Wittenaar H, Raj IS, van der Schans CP, Hobbelen H. Reliability and validity of ultrasound to estimate muscles: A comparison between different transducers and parameters. Clin Nutr ESPEN. 2020;35:146–52. https://doi.org/10.1016/j.clnesp.2019.10.009.
Norman K, Herpich C, Müller-Werdan U. Role of phase angle in older adults with focus on the geriatric syndromes sarcopenia and frailty. Rev Endocr Metab Disord. 2023;24:429–37. https://doi.org/10.1007/s11154-022-09772-3.
Duarte Martins A, Paulo Brito J, Batalha N, Oliveira R, Parraca JA, Fernandes O. Phase angle as a key marker of muscular and bone quality in community-dwelling independent older adults: A cross-sectional exploratory pilot study. Heliyon. 2023;9: e17593. https://doi.org/10.1016/j.heliyon.2023.e17593.
Čular D, Babić M, Zubac D, Kezić A, Macan I, Peyré-Tartaruga LA, et al. Tensiomyography: from muscle assessment to talent identification tool. Front Physiol. 2023;14:1163078. https://doi.org/10.3389/fphys.2023.1163078.
Lohr C, Schmidt T, Medina-Porqueres I, Braumann K-M, Reer R, Porthun J. Diagnostic accuracy, validity, and reliability of Tensiomyography to assess muscle function and exercise-induced fatigue in healthy participants. A systematic review with meta-analysis. J Electromyograph Kinesiol. 2019;47:65–87. https://doi.org/10.1016/j.jelekin.2019.05.005.
McGowen JM, Hoppes CW, Forsse JS, Albin SR, Abt J, Koppenhaver SL. The utility of myotonometry in musculoskeletal rehabilitation and human performance programming. J Athl Train. 2022. https://doi.org/10.4085/1062-6050-0616.21.
Morgan G, Martin R, Welch H, Williams L, Morris K. Objective assessment of stiffness in the gastrocnemius muscle in patients with symptomatic Achilles tendons. BMJ Open Sport Exerc Med. 2019;5: e000622. https://doi.org/10.1136/bmjsem-2019-000622.
Ilahi S, T. Masi A, White A, Devos A, Henderson J, Nair K. Quantified biomechanical properties of lower lumbar myofascia in younger adults with chronic idiopathic low back pain and matched healthy controls. Clinical Biomechanics. 2020;73:78–85. https://doi.org/10.1016/j.clinbiomech.2019.12.026.
Brazier J, Bishop C, Simons C, Antrobus M, Read PJ, Turner AN. Lower extremity stiffness: effects on performance and injury and implications for training. Strength Conditioning J. 2014;36:103–12. https://doi.org/10.1519/SSC.0000000000000094.
White A, Abbott H, Masi AT, Henderson J, Nair K. Biomechanical properties of low back myofascial tissue in younger adult ankylosing spondylitis patients and matched healthy control subjects. Clin Biomech. 2018;57:67–73. https://doi.org/10.1016/j.clinbiomech.2018.06.006.
Wilson MT, Ryan AMF, Vallance SR, Dias-Dougan A, Dugdale JH, Hunter AM, et al. Tensiomyography derived parameters reflect skeletal muscle architectural adaptations following 6-weeks of lower body resistance training. Front Physiol. 2019;10:1493. https://doi.org/10.3389/fphys.2019.01493.
Wickiewicz TL, Roy RR, Powell PL, Perrine JJ, Edgerton VR. Muscle architecture and force-velocity relationships in humans. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:435–43. https://doi.org/10.1152/jappl.1984.57.2.435.
Charles J, Kissane R, Hoehfurtner T, Bates KT. From fibre to function: are we accurately representing muscle architecture and performance? Biol Rev Camb Philos Soc. 2022;97:1640–76. https://doi.org/10.1111/brv.12856.
Wong V, Spitz RW, Bell ZW, Viana RB, Chatakondi RN, Abe T, et al. Exercise induced changes in echo intensity within the muscle: a brief review. J Ultrasound. 2020;23:457–72. https://doi.org/10.1007/s40477-019-00424-y.
Fukumoto Y, Ikezoe T, Yamada Y, Tsukagoshi R, Nakamura M, Mori N, et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol. 2012;112:1519–25. https://doi.org/10.1007/s00421-011-2099-5.
Ema R, Akagi R, Wakahara T, Kawakami Y. Training-induced changes in architecture of human skeletal muscles: Current evidence and unresolved issues. J Phys Fitness Sports Med. 2016;5:37–46. https://doi.org/10.7600/jpfsm.5.37.
Cordeiro Lde S, Linhares DG, Barros dos Santos AO, Lima dos Santos L, de Castro JBP, Vale RG de S. Influence of resistance training on muscle architecture in older adults: A systematic review and meta-analysis of randomized controlled trials. Arch Gerontol Geriatr. 2023;112:105020. https://doi.org/10.1016/j.archger.2023.105020.
Létocart AJ, Mabesoone F, Charleux F, Couppé C, Svensson RB, Marin F, et al. Muscles adaptation to aging and training: architectural changes – a randomised trial. BMC Geriatr. 2021;21:48. https://doi.org/10.1186/s12877-020-02000-0.
Vezzoli A, Mrakic-Sposta S, Montorsi M, Porcelli S, Vago P, Cereda F, et al. Moderate intensity resistive training reduces oxidative stress and improves muscle mass and function in older individuals. Antioxidants. 2019;8:431. https://doi.org/10.3390/antiox8100431.
Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol. 2007;103:1565–75. https://doi.org/10.1152/japplphysiol.00578.2007.
Lindberg K, Lohne-Seiler H, Fosstveit SH, Sibayan EE, Fjeller JS, Løvold S, et al. Effectiveness of individualized training based on force–velocity profiling on physical function in older men. Scand J Med Sci Sports. 2022;32:1013–25. https://doi.org/10.1111/sms.14157.
Din USU, Brook MS, Selby A, Quinlan J, Boereboom C, Abdulla H, et al. A double-blind placebo controlled trial into the impacts of HMB supplementation and exercise on free-living muscle protein synthesis, muscle mass and function, in older adults. Clin Nutr. 2019;38:2071–8. https://doi.org/10.1016/j.clnu.2018.09.025.
Allison SJ, Brooke-Wavell K, Folland J. High and odd impact exercise training improved physical function and fall risk factors in community-dwelling older men. J Musculoskelet Neuronal Interact. 2018;18:100–7.
Rantalainen T, Hoffrén M, Linnamo V, Heinonen A, Komi PV, Avela J, et al. Three-month bilateral hopping intervention is ineffective in initiating bone biomarker response in healthy elderly men. Eur J Appl Physiol. 2011;111:2155–62. https://doi.org/10.1007/s00421-011-1849-8.
Englund DA, Kirn DR, Koochek A, Zhu H, Travison TG, Reid KF, et al. Nutritional supplementation with physical activity improves muscle composition in mobility-limited older adults, the vive2 study: a randomized, double-blind, placebo-controlled trial. J Gerontol: Ser A. 2018;73:95–101. https://doi.org/10.1093/gerona/glx141.
Manini TM, Buford TW, Lott DJ, Vandenborne K, Daniels MJ, Knaggs JD, et al. Effect of dietary restriction and exercise on lower extremity tissue compartments in obese, older women: a pilot study. J Gerontol A Biol Sci Med Sci. 2014;69:101–8. https://doi.org/10.1093/gerona/gls337.
Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14:362–6. https://doi.org/10.1007/s12603-010-0081-2.
Walts CT, Hanson ED, Delmonico MJ, Yao L, Wang MQ, Hurley BF. Do sex or race differences influence strength training effects on muscle or fat? Med Sci Sports Exerc. 2008;40:669–76. https://doi.org/10.1249/MSS.0b013e318161aa82.
Otsuka Y, Yamada Y, Maeda A, Izumo T, Rogi T, Shibata H, et al. Effects of resistance training intensity on muscle quantity/quality in middle-aged and older people: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2022;13:894–908. https://doi.org/10.1002/jcsm.12941.
Kalapotharakos VI, Michalopoulou M, Godolias G, Tokmakidis SP, Malliou PV, Gourgoulis V. The effects of high- and moderate-resistance training on muscle function in the elderly. J Aging Phys Act. 2004;12:131–43. https://doi.org/10.1123/japa.12.2.131.
Madrid DA, Beavers KM, Walkup MP, Ambrosius WT, Rejeski WJ, Marsh AP, et al. Effect of exercise modality and weight loss on changes in muscle and bone quality in older adults with obesity. Exp Gerontol. 2023;174:112126. https://doi.org/10.1016/j.exger.2023.112126.
Waters DL. Intermuscular adipose tissue: a brief review of etiology, association with physical function and weight loss in older adults. Ann Geriatr Med Res. 2019;23:3–8. https://doi.org/10.4235/agmr.19.0001.
Ikenaga M, Yamada Y, Kose Y, Morimura K, Higaki Y, Kiyonaga A, et al. Effects of a 12-week, short-interval, intermittent, low-intensity, slow-jogging program on skeletal muscle, fat infiltration, and fitness in older adults: randomized controlled trial. Eur J Appl Physiol. 2017;117:7–15. https://doi.org/10.1007/s00421-016-3493-9.
Cadore EL, Pinto RS, Kruel LFM. Neuromuscular adaptations to strength and concurrent training in elderly men. Revista Brasileira de Cineantropometria e Desempenho Humano. 2012;14:483–95. https://doi.org/10.5007/1980-0037.2012v14n4p483.
Aas SN, Breit M, Karsrud S, Aase OJ, Rognlien SH, Cumming KT, et al. Musculoskeletal adaptations to strength training in frail elderly: a matter of quantity or quality? J Cachexia Sarcopenia Muscle. 2020;11:663–77. https://doi.org/10.1002/jcsm.12543.
Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–48. https://doi.org/10.2165/00007256-200434050-00005.
Wilson MT, Hunter AM, Fairweather M, Kerr S, Hamilton DL, Macgregor LJ. Enhanced skeletal muscle contractile function and corticospinal excitability precede strength and architectural adaptations during lower-limb resistance training. Eur J Appl Physiol. 2023;123:1911–28. https://doi.org/10.1007/s00421-023-05201-8.
Garcia-Manso J, Rodriguez-Matoso D, Sarmiento S, de Saa Y, Vaamonde D, Rodriguez-Ruiz D, et al. Effect of high-load and high-volume resistance exercise on the tensiomyographic twitch response of biceps brachii. J Electromyogr Kinesiol. 2012;22:612–9. https://doi.org/10.1016/j.jelekin.2012.01.005.
Kojić F, Ranisavljev I, Ćosić D, Popović D, Stojiljković S, Ilić V. Effects of resistance training on hypertrophy, strength and tensiomyography parameters of elbow flexors: role of eccentric phase duration. Biol Sport. 2021;38:587–94. https://doi.org/10.5114/biolsport.2021.99323.
Shin H-J, Kim S-H, Hahm S-C, Cho H-Y. Thermotherapy plus neck stabilization exercise for chronic nonspecific neck pain in elderly: a single-blinded randomized controlled trial. Int J Environ Res Public Health. 2020;17:5572. https://doi.org/10.3390/ijerph17155572.
Chuang L, Wu C, Lin K. Reliability, validity, and responsiveness of myotonometric measurement of muscle tone, elasticity, and stiffness in patients with stroke. Arch Phys Med Rehabil. 2012;93:532–40. https://doi.org/10.1016/j.apmr.2011.09.014.
Thomas E, Ficarra S, Nakamura M, Paoli A, Bellafiore M, Palma A, et al. Effects of Different Long-Term Exercise Modalities on Tissue Stiffness. Sports Med- Open. 2022;8:71. https://doi.org/10.1186/s40798-022-00462-7.
Sardinha LB, Rosa GB. Phase angle, muscle tissue, and resistance training. Rev Endocr Metab Disord. 2023;24:393–414. https://doi.org/10.1007/s11154-023-09791-8.
Souza MF, Tomeleri CM, Ribeiro AS, Schoenfeld BJ, Silva AM, Sardinha LB, et al. Effect of resistance training on phase angle in older women: a randomized controlled trial. Scand J Med Sci Sports. 2017;27:1308–16. https://doi.org/10.1111/sms.12745.
Herda AA, Nabavizadeh O. Short-term resistance training in older adults improves muscle quality: A randomized control trial. Exp Gerontol. 2021;145: 111195. https://doi.org/10.1016/j.exger.2020.111195.
Correa C, LaRoche D, Cadore E, Reischak-Oliveira A, Bottaro M, Kruel L, et al. 3 Different Types of Strength Training in Older Women. Int J Sports Med. 2012;33:962–9. https://doi.org/10.1055/s-0032-1312648.
Akima H, Yoshiko A, Radaelli R, Ogawa M, Shimizu K, Tomita A, et al. Comparison of muscle quality and functional capacity between Japanese and Brazilian older individuals. PLoS ONE. 2020;15:e0243589. https://doi.org/10.1371/journal.pone.0243589.
Paris MT, Mourtzakis M. Muscle composition analysis of ultrasound images: a narrative review of texture analysis. Ultrasound Med Biol. 2021;47:880–95. https://doi.org/10.1016/j.ultrasmedbio.2020.12.012.
Acknowledgements
Not applicable.
Funding
N.V received a University of Deusto grant from the researcher education program (Award number: FPI UD_2022_10).
Author information
Authors and Affiliations
Contributions
NV and XR conceived the idea and design for the article. NV and XR performed the literature search, data acquisition, analysis, and/or interpretation. NV, XR, AM-Z and BG-Z drafted and/or critically revised the work. All authors have read, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Virto, N., Río, X., Méndez-Zorrilla, A. et al. Non invasive techniques for direct muscle quality assessment after exercise intervention in older adults: a systematic review. BMC Geriatr 24, 642 (2024). https://doi.org/10.1186/s12877-024-05243-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05243-3