Abstract
Background
To assess the prevalence of anemia before and after antiretroviral therapy (ART) initiation and to identify impact of anemia on mortality among HIV-infected patients in China during the Treat-All era.
Methods
All HIV-infected patients who newly initiated ART between January 1, 2017 and December 31, 2020 were enrolled and followed up to December 31, 2021 in China. We analyzed the prevalence of anemia before and after ART initiation. Generalized estimating equations were fitted to determine factors associated with anemia after ART. Time-dependent cox proportional hazards models were performed to estimate the effect of anemia on death.
Results
Of 436,658 patients at the baseline of ART initiation, the overall prevalence of anemia was 28.6%. During a median 2.65 (IQR: 1.80–3.51) years of follow-up after ART initiation, 376,325 (86.2%) patients had at least one Hb measurement (a total of 955,300 hemoglobin measurements). The annual prevalence of anemia after ART was 17.0%, 14.1%, 13.4%, 12.6% and 12.7%, respectively. Being anemic at the baseline of ART initiation (adjusted odds ratio, aOR = 6.80, 95% confidence interval (CI): 6.67–6.92) was the strongest factor associated with anemia after ART. Anemia status after ART showed a strong association with death after multivariable adjustment (mild anemia: adjusted hazard ratio (aHR) = 2.65, 95% CI: 2.55–2.76; moderate anemia: aHR = 4.60; 95% CI:4.40–4.81; severe anemia: aHR = 6.41; 95% CI:5.94–6.91).
Conclusions
In the era of ART universal access, pre-ART anemia was common among HIV-infected patients. Notably, a certain proportion of anemia still persisted after ART, and was significantly associated with death. We recommend strengthening the monitoring of patients at risk of anemia, especially in patients with baseline anemia or during the first year of ART, and timely treatment for correcting anemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia has always been a global public health issue, affecting nearly 23% of the world’s population and accounting for approximately 50.3 million healthy life years lost according to the Global Burden of Disease 2019 statistics [1]. Notably, anemia is of particular concern in HIV-infected patients. HIV-infected patients experience a higher risk of anemia than the general population [2]. Anemia, as one of the most common complications in HIV-infected patients [3, 4], is considered to be a predictor of poor health outcomes such as apparent fatigue, poor quality of life, rapid disease progression, and increased mortality [5].
The causes of anemia in HIV-infected patients are multifactorial, including HIV infection, aging, poor nutritional status, advanced disease, lower CD4+ T cell count as well as antiretroviral drugs [6, 7]. Common mechanisms of suffering from anemia in the general population such as nutritional deficiencies (including iron, vitamin B12 and folate), endemic anemia as well as infections including malaria and tuberculosis also occur in HIV-infected patients [8, 9]. Although antiretroviral therapy (ART) has been generally recognized as an effective treatment for improving anemia, several antiretroviral drugs are associated with anemia. Zidovudine (AZT), as one of the most critical components in the early history of ART, has been dramatically reduced in use due to its hematologic toxicity by bone marrow suppression [7]. Recent studies demonstrate that integrase strand transfer inhibitor (INSTI)-based regimens may also increase the risk of anemia [10, 11]. Of note, the selection of antiretroviral drugs is influenced not only by toxicity but also by complex factors such as drug availability, economic status, indications and prescribing physicians [12].
In the era of ART universal access, HIV-infected patients still face a higher burden of anemia. Recent findings reported a global prevalence of anemia among HIV-infected adults at 46.6% [12], at 19–33% in developed countries [11, 13, 14] and 32–67% in developing countries [15,16,17,18]. The prevalence of anemia varies markedly across different socioeconomic and clinical settings [12, 19]. A study among ART-naïve patients showed that the prevalence of anemia was 30.5% among patients with CD4+ T cell count < 200 cells/μL, and 60.1% among patients aged ≥ 60 years [20]. Currently, the increasing aging patients and the high proportion of late diagnosis among HIV-infected patients might contribute to the anemia burden [21, 22]. In addition, another study from North America showed that the prevalence of anemia among patients receiving ART declined from 33% to 2007 to 20% in 2017 [11]. Thus, universal access to ART and the use of AZT free treatment combination may partially alleviate the burden of anemia [23].
In China, the total number of patients living with HIV diagnosed in 2022 reached 1223 thousand since the first AIDS case was reported in 1985 [24]. The Chinese criterion for being eligible for ART initiation has been adjusted several times from CD4 ≤ 200 cells/μL in 2004, and revised to regardless of CD4 + T cell count in 2016. By the end of 2022, ART coverage achieved 92.8% [24]. Only few small studies from early ART era reported the prevalence of anemia among Chinese HIV-infected patients, ranging from 9.8 to 51.9% [20, 25, 26]. So far nationwide cohort studies on the prevalence of anemia are lacking in the Treat-all era in China. In addition, previous studies only confirmed the association between baseline anemia and mortality [25, 27], despite the fact that anemia during ART is time-dependent and may influence the association. The aim of this study is to evaluate the burden of anemia before and after ART and the impact of time-dependent anemia on outcomes in the national ART program.
Methods
Study design and participants
We conducted an observational longitudinal cohort study among HIV-infected patients throughout the country who newly initiated ART from January 1, 2017 to December 31, 2020 in China (Fig. 1). The follow-up endpoint of this study was December 31, 2021. Eligible patients were aged 15 years or older and had hemoglobin (Hb) measurements at the ART initiation. In the analysis of ART follow-up section, we selected those with at least one follow-up Hb measurement during the study observation period. This study was performed in accordance with the Declaration of Helsinki. This study was reviewed and approved by the Institutional Review Board of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention. Since all data were de-identified and provided in the aggregated form, the informed consent was waived.
Data resources
All data we used was retrieved from the Chinese National Free ART Program (NFATP) database, which has been described previously [28]. This database is a web-based ART data collection system, containing related information on all HIV-infected patients receiving ART in mainland China. The information collected includes socio-demographic, clinical, and laboratory data. CD4+ T cell count and routine laboratory tests including routine blood tests, kidney and liver function were required to test at ART baseline. Routine laboratory test was repeated by the clinician as needed during the treatment follow-up. CD4+ T cell count and viral load were provided every year after ART initiation. Clinical visits were conducted at 0.5, 1, 2, 3 months after ART initiation and every 3 months thereafter.
Definitions of outcomes and other variables
Primary outcomes were the prevalence of anemia at the baseline of ART initiation and after ART. Secondary outcomes were the effect of anemia on death after ART. When assessing the annual prevalence of anemia after ART, the last Hb test was selected if two or more measurements were available each year. Anemia was defined as Hb level less than 13 g/dL for males or less than 12 g/dL for females according to the World Health Organization criteria [29]. It was further classified into mild anemia (11–12.9 g/dL in males or 11–11.9 g/dL in females), moderate anemia (8–10.9 g/dL), and severe anemia (< 8 g/dL).
Body mass index (BMI) scores were generated by the formula of (weight in kilograms)/(height in meters) [2]. Estimated glomerular filtration rate (eGFR) was generated by the Modification of Diet and in Renal Disease (MDRD) equation, eGFR = 186×serum creatinine (mg/dL) − 1.154 × [age(years)] − 0.203 × [0.742 if female] × [1.212 if black]. OIs were defined according to the Chinese Diagnosis criteria for HIV/AIDS [30]. Detailed OI data at ART baseline were only available from the designated hospitals/clinics. Based on available antiretroviral drugs in China, ART regimen was categorized into non-nucleoside reverse transcriptase inhibitor (NNRTI), protease inhibitor (PI), INSTI, and abnormal prescription or missing. Depending on the use of NRTI, the backbone of ART was divided into five groups, tenofovir disoproxil fumarate (TDF), AZT, abacavir (ABC), tenofovir alafenamide (TAF) and abnormal prescription or missing. The 2016 version of Chinese free ART guideline recommended TDF or AZT + Lamivudine + Efavirenz as the first-line regimen, with TDF preferred over AZT. And AZT should not be used in HIV-infected patients with Hb less than 90 g/L [31]. Financially affordable patients could access drugs beyond the scope of the free catalog. Patients with specific conditions such as severe anemia, hepatic or renal abnormalities, or severe opportunistic infections are recommended to delay initiation of ART until their condition improves [32].
Statistical analyses
Continuous variables were described with median and interquartile ranges (IQR) or mean ± standard deviation. Categorical variables were presented as frequencies and proportions. The baseline characteristics of the cohort were described, stratified by the magnitude of anemia at the baseline of ART initiation. Then the annual prevalence of anemia after ART initiation was calculated.
Generalized estimating equation models (GEE) were used to explore the factors associated with anemia after ART initiation. The dependent variable was the status of anemia at each follow-up visit. Explanatory variables included fixed characteristics and time-varying variables. The fixed variables included age, gender, route of HIV transmission, BMI, eGFR and OIs at ART initiation. CD4+ T cell count, viral load, NRTI backbone, ART regimen and duration of ART were considered as time-dependent variables. Univariate and multivariate GEE analyses were performed on all covariates to calculate the odds ratio (OR) and 95% confidence interval (CI) for anemia. Time-varying Cox proportional hazards models were used to evaluate the effect of anemia on death. CD4+ T cell count, viral load, NRTI backbone, ART regimen and anemia status were considered as time-dependent variables. The observation time for each patient was calculated from the ART initiation to the date of death, dropout or the follow-up endpoint of this study, whichever came first. Two-sided P-values < 0.05 were considered statistically significant. All calculations were performed using SAS version 9.1 (SAS Institute, Cary, United States of America).
Results
A total of 559,780 patients entered the NFATP from January 1, 2017, to December 31, 2020, 123,122 patients were excluded due to missing Hb measurement at ART initiation. We assessed the baseline homogeneity of included and excluded patients, and found the difference in baseline information was ± 5% (data not shown). Finally, 436,658 patients entered the study cohort (Fig. 1).
At the baseline of ART initiation, the overall prevalence of anemia in the national cohort was 28.6%, with mild, moderate, and severe anemia for 16.3%, 10.5%, and 1.9%, respectively. As shown in Table 1, the proportion of anemia was higher in concurrent OIs group, the older age group, the lower baseline eGFR, BMI or CD4+ T cell count group. In patients with the concurrent OIs, baseline eGFR < 60ml/min/1.73m2, baseline BMI < 18.5 kg/m2, baseline CD4+ T cell count < 200 cells/μL or age ≥ 50 years, the proportion of total anemia was 61.4%, 58.2%, 50.6%, 47.8% and 41.6%, respectively.
Anemia after ART initiation
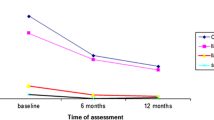
A total of 376,325 (86.2%) patients who had at least one Hb follow-up were included in the analysis (Fig. 1), with a median follow-up time was 2.65 (IQR: 1.80–3.51) years. 955,330 Hb measurements were available and the mean frequency of Hb testing after ART initiation was 2.06 ± 1.09. The annual prevalence of anemia was 17.0%, 14.1%, 13.4%, 12.6% and 12.7% from the first year to the fifth year after ART (Fig. 2).
In multivariate GEE model analysis (Table 2), being anemic at the baseline of ART initiation was the strongest factor associated with the increased odds of anemia during the ART follow-up (aOR = 6.80, 95% CI: 6.67–6.92). Compared with NNRTI-based regimen, the use of INSTI decreased the risk of anemia (aOR = 0.78, 95% CI: 0.74–0.83), but PI-based regimen (aOR = 1.43, 95% CI: 1.39–1.46) was more likely to have anemia after ART. The use of ABC (aOR = 1.47, 95% CI: 1.37–1.57) or AZT (aOR = 2.25, 95% CI: 2.19–2.30) had a significantly higher risk of anemia than TDF-contained regimen. Other factors including older age, female, acquired HIV through heterosexual contact or injecting drug use, low BMI, low eGFR, low CD4+ T cell count and high viral load were independent predictors of anemia.
Anemia and mortality
During the follow-up period, 15,562 deaths were identified and the overall mortality rate was 1.52/100 person-years (PY). The mortality rate was 0.82/100PY in those who were not anemic. Stratified by the magnitude of anemia, the mortality rate was 3.40,5.45,8.17/100PY in those with mild, moderate and severe anemia. After adjusting for confounders in the time-varying Cox proportional hazards model, the severity of anemia was the strongest predictor for mortality. Compared with non-anemic patients, mild anemia (aHR = 2.65; 95% CI: 2.55–2.76), moderate anemia (aHR = 4.60; 95% CI:4.40–4.81), and severe anemia (aHR = 6.41; 95% CI:5.94–6.91) were strongly increased the association with mortality (Table 3).
Discussion
This is the first study of a nationwide ART cohort to assess the burden of anemia in HIV-infected patients in China during the current ART era. We observed that pre-ART anemia was common, but severe anemia was infrequent (1.9%). Although anemia was improved after ART initiation, a certain percentage of patients were still anemic and baseline anemia was the strongest risk factor. Furthermore, we found that anemia status after ART was a significant predictor of death. Thus, the issue of anemia in both ART-naïve and ART-experienced patients still needs to be taken seriously.
In China, the prevalence of anemia among the general population was 8.93% according to the Global Burden of Disease Study [32]. In our study, pre-ART anemia prevalence among HIV-infected patients was 28.6%, which is much higher than that in the general population. Comparable results have been documented in other countries, including those studies conducted in Brazil (33%) [14], Ethiopia (31.8%) [17] and India (25.5%) [33]. However, a much higher proportion of anemia was reported in Uganda (67.4%) [34] and South Africa (70.5%) [35]. Another study nested within the PEARLS cohort study conducted in nine countries in diverse geographic settings found significant differences in the frequency of anemia, such as Malawi (67%), Haiti (67%), Thailand (47%) and Peru (37%) [14]. The burden of anemia in resource-limited countries might be higher, possibly due to nutrient deficiencies, more frequent infectious diseases and limited medical resources [36].
Moreover, we found that anemia was more prevalent in patients with older age, poor nutritional and clinical status. Similarly, a study in Uganda and Zimbabwe found that HIV-infected patients greater than 50 years were 2.6 times more likely to be anemic than patients of reproductive age [19]. This may not only be related to an age-related increase in comorbidities, but also aging itself may increase hematopoietic stem cell resistance to erythropoietin and proinflammatory cytokine expression [37]. Furthermore, HIV infection may accentuate aging progress through mechanisms such as chronic immune activation and inflammatory responses [38]. Additionally, previous studies support our finding that anemia was common among patients with lower CD4+ T cell count, especially in patients with CD4+ T cell count < 200 cells/μL [39]. Evidence has reported that lower CD4+ T cell count is associated with poor immune recovery, active viral replication and advanced disease, which are all contributing factors to anemia [40, 41].
Consistent with previous evidence [11], we observed a remarkable reduction of anemia after ART initiation, especially in the first year. In our study, the proportion of anemia declined from 27.3 to 17.0% within 12 months of ART. Similar findings were observed in a separate study in Ethiopia that the prevalence of anemia decreased from 42.9% at baseline to 14.3% at 12 months after ART [42]. Based on data from 34 ART cohorts, a systematic review showed that Hb continued to increase after initiating non-AZT-containing ART, the most significant increase was seen in the first year, with Hb increasing by 2.0 g/dL at 12 months and by 2.5 g/dL at 36 months [23]. The improvement of anemia after ART might be explained through inhibiting HIV replication, promoting immune reconstitution, and reducing the occurrence of OIs [12].
Our study revealed similar results to other studies, that being anemic at ART initiation was the strongest factor associated with anemia after ART [23]. A study conducted in rural Tanzania observed that there were one-third were still anemic after 12 months of ART among patients with anemia at ART initiation [43]. The persistence of anemia in HIV-infected patients may possibly be due to multifactorial causes [8], such as inflammatory states, nutritional deficiencies, co-infection with other viruses and antiretroviral drugs. necessitating additional intervention in addition to ART to correct anemia. In addition, we found that the use of INSTI decreased the risk of anemia. However, a study in the US showed an inconsistent outcome that INSTI increased the risk of anemia by 26% compared to NNRTI, which was considered due to the fact that patients with failed viral suppression or worse clinical status are more likely to switch to an INSTI-based regimen [10]. Our study also showed that patients with higher viral load are more likely to be anemic. This is consistent with prior studies that not only HIV infection but also active HIV viral replication could increase the risk of anemia [8].
Our findings provided evidence that the severity of anemia after ART was a significant predictor of mortality in HIV-infected patients. A long-term cohort in China showed that compared to patients who were not anemic at baseline, mild and moderate anemia increased the hazard of death by 60% and severe anemia by 86% [44]. Another analysis from EuroSIDA quantified the effect of Hb on death in HIV-infected patients, then found that the risk of death tended to increase by 57% with 1 g/dL decrease in Hb [45]. Based on similar findings, emerging studies attempted to use baseline Hb to predict prognostic outcomes. A nested case-control study found that adding baseline Hb to a prognostic model containing both CD4+ T cell count and viral load significantly improved the accuracy in predicting the risk of death in HIV-infected patients [27].
Our study has some limitations. Firstly, we only included those who initiated ART. Patients with severe anemia are more likely to be excluded from initiating ART until their condition improves to meet the criteria for initiating ART, which may underestimate the burden of anemia. Secondly, potential selection bias may have been introduced. Some patients were excluded due to a lack of Hb measurement at baseline and follow-up, although we found differences in baseline characteristics between included and excluded subjects to be within ± 5%. Additionally, ART regimen that patients received was not randomly assigned. Baseline characteristics of patients receiving different ART regimens may have differed. Therefore, we used multivariate analysis to correct for the effects of potential bias. Finally, all the data in our study were drawn from regular record registers. Due to the limited information reported, some other potential influencing factors such as socio-economic status and lifestyle, were not introduced in this study. And types of anemia before and after ART could not be classified, which is essential for determining the etiology of the anemia and for guiding precise interventions. Further research is needed.
Conclusions
The findings of our nationwide cohort study indicate that pre-ART anemia was common among HIV-infected patients during the Treat-All era. Despite a significant reduction in the prevalence of anemia after ART, a certain proportion of anemia still persisted. Moreover, time-dependent anemia was significantly associated with death. We recommend strengthening the monitoring of patients at risk of anemia, especially in patients with baseline anemia or during the first year of ART. Timely detection of anemia and treatment for correcting anemia during ART is critical to improving the burden of anemia and prognosis of patients living with HIV.
Data Availability
The datasets used and/or analyzed during the current study were available from the corresponding author on reasonable request.
Abbreviations
- ABC:
-
Abacavir
- aHR:
-
Adjusted hazard ratio
- aOR:
-
Adjusted odds ratio
- ART:
-
Antiretroviral therapy
- AZT:
-
Zidovudine
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- eGFR:
-
Estimated glomerular filtration rate
- Hb:
-
Hemoglobin
- INSTI:
-
Integrase strand transfer inhibitor
- IQR:
-
Interquartile ranges
- NNRTI:
-
Non-nucleoside reverse transcriptase inhibitor
- OIs:
-
Opportunistic infections
- PI:
-
Protease inhibitor
- TAF:
-
Tenofovir alafenamide
- TDF:
-
Tenofovir disoproxil fumarate
References
Safiri S, Kolahi AA, Noori M, Nejadghaderi SA, Karamzad N, Bragazzi NL, et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: results from the global burden of Disease Study 2019[J]. J Hematol Oncol. 2021;14(1):185. https://doi.org/10.1186/s13045-021-01202-2
Kong AM, Pozen A, Anastos K, Kelvin EA, Nash D. Non-HIV Comorbid Conditions and Polypharmacy among People living with HIV Age 65 or older compared with HIV-Negative individuals Age 65 or older in the United States: a retrospective claims-based Analysis[J]. AIDS Patient Care STDS. 2019;33(3):93–103. https://doi.org/10.1089/apc.2018.0190
Fangman JJ, Scadden DT. Anemia in HIV-infected adults: epidemiology, pathogenesis, and clinical management[J]. Curr Hematol Rep. 2005;4(2):95–102.
Moore RD. Human immunodeficiency virus infection, anemia, and survival[J]. Clin Infect Dis. 1999;29(1):44–9. https://doi.org/10.1086/520178
Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies[J]. Clin Infect Dis. 2004;38(10):1454–63. https://doi.org/10.1086/383031
Tigabu A, Beyene Y, Getaneh T, Chekole B, Gebremaryam T, Sisay CE, et al. Incidence and predictors of anemia among adults on HIV care at South Gondar Zone Public General Hospital Northwest Ethiopia, 2020; retrospective cohort study[J]. PLoS ONE. 2022;17(1):e259944. https://doi.org/10.1371/journal.pone.0259944
Tamir Z, Alemu J, Tsegaye A. Anemia among HIV infected individuals taking ART with and without zidovudine at Addis Ababa, Ethiopia[J]. Ethiop J Health Sci. 2018;28(1):73–82. https://doi.org/10.4314/ejhs.v28i1.9
Marchionatti A, Parisi MM. Anemia and thrombocytopenia in people living with HIV/AIDS: a narrative literature review[J]. Int Health. 2021;13(2):98–109.
Beyene HB, Tadesse M, Disassa H, Beyene MB. Concurrent Plasmodium infection, anemia and their correlates among newly diagnosed people living with HIV/AIDS in Northern Ethiopia[J]. Acta Trop. 2017;169:8–13.
Harding BN, Whitney BM, Nance RM, Crane HM, Burkholder G, Moore RD, et al. Antiretroviral drug class and anaemia risk in the current treatment era among people living with HIV in the USA: a clinical cohort study[J]. BMJ Open. 2020;10(3):e31487.
Lang R, Gill MJ, Coburn SB, Grossman J, Gebo KA, Horberg MA, et al. The changing prevalence of anemia and risk factors in people with HIV in North America who have initiated ART, 2007–2017[J]. AIDS. 2023;37(2):287–98.
Cao G, Wang Y, Wu Y, Jing W, Liu J, Liu M. Prevalence of anemia among people living with HIV: a systematic review and meta-analysis[J]. EClinicalMedicine. 2022;44:101283.
Harding BN, Whitney BM, Nance RM, Crane HM, Burkholder G, Moore RD, et al. Antiretroviral drug class and anaemia risk in the current treatment era among people living with HIV in the USA: a clinical cohort study[J]. BMJ Open. 2020;10(3):e31487. https://doi.org/10.1136/bmjopen-2019-031487
Shivakoti R, Yang WT, Gupte N, Berendes S, Rosa AL, Cardoso SW, et al. Concurrent Anemia and elevated C-Reactive protein predicts HIV Clinical Treatment failure, including tuberculosis, after antiretroviral therapy Initiation[J]. Clin Infect Dis. 2015;61(1):102–10.
Khatri S, Amatya A, Shrestha B. Nutritional status and the associated factors among people living with HIV: an evidence from cross-sectional survey in hospital based antiretroviral therapy site in Kathmandu, Nepal[J]. BMC Nutr. 2020;6:22. https://doi.org/10.1186/s40795-020-00346-7
Sah SK, Dahal P, Tamang GB, Mandal DK, Shah R, Pun SB. Prevalence and predictors of Anemia in HIV-Infected persons in Nepal[J]. HIV AIDS (Auckl). 2020;12:193–200. https://doi.org/10.2147/HIV.S244618
Duguma N, Tesfaye KG, Adissu MW, Bimerew LG. Hematological parameters abnormalities and associated factors in HIV-positive adults before and after highly active antiretroviral treatment in Goba Referral Hospital, southeast Ethiopia: a cross-sectional study[J]. SAGE Open Med. 2021;9:393096305. https://doi.org/10.1177/20503121211020175
Baye M, Fisseha B, Bayisa M, Abebe SM, Janakiraman B. Experience of fatigue and associated factors among adult people living with HIV attending ART clinic: a hospital-based cross-sectional study in Ethiopia[J]. BMJ Open. 2020;10(10):e42029. https://doi.org/10.1136/bmjopen-2020-042029
Jaganath D, Walker AS, Ssali F, Musiime V, Kiweewa F, Kityo C, et al. HIV-associated anemia after 96 weeks on therapy: determinants across age ranges in Uganda and Zimbabwe[J]. AIDS Res Hum Retroviruses. 2014;30(6):523–30. https://doi.org/10.1089/aid.2013.0255
Shen Y, Wang Z, Lu H, Wang J, Chen J, Liu L, et al. Prevalence of anemia among adults with newly diagnosed HIV/AIDS in China[J]. PLoS ONE. 2013;8(9):e73807. https://doi.org/10.1371/journal.pone.0073807
Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: estimates and projections for 2000–2020[J]. PLoS ONE. 2018;13(11):e207005. https://doi.org/10.1371/journal.pone.0207005
The Lancet HIV. Time to tackle late diagnosis. Lancet HIV. 2022;9(3):e139. https://doi.org/10.1016/S2352-3018(22)00040-6
Zhou J, Jaquet A, Bissagnene E, Musick B, Wools-Kaloustian K, Maxwell N, et al. Short-term risk of anaemia following initiation of combination antiretroviral treatment in HIV-infected patients in countries in sub-saharan Africa, Asia-Pacific, and central and South America[J]. J Int AIDS Soc. 2012;15(1):5. https://doi.org/10.1186/1758-2652-15-5
Han MJ. Analysis of the HIV epidemic in China and prospects for prevention and treatment[J]. Chin J AIDS STD. 2023;29(03):247–50.
Dai G, Xiao J, Gao G, Chong X, Wang F, Liang H, et al. Anemia in combined antiretroviral treatment-naive HIV-infected patients in China: a retrospective study of prevalence, risk factors, and mortality[J]. Biosci Trends. 2017;10(6):445–53.
Jin Y, Meng X, Liu S, Yuan J, Guo H, Xu L, et al. Prevalence trend and risk factors for anemia among patients with human immunodeficiency virus infection receiving antiretroviral therapy in rural China[J]. J Tradit Chin Med. 2019;39(1):111–7.
Wang D, Hou X, Yu X, Wang T, Ye Z, Li J, et al. Plasma hemoglobin and the risk of death in HIV/AIDS patients treated with antiretroviral therapy[J]. Aging. 2021;13(9):13061–72.
Zhang F, Dou Z, Ma Y, Zhang Y, Zhao Y, Zhao D, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study[J]. Lancet Infect Dis. 2011;11(7):516–24. https://doi.org/10.1016/S1473-3099(11)70097-4
World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity, https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1/; 2011 [accessed 21 Jun 2023].
Health Commission of the people’s Republic of China. Diagnosis criteria for HIV/AIDS, http://www.nhc.gov.cn/fzs/s7852d/201901/9493bdd1549b4908be18eb6007b009d.shtml/2019 [accessed 18 May 2023].
China free antiretroviral therapy manual. Beijing: Chinese Center for Disease Control and Prevention; 2016.
GBD 2021 Anaemia Collaborators. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: findings from the global burden of Disease Study 2021[J]. Lancet Haematol. 2023;10(9):e713–34.
Shet A, Antony J, Arumugam K, Kumar DS, Rodrigues R, DeCosta A. Influence of adverse drug reactions on treatment success: prospective cohort analysis of HIV-infected individuals initiating first-line antiretroviral therapy in India[J]. PLoS ONE. 2014;9(3):e91028. https://doi.org/10.1371/journal.pone.0091028
Ezeamama AE, Guwatudde D, Sikorskii A, Kabagambe EK, Spelts R, Vahey G, et al. Impaired hematologic status in relation to clinical outcomes among HIV-Infected adults from Uganda: a prospective cohort Study[J]. Nutrients. 2018;10(4). https://doi.org/10.3390/nu10040475
Kerkhoff AD, Wood R, Cobelens FG, Gupta-Wright A, Bekker LG, Lawn SD. Resolution of anaemia in a cohort of HIV-infected patients with a high prevalence and incidence of tuberculosis receiving antiretroviral therapy in South Africa[J]. BMC Infect Dis. 2014;14:3860. https://doi.org/10.1186/s12879-014-0702-1
Firnhaber C, Smeaton L, Saukila N, Flanigan T, Gangakhedkar R, Kumwenda J, et al. Comparisons of anemia, thrombocytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the Americas[J]. Int J Infect Dis. 2010;14(12):e1088–92. https://doi.org/10.1016/j.ijid.2010.08.002
Vanasse GJ, Berliner N. Anemia in elderly patients: an emerging problem for the 21st century[J]. Hematol Am Soc Hematol Educ Program. 2010;2010:271–5. https://doi.org/10.1182/asheducation-2010.1.271
Blanco JR, Negredo E, Bernal E, Blanco J. Impact of HIV infection on aging and immune status[J]. Expert Rev Anti Infect Ther. 2021;19(6):719–31. https://doi.org/10.1080/14787210.2021.1848546
Gedefaw L, Yemane T, Sahlemariam Z, Yilma D. Anemia and risk factors in HAART naive and HAART experienced HIV positive persons in south west Ethiopia: a comparative study[J]. PLoS ONE. 2013;8(8):e72202. https://doi.org/10.1371/journal.pone.0072202
Woldeamanuel GG, Wondimu DH. Prevalence of anemia before and after initiation of antiretroviral therapy among HIV infected patients at Black Lion Specialized Hospital, Addis Ababa, Ethiopia: a cross sectional study[J]. BMC Hematol. 2018;18:7. https://doi.org/10.1186/s12878-018-0099-y
Parinitha S, Kulkarni M. Haematological changes in HIV infection with correlation to CD4 cell count[J]. Australas Med J. 2012;5(3):157–62. https://doi.org/10.4066/AMJ.20121008
Assefa M, Abegaz WE, Shewamare A, Medhin G, Belay M. Prevalence and correlates of anemia among HIV infected patients on highly active anti-retroviral therapy at Zewditu Memorial Hospital, Ethiopia[J]. BMC Hematol. 2015;15:6. https://doi.org/10.1186/s12878-015-0024-6
Johannessen A, Naman E, Gundersen SG, Bruun JN. Antiretroviral treatment reverses HIV-associated anemia in rural Tanzania[J]. BMC Infect Dis. 2011;11:190. https://doi.org/10.1186/1471-2334-11-190
Jin M, Wang Y, Li J, Wu Z, Liu X, Wang H, et al. Anemia is independently associated with mortality in people living with human immunodeficiency virus/acquired immune deficiency syndrome: a propensity score matching-based retrospective cohort study in China[J]. Front Med (Lausanne). 2023;10:1055115. https://doi.org/10.3389/fmed.2023.1055115
Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group[J]. AIDS. 1999;13(8):943–50. https://doi.org/10.1097/00002030-199905280-00010
Acknowledgements
The authors thank the many healthcare providers across China for their dedication in providing conscientious treatment and care to their HIV-positive patients and in completing the data reporting that made this research possible.
Funding
This work was supported by the China National AIDS Program, the National Science and Technology Major Project on Prevention and Treatment of Major Infectious Diseases including AIDS and Viral Hepatitis [grant number 2018ZX10721102].
Author information
Authors and Affiliations
Contributions
Lai Wei and Yan Zhao designed the study. Lai Wei performed the statistical analysis. Lai Wei and Yan Zhao interpreted the results and developed the initial manuscript draft. Lai Wei, Yan Zhao, Xiumin Gan, Decai Zhao, Yasong Wu, Zhihui Dou and Ye Ma contributed to manuscript revisions. All authors approved the final version for publication. Yan Zhao had full access to all the data and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. This study was reviewed and approved by the Institutional Review Board of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention. Since all data were de-identified and provided in the aggregated form, the informed consent was waived by the ethics committee of Institutional Review Board of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention.
Consent for publication
Not applicable as all data are presented in the aggregate.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, L., Zhao, Y., Gan, X. et al. The burden of anemia among Chinese HIV-infected patients following the initiation of antiretroviral therapy in the treat-all era: a nationwide cohort study. BMC Infect Dis 23, 704 (2023). https://doi.org/10.1186/s12879-023-08675-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08675-1