Abstract
Introduction
Lower leg pain and symptoms, and poor leg circulation are common in older adults. These can significantly affect their function and quality of life. Neuromuscular electrical stimulation (NMES) applied via the feet as ‘foot NMES’ activates the leg musculovenous pump. This study investigated the effects of foot NMES administered at home using Revitive® among community-dwelling older adults with lower leg pain and/or other lower leg symptoms such as cramps, or sensations of tired, aching, and heavy feeling legs.
Methods
A randomised placebo-controlled study with three groups (2 NMES, 1 Sham) and three assessments (baseline, week 8, week 12 follow-up) was carried out. Self-reported function using Canadian occupational performance measure (COPM), leg pain, overall leg symptoms score (heaviness, tiredness, aching, or cramps), and ankle blood flow were assessed. Analysis of covariance (ANCOVA) and logistic regression were used to compare the groups. Statistical significance was set at p < 0.05 (two-sided 5%).
Results
Out of 129 participants enrolled, 114 completed the study. The improvement in all outcomes were statistically significant for the NMES interventions compared to Sham at both week 8 (p < 0.01) and week 12 (p < 0.05). The improvement in COPM met the minimal clinically important difference (MCID) for the NMES interventions compared to Sham at both week 8 (p < 0.005) and week 12 (p < 0.05). Improvement in leg pain met MCID at week 8 compared to Sham (p < 0.05). Ankle blood flow increased approximately 3-fold during treatment compared to Sham. Compliance with the interventions was high and no device-related adverse events were reported.
Conclusions
The home-based foot NMES is safe, and significantly improved self-reported function, leg pain and overall leg symptoms, and increased ankle blood flow compared to a Sham among older adults.
Trial registration
The trial was prospectively registered in ISRCTN on 17/06/2019 with registration number ISRCTN10576209. It can be accessed at https://www.isrctn.com/ISRCTN10576209.
Key points
• Eight weeks of daily home-based foot NMES therapy delivered using Revitive devices significantly improved self-reported function, leg pain and other leg symptoms in community-dwelling older adults when compared to a Sham.
• Significant improvements in self-reported function and leg symptoms were sustained when measured at the follow-up four weeks after the intervention period.
• During use, the foot NMES induced approximately a three-fold increase in ankle blood flow volume and intensity of blood flow when compared to a Sham.
• No device-related adverse events were reported.
• Compliance with the foot NMES intervention was high.
Similar content being viewed by others
Introduction
‘Healthy ageing’ is vital as the world population is becoming older. As life expectancy increases an active lifestyle is key to maintaining functional ability and to promote healthy ageing, thereby minimising the impact on quality of life (QoL) [1, 2]. Lower leg symptoms such as pain and cramps, and sensations of tired, aching, and heavy feeling legs are common in older adults and can significantly impact on their functional QoL [3,4,5,6,7,8]. It is often difficult to associate the causes of such symptoms to a specific diagnosis, especially when multiple comorbidities relating to different medical conditions are present. Functional limitations due to ageing, a sedentary lifestyle or underlying comorbidities (e.g. peripheral vascular disease (PVD)) can perpetuate a ‘vicious circle’ leading to a more sedentary life, increased leg symptoms and a further decline in function [3,4,5, 9, 10].
Reduced lower leg muscle strength is a predictor of disability among older adults [11, 12]. Alongside poor blood flow, due to deterioration of the calf muscle pump, weakness in the legs is a common problem seen among older adults [11, 12]. Neuromuscular electrical stimulation (NMES) is a widely used modality for muscle strengthening and functional rehabilitation across various clinical populations, with a significant body of supporting literature [13,14,15,16,17]. Additionally, NMES treatment can boost blood circulation in asymptomatic people [18, 19], as well as in those with underlying circulatory deficits [20,21,22,23,24]. Instead of delivering through the conventional body pads, when NMES is applied as a novel ‘foot NMES’ via the plantar surface, the induced muscle contractions activate the musculovenous pump thus stimulating blood flow during treatment [19]. Whilst improved blood flow helps maintain leg circulatory health, muscle contractions can help improve strength and mobility [18, 25,26,27,28,29,30,31], demonstrating clinical benefits in patients with PVDs such as chronic venous disease (CVD) and peripheral arterial disease (PAD) by relieving symptoms, and improving functional QoL [32,33,34].
There is a paucity of research investigating the effects of home-based foot NMES on leg pain and other leg symptoms, and the functional QoL among community-dwelling older adults. Contemporary research lacks sham-controlled studies and are focused mainly on clinician delivered NMES with various patient groups despite home-based care becoming increasingly important for reducing the pressures on healthcare providers [35,36,37]. Therefore, the objective of this study was to investigate the effects of an eight-week foot NMES treatment program administered at home using two different Revitive® devices on older adults with leg pain and/or leg symptoms and to compare them to a Sham. The study protocol has been published [38].
Methods
This study is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines [39].
Study design
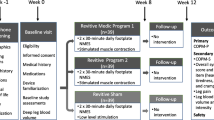
This study investigated the effects of two different foot NMES programmes against a Sham. It was a single-centre (Physiotherapy research laboratory at University of Hertfordshire, Hatfield, UK), participant-blind, parallel-group, randomised, placebo-controlled (Sham group), interventional study of 12-week duration (8-week intervention, and 4-week follow-up) with three assessments (baseline, week 8, and week 12) and three groups, each receiving a different type of foot NMES:
-
Group 1 – Revitive®Sham.
-
Group 2 – Revitive®Medic© Program 1.
-
Group 3 – Revitive®Program 2.
An overview of the study structure is given in Fig. 1. More details can be found in the published study protocol [38].
Participants
The study was advertised to the public using multiple local newspapers. Participants were community-dwelling adults aged over 65 years who reported one or more of the following symptoms in one or both legs: heaviness, tiredness, aching, or cramps. Exclusion criteria (self-reported) included: severe diabetic neuropathy; lumbar radiculopathy; restless legs syndrome; nervous system disorders; active cancer; contra-indications to NMES such as implanted electronic device (e.g., cardiac pacemaker); significant recent injury to the leg(s) (within the last six months); symptoms related to autoimmune, rheumatological, systemic illnesses or musculoskeletal conditions; those currently using Revitive®; being non-ambulant; inability to communicate in English; and inability to provide informed consent. The participants did not need to present with any specific diagnosis and the recruitment was solely based on self-reported symptoms.
Randomisation and blinding
Participants were randomised in a 1:1:1 ratio using computer-generated blocks of nine generated by the third author. The first author (principal investigator, PI) enrolled and assigned the participants to the interventions. Allocation was blinded by concealment of the randomisation schedule. All three Revitive® devices (Fig. 1) and their user manuals were identical. Conducting a double-blind study was problematic, as the assessor could identify the intervention groups during blood flow measurements due to visible muscle contractions (unlike for Sham). The participants remained blinded throughout the study. All data were processed and analysed by an independent statistician (second author).
Intervention
The study investigated the effects of two different Revitive® foot NMES programmes, against a Sham. The devices featured a mechanical foot rocker function (patented IsoRocker). Revitive® Medic© Program 1 comprised 15 NMES waveforms (patented). Revitive® Program 2 comprised 6 NMES waveforms (patent pending). The stimulation intensity was variable (1 to 99) and was controlled by the user. The comparator was Revitive® Sham, which was identical to Program 1, except that the stimulation intensity was limited to ‘2’ (delivered in 99 increments). The Sham devices delivered a weak yet perceivable sensation thus promoting a placebo effect among the recipients in that group rather than delivering a ‘no treatment’ control intervention. All devices were timed to run continuously for 30 min. Participants completed two 30-minute sessions at home daily for eight weeks, with the mechanical foot rocker enabled. They were advised to maintain a strong but comfortable stimulation intensity. Participants were told that the perception of stimulation may vary between people or may not be felt at all, and that the sensation often becomes less noticeable over time. All participants were supported throughout the study for any technical or clinical concerns.
NMES parameters
The Revitive® foot NMES devices used in this study comprise biphasic waveforms with frequencies ranging from 20 to 43.7 Hz. The pulse durations of the waveforms range from 450 to 970 µs. The maximum current output of the devices is 15 mA RMS at 500Ω resistance. The ‘ON’ and ‘OFF’ duration of NMES is waveform dependent and ranges from 1.9 to 8.3 s and 1.0 to 1.5 s respectively.
Primary outcome
The primary outcome was change in the Canadian Occupational Performance Measure Performance (COPM-P) from baseline to Week 8 for Revitive® Medic© Program 1 versus Sham. The COPM-P is a self-evaluation measure of each participant’s current physical/functional performance on self-selected activities. The COPM [40] is a valid, reliable, and responsive outcome measure widely used in clinical research among older adults [41,42,43]. The COPM was administered by the investigator in an open dialogue with the participants during their study visits. The COPM functional activities most commonly reported by the participants in this study were sleeping, walking, sitting, standing, and stair climbing.
Secondary outcomes
The secondary outcomes were COPM satisfaction (COPM-S), which measures the individual’s satisfaction with their COPM-P, leg pain measured using numerical pain rating scale (NPRS), total daily overall leg symptoms score [44] (the number of symptom days multiplied by the average intensity, summed across all four symptom domains: heaviness, tiredness, aching, and cramps), and deep ankle blood flow volume and intensity of flow measured using Doppler ultrasound before and during NMES application. Like the primary outcome, all secondary outcomes were administered by the same investigator (first author) during the study visits. Blood flow was recorded (first author is trained in ultrasound blood flow measurements) with the mechanical rocker disabled (to reduce noise), using ‘Esaote MyLab70 XVG’ ultrasound scanner with ‘LA523’ probe (4–13 MHz, Esaote S.p.A, Genoa, Italy).
All outcomes except blood flow were assessed at weeks 0, 8, and 12. Blood flow was recorded before and during NMES application at Week 0 only as it was a spot measurement unlike the other outcomes, and based on previous pilot experiments, the authors did not expect the relative change in blood flow during the NMES application to change over the study duration.
Adverse events monitoring
The Revitive NMES is a commercially available over the counter (OTC) device used extensively in the community. The risk of serious adverse events (SAE) is very rare. The occurrence of all adverse events (AE), whether or not device-related, was recorded throughout this study. Participants were instructed to report all AEs to the investigator as soon as possible. They were also asked about the occurrence of such events at study visits. AEs of special interest were identified as events with a potential causal association to the use of the study device. Any SAEs were investigated by an independent medical monitor and reported to the Ethics Committee and regulatory authorities.
Compliance monitoring
The participants were instructed to keep a daily record of treatment sessions undertaken. In the rare event that one or more sessions is missed, participants were required to keep a written record of those events and report them to the investigator at the Week 8 visit. The investigator documented the total number of sessions missed by each participant during their intervention period. All data were included in the statistical analysis.
Statistical analysis
Statistical analyses were conducted (using SAS version 9.4) for intent-to-treat (ITT), modified intent-to-treat (MITT) and per protocol (PP) populations; MITT and PP analyses were considered secondary. The MITT population included all participants who used their intervention at least once (ITT population), and if the condition being assessed was present at baseline. The PP population included participants who demonstrated a minimum 75% compliance with the intervention (missing no more than 28 treatments out of 112 over eight weeks). Missing data were imputed using multiple imputation under a ‘missing at random’ assumption using a monotone regression model.
Analysis of covariance (ANCOVA) with baseline value as a covariate and treatment group as a classification variable was used to compare Program 1 versus Sham and Program 2 versus Sham at weeks 8 and 12. If model assumptions were not met, Wilcoxon rank sum test was used. To control for multiplicity, a hierarchical testing procedure was used, whereby the statistical significance of Program 1 versus Sham was evaluated, and if this achieved significance (p < 0.05), the comparison of Program 2 versus Sham was evaluated. Logistic regression was used to compare the proportion of ‘responders’ (participants who achieved a minimal clinically important difference (MCID) in the outcome: 2-point improvements in COPM and leg pain) in each test group versus Sham. Treatment effect was estimated as an odds ratio (test/Sham), with 95% CIs and p-value. An odds ratio > 1 indicated a better outcome in the test group. Ultrasound images were computationally analysed using MATLAB (MathWorks, Massachusetts) algorithms to process colour image data into numerical data prior to statistical analysis, using a published method [45].
Interim analysis and sample size
An interim analysis of COPM-P was conducted with the first 10 participants from each group (total 30) to confirm sample size. An improvement of two points in individual COPM-P score was considered the MCID [43, 46, 47]. An absolute difference of 30% in the proportion of participants that met the MCID between Revitive® Sham, and Programs 1 or 2 was considered necessary to demonstrate a clinically meaningful difference for either active intervention [48,49,50,51]. The responder rate (participants who met the MCID) was calculated for Sham, and an absolute risk difference was defined for determining the required responder rates for Programs 1 and 2. Based on this model 39 participants were needed in each group (80% power, two-sided 5% significance, medium to large effect size of 0.643). Pearson Chi-square test at two-sided significance level (p < 0.05) was used for this comparison. Participant data from the interim analysis was included in the final analysis, as an identical protocol was followed throughout. No hypothesis test for stopping for futility or efficacy was conducted at the interim analysis, therefore the potential for inflation of Type I or Type II errors was considered negligible.
Post-hoc analysis
A post-hoc responder analysis was performed for leg pain, where an improvement by at least 30% compared to baseline was considered the MCID, instead of the conventional 2-point absolute change. The rationale for this analysis was that in studies with higher levels of variability in baseline pain (such as this study where there was no ‘minimum pain’ entry criterion), the relationship between percentage change and participant perception of improvement is more consistent than the relationship between raw change and perception of improvement [52, 53]. Therefore, the relative change provides additional and pertinent evidence.
Results
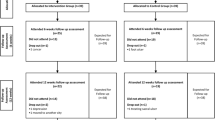
The study flowchart is given in Fig. 2. The demographic and anthropometric data are detailed in Table 1. There were no notable differences in these characteristics between the three treatment groups. Patient self-reported comorbidities are given in Additional Table 1.
Compliance, adverse events, and dropouts
Among the 129 participants enrolled, 127 formed the ITT population, of which 119 completed Week 8 assessments (8 dropouts) and 114 completed Week 12 assessments (further 5 dropouts) (Fig. 2). Compliance with the interventions were high, with > 92% in the two test groups and > 88% in the Sham group completing at least 75% of the required number of treatments over eight weeks. Excluding ten non-compliant participants (5 from Sham, 2 from Program 1, and 3 from Program 2), 117 were analysed in the PP population. The dropouts were due to unrelated AEs (UAE) and/or due to non-compliance. No serious adverse events (SAE) were reported and none of the AEs reported were determined as having any causal relationship to the interventions. The recruitment started on 01 July 2019 and finished on 30 June 2022. The study was successfully completed on 31 October 2022.
Main results
Tables 2 and 3 summarise the results of statistical analyses from all primary and secondary outcomes. The results of sensitivity analysis and PP analysis were consistent with the primary analyses for all instances (not reported). The mean/median percentage changes in scores from baseline for each outcome are reported in Additional Table 2.
Primary outcome – COPM performance
The commonly reported COPM functional activities in this study are detailed in Additional Table 3. The improvement in COPM-P was statistically significantly greater in both test groups compared to Sham, at both Week 8 and Week 12 (p < 0.001) in the change score analysis. Size of this difference was 1-point or greater. The percentage of participants achieving MCID (responders achieving 2-point change) at week 8 was 61% for Program 1 and 60% for Program 2, compared to 21% for Sham (absolute risk difference of approximately 40%). Hence, the odds of achieving MCID in either test group was approximately 4.8 times the odds of achieving MCID in Sham (p < 0.005). At week 12 follow-up, the responder rates remained significantly greater in both test groups, with odds ratios of 3.3 for Program 1 and 4.4 for Program 2 versus Sham (p < 0.05).
Secondary outcomes
COPM satisfaction
The COPM-S results were similar to that of COPM-P, with both change score and responder (MCID) analyses showing statistically significantly greater improvement in both test groups compared to Sham, at both Week 8 and Week 12 (p < 0.005). Size of this difference was 1-point or greater. The odds ratios for achieving MCID were 8.80 for Program 1 versus Sham (p < 0.0001) and 7.16 for Program 2 versus Sham (p = 0.0004) at week 8, and 4.80 for Program 1 versus Sham (p = 0.0027) and 4.86 for Program 2 versus Sham (p = 0.0029) at week 12.
Leg pain ITT analysis
The improvement in leg pain change score was statistically significantly greater in both test groups compared to Sham, at both week 8 (p < 0.01) and week 12 (p < 0.05). Size of this difference was greater than 1-point. The odds ratios for achieving MCID were 2.8 for Program 1 versus Sham (p = 0.0523) and 4.2 for Program 2 versus Sham (p = 0.0113). At week 12 there were no significant differences between the groups (p > 0.05).
Leg pain MITT responder analysis
Fourteen participants did not report leg pain (as it was not a mandatory inclusion criterion) at baseline (5 participants each from Programs 1&2, and 4 participants from Sham). These 14 participants were excluded from the MITT analysis, which was considered more clinically meaningful (for all other outcomes ITT analysis was equal to MITT analysis). The participants excluded from the MITT population were equally distributed across treatment groups and did not experience any worsening of their pain during the study (pain scores remained at zero throughout). The MITT analysis showed that the relative benefit in either test group was greater compared to Sham. The odds ratios for achieving MCID were 2.91 for Program 1 versus Sham (p = 0.0426) and 4.03 for Program 2 versus Sham (p = 0.0174). At week 12 there was no significant difference for Program 1 versus Sham (p > 0.05).
Leg pain post-hoc responder analysis
As stated, for the post-hoc responder analysis for leg pain, the definition of a responder was: a participant whose pain score improved by at least 30% from baseline (instead of the conventional 2-point absolute change). For both ITT and MITT analyses, the percentage of participants achieving a 30% reduction in pain after 8 weeks of device use was statistically significantly greater in Program 1 and Program 2 compared to Sham (p < 0.05). The odds ratios for achieving MCID were 3.36 for Program 1 versus Sham (p = 0.016) and 3.03 for Program 2 versus Sham (p = 0.0257) for the post-hoc ITT analysis, and 4.05 for Program 1 versus Sham (p = 0.0075) and 3.25 for Program 2 versus Sham (p = 0.0247) for the post-hoc MITT analysis. At week 12 there was no significant difference for Program 1 versus Sham (p > 0.05).
Leg symptoms score
The improvement in overall leg symptoms scores were statistically significantly greater in both test groups compared to Sham, at both Week 8 and Week 12. The size of the difference at Week 8 was greater than 4 points (p < 0.001) (-10.17 in Program 1, -10.49 in Program 2 compared to -5.95 in Sham). The treatment benefit decreased by Week 12 but was still statistically significant (p < 0.05). No responder analysis was carried out for this outcome as MCID have not been established. Analysis of the individual components of the leg symptoms score is given in Additional Table 4.
Blood flow volume and intensity
In groups using active devices, ankle blood flow volume increased approximately 3-fold (mean of 2.58 at baseline to 7.51 during use). Similarly, blood flow intensity increased 3-fold in the active groups (mean of 223 at baseline to 730 during use). There were no notable changes for either measure in the Sham group.
Discussion
Although the participants in this study did not necessarily have a formal diagnosis, the reported leg symptoms of pain and cramps, and sensations of aching, heaviness, and tiredness are usually consistent with impairment in blood circulation relating to PVDs such as CVD or PAD (although a diagnostic correlation is unclear) [3,4,5,6, 10]. Such conditions are common yet underdiagnosed among community-dwelling older adults [54, 55]. Up to 80% of the population suffer from at least a mild form of CVD and over 230 million people worldwide are affected by PAD causing a significant global health and economic impact [33, 56, 57]. The vascular system degenerates with age indicating an increased risk of severe cardiovascular events such as stroke and myocardial infarction [56, 57], which gets worsened by declining physical activity and deterioration of functional QoL [58]. When vascular diseases remain underdiagnosed and the emanating symptoms receive little medical attention, alternative home-based ‘over the counter’ treatments such as ‘foot NMES’ that provide effective early intervention for the management of leg symptoms become particularly important [32, 33, 59, 60]. Moreover, recent studies have shown that referral and participation in recommended treatments for PAD (such as supervised exercise therapy (SET)) is very low and therefore they remain highly underutilised due to challenges in awareness, access, and implementation [61, 62].
Foot NMES devices can be purchased over the counter without prescription and are designed for self-use at home without supervision. This pragmatic study was therefore conducted in a real-world setting, where the participants self-administered the treatment at home and were not required to make any alteration to their medication, diet, or exercise. This study has, to our knowledge, for the first time provided real-world data informing the applicability of foot NMES in community-dwelling older adults for the management of leg pain and other leg symptoms, and for improving their everyday functional performance. The results will therefore inform clinical practice.
Improvements in both COPM performance and satisfaction were highly clinically significant, with the percentage of responders nearly three times greater in the two Revitive® test groups compared to Sham. Similarly, the odds of achieving MCID for leg pain were nearly three-times greater in the two Revitive® test groups compared to Sham. The significant reduction in overall leg symptoms scores further supported these findings. The study demonstrated that these foot NMES devices are safe to use at home without supervision and that compliance was high, which corroborates earlier research [32,33,34]. Overall, the significant improvements and clinically relevant changes in the subjective outcomes indicate that the benefits delivered by foot NMES in real-world use are clinically relevant (results met MCID for COPM and leg pain) and were meaningful to the study participants. The study also demonstrated a sizeable ‘placebo effect’ for most outcomes. However, this is not unusual for a sham-controlled study given that placebo effect may exist with ‘perceived intervention’, mainly due to ‘expectation’ [63, 64]. On the other hand, the placebo effect together with high compliance showed that participants were adequately blinded to the Sham. Notwithstanding the placebo effect, the real treatment effect was statistically significantly greater and clinically meaningful.
This study had various strengths and some limitations. To the authors’ knowledge this is the first study of its kind on this important topic. The study featured a sham control and was adequately statistically powered. The primary outcome was ‘patient-centred’, evaluating self-reported functions that were important to the participants. Compliance with the intervention was high and the dropout rates were low. One limitation was that the study was only participant-blinded, the assessor blinding would have been problematic for reasons previously identified. The follow-up was short, but many of the outcomes were still significant at 12 weeks.
Conclusions
The home-based foot NMES therapy using Revitive® Medic© Program 1 and Revitive® Program 2 over an 8-week period significantly improved self-reported function, reduced leg pain, relieved leg symptoms, and increased ankle blood flow (during treatment) compared to Sham. Compliance with the intervention was high (> 92% in the test groups) indicating that the device was well tolerated and was sufficiently easy to use and manage. No device-related adverse events were reported, which demonstrated the high degree of safety. It is anticipated that with continued foot NMES use more sustained and greater treatment effects may be achieved; however, this should be investigated by further studies with potentially longer follow-up, which per se was beyond the scope of this study.
Data availability
Anonymised summary data will be available from the corresponding author upon reasonable request.
Abbreviations
- AE:
-
Adverse event
- ANCOVA:
-
Analysis of covariance
- COPM-P:
-
Canadian occupational performance measure – performance
- COPM-S:
-
Canadian occupational performance measure – satisfaction
- CVD:
-
Chronic venous disease
- ITT:
-
Intention to treat
- HSETECDA:
-
University of Hertfordshire Health, Science, Engineering and Technology Ethics Committee with Delegated Authority
- MCID:
-
Minimal clinically important difference
- MITT:
-
Modified intention to treat
- NMES:
-
Neuromuscular electrical stimulation
- NPRS:
-
Numerical pain rating scale
- PP:
-
Per protocol
- PAD:
-
Peripheral arterial disease
- PVD:
-
Peripheral vascular disease
- QoL:
-
Quality of life
- SAE:
-
Serious adverse event
- SET:
-
Supervised exercise therapy
- UAE:
-
Unrelated adverse event
References
von Berens Å, Fielding RA, Gustafsson T, Kirn D, Laussen J, Nydahl M, et al. Effect of exercise and nutritional supplementation on health-related quality of life and mood in older adults: the VIVE2 randomized controlled trial. BMC Geriatr. 2018;18:1–8.
Puente-Fernández J, Larumbe-Zabala E, Jiménez A, Liguori G, Rossato CJL, Mayo X, et al. No impact of combining multi-ingredient supplementation with exercise on body composition and physical performance, in healthy middle-aged and older adults: a systematic review and meta-analysis. Exp Gerontol. 2023;172:112079.
Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guideline on the management of patients with Lower Extremity Peripheral Artery Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation. 2017;135(12):e686–725.
McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159(4):387–92.
Criqui MH, Matsushita K, Aboyans V, Hess CN, Hicks CW, Kwan TW, et al. Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: A Scientific Statement from the American Heart Association. Circulation. 2021;144(9):e171–91.
Berger D. Leg discomfort: beyond the joints. Med Clin. 2014;98(3):429–44.
Jones RH, Carek PJ. Management of varicose veins. Am Family Phys. 2008;78(11):1289–94.
Raetz J, Wilson M, Collins K. Varicose veins: diagnosis and treatment. Am Family Phys. 2019;99(11):682–8.
Hamburg NM, Creager MA. Pathophysiology of intermittent claudication in Peripheral Artery Disease. Circulation Journal: Official J Japanese Circulation Soc. 2017;81(3):281–9.
Mills JL, Armstrong D. Chronic venous insufficiency. BMJ Best Practice. 2020.
Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50(3):461–7.
Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002;31(2):119–25.
Anthony J. Neuromuscular electrical stimulation (NMES). In: Watson T, Nussbaum EL, editors. Electrophysical agents: evidence-based practice. Volume 13. ed. London: Elsevier; 2021. pp. 296–327.
Nussbaum EL, Houghton P, Anthony J, Rennie S, Shay BL, Hoens AM. Neuromuscular Electrical Stimulation for Treatment of Muscle Impairment: critical review and recommendations for clinical practice. Physiother Can. 2017;69(5):1–76.
Adams V. Electromyostimulation to fight atrophy and to build muscle: facts and numbers. Wiley Online Library; 2018. pp. 631–4.
Kucio E, Niesporek J, Kucio C. Application of neuromuscular electrical stimulation of the lower limb skeletal muscles in the rehabilitation of patients with chronic heart failure and chronic obstructive pulmonary disease. Family Med Prim Care Rev. 2017(1):71–4.
Langeard A, Bigot L, Chastan N, Gauthier A. Does neuromuscular electrical stimulation training of the lower limb have functional effects on the elderly? A systematic review. Exp Gerontol. 2017;91:88–98.
Man WIO, Lepar GS, Morrissey MC, Cywinski JK. Effect of neuromuscular electrical stimulation on foot/ankle volume during standing. Med Sci Sports Exerc. 2003;35(4):630–4.
Varatharajan L, Williams K, Moore H, Davies AH. The effect of footplate neuromuscular electrical stimulation on venous and arterial haemodynamics. Phlebology. 2015;30(9):648–50.
Williams KJ, Moore HM, Ellis M, Davies AH. Pilot trial of neuromuscular stimulation in human subjects with chronic venous disease. Vasc Health Risk Manag. 2021;17:771–8.
Zhang Q, Styf J, Ekstrom L, Holm AK. Effects of electrical nerve stimulation on force generation, oxygenation and blood volume in muscles of the immobilized human leg. Scand J Clin Lab Investig. 2014;74(5):369–77.
Hajibandeh S, Hajibandeh S, Antoniou GA, Scurr JR, Torella F. Neuromuscular electrical stimulation for the prevention of venous thromboembolism. Cochrane Database Syst Rev. 2017(11).
Williams K, Ravikumar R, Gaweesh A, Moore H, Lifsitz A, Lane T et al. A review of the evidence to support neuromuscular electrical stimulation in the prevention and management of venous disease. Thrombosis and Embolism: from Research to Clinical Practice: Volume 1. 2017:377 – 86.
Ellul C, Formosa C, Gatt A, Hamadani AA, Armstrong DG. The effectiveness of calf muscle Electrostimulation on Vascular Perfusion and walking capacity in patients living with type 2 diabetes Mellitus and Peripheral Artery Disease. Int J Lower Extremity Wounds. 2017;16(2):122–8.
Bolter CP, Critz JB. Changes in thoracic and right duct lymph flow and enzyme content during skeletal muscle stimulation. Archives internationales de physiologie et de biochimie. 1976;84(1):115–28.
Faghri PD, Votto JJ, Hovorka CF. Venous hemodynamics of the lower extremities in response to electrical stimulation. Arch Phys Med Rehabil. 1998;79(7):842–8.
Kaplan RE, Czyrny JJ, Fung TS, Unsworth JD, Hirsh J. Electrical foot stimulation and implications for the prevention of venous thromboembolic disease. Thromb Haemost. 2002;88(2):200–4.
Reed BV. Effect of high voltage pulsed electrical stimulation on microvascular permeability to plasma proteins. A possible mechanism in minimizing edema. Phys Ther. 1988;68(4):491–5.
Snyder AR, Perotti AL, Lam KC, Bay RC. The influence of high-voltage electrical stimulation on edema formation after acute injury: a systematic review. J Sport Rehabilitation. 2010;19(4):436–51.
Stick C, Grau H, Witzleb E. On the edema-preventing effect of the calf muscle pump. Eur J Appl Physiol Occup Physiol. 1989;59(1–2):39–47.
Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev. 2017;97(2):495–528.
Babber A, Ravikumar R, Onida S, Lane TRA, Davies AH. Effect of footplate neuromuscular electrical stimulation on functional and quality-of-life parameters in patients with peripheral artery disease: pilot, and subsequent randomized clinical trial. Br J Surg. 2020;107(4):355–63.
Ravikumar R, Lane TR, Babber A, Onida S, Davies AH. A randomised controlled trial of neuromuscular stimulation in non-operative venous disease improves clinical and symptomatic status. Phlebology. 2021;36(4):290–302.
Ravikumar R, Williams KJ, Babber A, Lane TRA, Moore HM, Davies AH. Randomised controlled trial: potential benefit of a Footplate Neuromuscular Electrical Stimulation device in patients with chronic venous disease. Eur J Vasc Endovasc Surg. 2017;53(1):114–21.
Loellgen H, Zupet P, Bachl N, Debruyne A. Physical activity, exercise prescription for health and home-based rehabilitation. Sustainability. 2020;12(24):10230.
McDermott MM, Guralnik JM, Criqui MH, Ferrucci L, Zhao L, Liu K, et al. Home-based walking exercise in peripheral artery disease: 12‐month follow‐up of the GOALS randomized trial. J Am Heart Assoc. 2014;3(3):e000711.
Organization WH. WHO guideline on self-care interventions for health and well-being. World Health Organization; 2021.
Kumaran B, Targett D, Watson T. The effect of an 8-week treatment program using a novel foot neuromuscular electrical stimulator on physical function, leg pain, leg symptoms, and leg blood flow in community-dwelling older adults: a randomized sham-controlled trial. Trials. 2022;23(1):873.
Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18.
Law M, Baptiste S, McColl MA, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57(2):82–7.
Larsen EA, Carlsson G. Utility of the Canadian Occupational performance measure as an admission and outcome measure in interdisciplinary community-based geriatric rehabilitation. Scand J Occup Ther. 2012;19(2):204–13.
Carswell A, McColl MA, Baptiste S, Law M, Polatajko H, Pollock N. The Canadian Occupational performance measure: a research and clinical literature review. Can J Occup Ther. 2004;71(4):210–22.
Tuntland H, Aaslund MK, Espehaug B, Forland O, Kjeken I. Reablement in community-dwelling older adults: a randomised controlled trial. BMC Geriatr. 2015;15:145.
Howlader MH, Smith PDC. Symptoms of chronic venous disease and association with systemic inflammatory markers. J Vasc Surg. 2003;38(5):950–4.
Kumaran B, Herbland A, Watson T. Continuous-mode 448 kHz capacitive resistive monopolar radiofrequency induces greater deep blood flow changes compared to pulsed mode shortwave: a crossover study in healthy adults. Eur J Physiotherapy. 2017;19(3):137–46.
Eyssen IC, Steultjens MP, Oud TA, Bolt EM, Maasdam A, Dekker J. Responsiveness of the Canadian occupational performance measure. J Rehabil Res Dev. 2011;48(5):517–28.
Nielsen TL, Andersen NT, Petersen KS, Polatajko H, Nielsen CV. Intensive client-centred occupational therapy in the home improves older adults’ occupational performance. Results from a Danish randomized controlled trial. Scand J Occup Ther. 2019;26(5):325–42.
Arnold LM, Williams DA, Hudson JI, Martin SA, Clauw DJ, Crofford LJ, et al. Development of responder definitions for fibromyalgia clinical trials. Arthr Rhuem. 2012;64(3):885–94.
Cornec D, Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, Berthelot J-M, Perdriger A, et al. Development of the Sjögren’s syndrome Responder Index, a data-driven composite endpoint for assessing treatment efficacy. Rheumatology. 2015;54(9):1699–708.
Hewitt DJ, Ho TW, Galer B, Backonja M, Markovitz P, Gammaitoni A, et al. Impact of responder definition on the enriched enrollment randomized withdrawal trial design for establishing proof of concept in neuropathic pain. PAIN®. 2011;152(3):514–21.
Simon LS, Evans C, Katz N, Bombardier C, West C, Robbins J, et al. Preliminary development of a responder index for chronic low back pain. J Rhuematol. 2007;34(6):1386–91.
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58.
Olsen MF, Bjerre E, Hansen MD, Hilden J, Landler NE, Tendal B, et al. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017;15(1):1–18.
McDermott MM. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circul Res. 2015;116(9):1540–50.
Rejeski WJ, Axtell R, Fielding R, Katula J, King AC, Manini TM, et al. Promoting physical activity for elders with compromised function: the lifestyle interventions and independence for elders (LIFE) study physical activity intervention. Clin Interv Aging. 2013;8:1119–31.
Peltokangas M, Verho J, Mattila VM, Romsi P, Vehkaoja A, Lekkala J, et al. Areas under peripheral pulse waves: a potential marker of atherosclerotic changes. Physiol Meas. 2018;39(2):025003.
Van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32(2):454–60.
McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Liao Y, et al. Greater sedentary hours and slower walking speed outside the home predict faster declines in functioning and adverse calf muscle changes in peripheral arterial disease. J Am Coll Cardiol. 2011;57(23):2356–64.
Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circul Res. 2021;128(12):1818–32.
Santiago FR, Ulloa J, Régnier C, Peudon T, Braund E, Fradet-Aubignat C, et al. The impact of lower limb chronic venous disease on quality of life: patient and physician perspectives. J Comp Eff Res. 2022;11(11):789–803.
Ehrman JK, Gardner AW, Salisbury D, Lui K, Treat-Jacobson D. Supervised Exercise Therapy for symptomatic peripheral artery disease: a review of current experience and practice-based recommendations. J Cardiopulm Rehabil Prev. 2023;43(1):15–21.
Khoury SR, Ratchford EV, Stewart KJ. Supervised exercise therapy for patients with peripheral artery disease: clinical update and pathways forward. Prog Cardiovasc Dis. 2022;70:183–9.
Brown WA. Expectation, the placebo effect and the response to treatment. Rhode Island Med J. 2015;98(5):19.
Miller FG, Rosenstein DL. The nature and power of the placebo effect. J Clin Epidemiol. 2006;59(4):331–5.
Acknowledgements
The authors want to thank all study participants.
Funding
The study was funded by Actegy Limited. The University of Hertfordshire received the funding from Actegy Limited to undertake this study.
Author information
Authors and Affiliations
Contributions
BK was the principal investigator and was responsible for the acquisition and processing of data and writing this manuscript. TW was responsible for the concept and overall supervision of the project and critical revision of the manuscript. BK and TW are responsible for the study design. DT planned and conducted data analyses. All authors have approved the final version of this manuscript and agree to be accountable for all aspects of the work, its accuracy, and integrity. The authors also confirm that all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The first author (BK) declares no competing interests. DT is an independent expert acting as a statistical consultant for Actegy Limited for this study (funder of the trial and manufacturer of Revitive®). TW has expert consultancy contract with Actegy Limited, which is unrelated to this study.
Ethical approval and consent to participate
This study was approved by the University of Hertfordshire Health, Science, Engineering and Technology Ethics Committee with Delegated Authority (HSETECDA). The study was conducted conforming with all required local and international medical device regulations. All experiments on humans were performed in accordance with relevant guidelines and regulations (such as the Declaration of Helsinki). Informed consent was received from all participants prior to the start of the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kumaran, B., Targett, D. & Watson, T. Benefits of home-based foot neuromuscular electrical stimulation on self-reported function, leg pain and other leg symptoms among community-dwelling older adults: a sham-controlled randomised clinical trial. BMC Geriatr 24, 683 (2024). https://doi.org/10.1186/s12877-024-05271-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05271-z