Abstract
Background
Trichomonas vaginalis (TV) accounts for the highest burden of curable, non-viral sexually transmitted infections worldwide. Prevalence in India ranges from 0.4 to 27.4% in women and 0.0-5.6% in men. In 2015, the prevalence of TV among pregnant women of rural Vellore was 3.11% using Sekisui OSOM® Trichomonas test and culture methods. Molecular methods are the most sensitive, rapid diagnostic tool for Sexually Transmitted Infection’s (STI) albeit cost hinders implementation of commercial platforms. To determine a sensitive, sustainable molecular method, we compared three targets (Adhesin AP65, cytoskeleton Beta-tubulin BTUB 9/2 and TVK 3/7) with the highest published diagnostic accuracy against microscopy, culture and Real Time PCR (RT- PCR).
Materials & methods
Six-hundred adult, sexually active women attending the Obstetrics-Gynaecology rural out-patient clinic the Rural Unit for Health and Social Affairs (RUHSA) from July 2020 - February 2021 were enrolled. A vaginal lateral and posterior fornix specimen was inoculated, onsite, into Biomed InPouch® TV culture and smeared onto a slide for fluorescence microscopy using Acridine orange. A flocked nylon swab specimen for PCR was used to determine the sensitivities of the Adhesin AP65, cytoskeleton Beta-tubulin BTUB 9/2 and TVK 3/7 gene targets. Seegene Allplex™ STI Essential Assay, S.Korea was used to confirm TV positives.
Results
Nine specimens (9/600, 1.5%) were positive for TV. There was a 100% correlation between Biomed InPouch TV® culture, PCR with TVK 3/7 and RT-PCR while a correlation of 66.6% with BTUB 9/2 and AP65 gene targets. Clinically, 77.7% (n = 7) presented with white-greenish discharge per vagina, 11% (n = 1) with infertility, 22.2% (n = 2) were asymptomatic. Eight of nine patients (88.9%) had co-infections with other bacterial STIs. Prevalence of TV coinfection with Neisseria gonorrhoea was 1.1%.
Conclusion
Current hospital-based prevalence of TV in rural Vellore was 1.5%. Repetitive DNA target TVK 3/7 was more sensitive than AP65 and BTUB 9/2 primers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
STIs are a serious public health disease wherein 50–80% infections are asymptomatic, facilitating disease progression and efficient transmission. They contribute to infertility, medical and psychological consequences and promote acquisition of Human Immunodeficiency Virus (HIV) and oncogenic cellular changes [1, 2].
Annually, an estimated 357 million new cases of four curable STIs affect individuals aged 15–49, with TV (142 million) being the most prevalent followed by Chlamydia trachomatis (131 million), Neisseria gonorrhoeae (78 million) and syphilis (6 million) [2].
Trichomoniasis causes vaginal irritation and foul smelling yellowish-green vaginal discharge. However, 70–80% of infected women and most men show minimal or no symptoms [3].
The prevalence of TV in India ranges from 0.4 to 27.4% in women and 0.0-5.6% in men. The tangible prevalence rates remain unknown as prevalence has been determined by various methodologies and in varied population groups.
Trichomoniasis is diagnosed by wet mount, culture, Papanicolaou smear, acridine orange fluorescent staining, Nucleic acid amplification tests (NAAT) and serological tests wherein Diamonds media culture is the gold standard. The commercially available InPouch® TV culture system (Biomed Diagnostics, San Jose, California) eliminates the difficulties of in-house preparation, and is highly specific with a low contamination risk [4].
However, NAAT is the recommended, rapid, sensitive and sustainable diagnostic method in the diagnosis of STI’s [5]. Commercial multiplex platforms are sensitive and reliable but cost hinders implementation in resource-limited settings. Various primer targets have been described for implementation as a conventional PCR, to promote a more widespread, cost-effective TV testing [6,7,8,9,10]. However, a single or combined best target has not been recommended. Thus, to strengthen the search for a single best target, three primers with higher sensitivity were chosen to be evaluated. In addition, against a published prevalence of 3.11% using Sekisui OSOM® Trichomonas test and culture in 2015 [11], this study was undertaken to determine the current prevalence, clinical presentations and co-infections.
Materials and methods
This prospective observational study was conducted among 600 symptomatic and asymptomatic adult women (> 18 years-75 years of age) attending the Obstetrics-Gynecology (OG) out-patient clinic at RUHSA, Vellore after obtaining written informed consent. The study population included both pregnant (92.8%) and non-pregnant women (7.2%). All laboratory testing was carried out at the tertiary hospital situated 26 km from the rural hospital. The study period was from November 2019 to September 2021.
Sample collection and onsite culture inoculation
Vaginal per-speculum examination was done by the Obstetrician. Two vaginal swab specimens were collected from the vaginal posterior and lateral fornices under aseptic condition using sterile cotton and nylon swabs [12]. One cotton swab specimen was inoculated onsite directly into InPouch®TV (BioMed, US) culture media while a second swab was smeared onto a glass slide and fixed with methanol for acridine orange stain microscopy. Another DNA/RNase free nylon flocked swab (Himedia, India) specimen was transported in 0.5 ml sterile saline within 6 h for DNA extraction. The inoculated InPouch®TV culture media was incubated upright at 37 °C in 5% CO2 and examined directly under low power (20-40X) for TV trophozoites daily for seven days. The trophozoites were identified by the characteristic pyriform shape with four anterior flagella and a lateral undulating membrane showing a jerky/twitching motility. The freshly prepared acridine orange dye-stained smears (5 mg/ml) were examined with a fluorescence microscope (470–490 nm filter, 40X). The doubtful smears were re-examined by a second examiner. The trophozoites were identified by a characteristic brick red color with a yellowish-green nucleus [13].
DNA extraction & polymerase chain reaction
DNA was extracted using QIAamp DNA minikit (Qiagen, Hilden, Germany). Conventional PCR was performed using three different primers AP65 (Nabweyambo et al.) [7], TVK3/TVK7 (Kengne et al.) [6] and BTUB9/BTUB2 (Madico et al.) [10]. The nucleotide sequences of the primers (Table 1) used in this study were obtained from Sigma-Aldrich, India.
PCR was standardized using ATCC 30001D Trichomonas vaginalis. The reaction mixture for AP65 adhesin monoplex PCR contained 10 µl of Master mix, 2 µl of 10 pmol forward and reverse primers and 2 µl template DNA.
The reaction mixture for multiplex PCR contained 10 µl of Master mix, 2 µl of 5pmol BTUB 9/2 forward and reverse primers, 2 µl of 5 pmol TVK3/7 forward and reverse primers and 2 µl template DNA.
The amplification conditions for both monoplex and multiplex PCR included one cycle of initial denaturation at 95 °C for 15 min, followed by 30 cycles of denaturation at 94 °C for 30s, annealing at 63 °C for 90s, and extension at 72 °C for 90s, followed by one cycle of final extension at 72 °C for 10 min.
The three PCRs were repeated from > 7 day culture extract of a randomly selected one-tenth of the specimens (61 samples) to confirm the diagnostic accuracy.
Coinfecting STI
All TV positives and 16 randomly selected TV negative samples were subjected to multiplex real time PCR (Allplex™ STI Essential Assay, Seegene, South Korea) to detect coinfecting bacterial STIs (Chlamydia trachomatis, Neisseria gonorrhoea, Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum).
Results
A total of 600 samples were collected from both pregnant women and women with urogenital symptoms attending the RUHSA OG out-patient clinic from July 2020 to February 2021. The period coincided with the first and second wave COVID-19 pandemic governmental lockdowns in India.
Of the 600 samples, nine were found to be positive for TV using InPouch®TV culture and PCR using TVK 3/7 primer while six of the nine TV positives were identified by Acridine orange microscopy, PCR with AP65 and BTUB 9/2 gene primers.
The hospital-based prevalence of TV in rural Vellore through this study was found to be 1.5%.
The age group of TV infected women ranged between 19-52yrs and was more common among 25–44 years of age. The distribution of T. vaginalis among different age group is shown in Table 2.
Among the 600 women, 63.3% (n = 380) presented with one or more of the following symptoms: white discharge per vagina, genital itching, dysuria, lower abdominal pain, dyspareunia, and infertility while 36.6% (n = 220) were asymptomatic.
Among the TV positive cases, seven were symptomatic, two were asymptomatic. Five (55.5%) cases were pregnant. The most common symptom among TV positive women was vaginal discharge (77.7%) followed by genital itching (44.4%) (Fig. 1).
Overall, 1.8% of symptomatic patients and 0.9% of asymptomatic patients were identified to have TV.
Motile trichomonads were visualized directly from the culture pouch under brightfield microscope. The time to positivity of InPouch®TV culture media was 0–3 days. Among the nine positive samples, six were found positive on day 0 in the culture media. In the remaining three, two samples turned positive on day 1 and one on day 2. Out of nine positives in culture, only six were identified by acridine orange microscopy (Fig. 2).
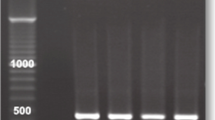
PCR was done using three different primer sets AP65, TVK 3/7 and BTUB 9/2 for the detection of TV (Fig. 3) and was compared with culture.
There was a 100% correlation between Biomed InPouch®TV culture and TVK 3/7 primer PCR. The diagnostic accuracy of direct specimen swab PCRs with culture is shown in Table 3.
Molecular assays were repeated from > 7 day culture extract of a randomly selected 52 isolates plus the nine culture positive samples (Total – 61 samples). All nine culture positive samples showed expected band in all three PCRs. None of the culture negative specimens tested among the 52 were found to be positive with all three PCRs.
Corresponding with the performance of the 3 primers, the limit of detection (LOD) determined with ATCC 30001D Trichomonas vaginalis was 0.2ng/µl with primer TVK3/7 and 0.7ng/µl with primer Adhesin AP65 and BTUB9/2.
Coinfecting bacterial STI with TV were identified using multiplex RT-PCR (Allplex™ STI Essential Assay, Seegene, South Korea). The percentage of TV coinfection with Neisseria gonorrhoeae was 1.1%. There was no Chlamydia trachomatis coinfection (Table 4).
Clinically, TVR 79 and TVR 358 were asymptomatic. Remaining 7 presented with history of vaginal discharge. TVR 347 and 383 had associated dysuria. TVR 137, 347, 383 and 536 had genital itching. Majority of the symptomatic TV positives had STI pathogen coinfections. TVR 536 who presented with infertility had coinfection of 4 organisms.
Discussion
The WHO estimated the global prevalence of trichomoniasis in 2016 as 5.3% in women and 0.5% in men while the overall prevalence in South-East Asia as 2.5% [2].
Socio-economic status and literacy, influence the prevalence. It is higher in rural (24.18%) than urban populations (15.71%) [14] and in low income (11%) and middle income countries (7%), than in high income countries (3%) [2]. Notably, certain high-risk populations: HIV-infected individuals, female sex workers and their male partners (73.2%) [15], incarcerated women(52.6%) [16], symptomatic females (24.18%) [14] exhibit high prevalence rates.
The advent of molecular techniques with higher sensitivity have contributed to the increased prevalence of TV compared to the prevalence with conventional wet mount and culture.
In this study, the hospital-based prevalence of TV among pregnant women in rural Vellore was found to be 0.91% as compared to the previous field study (3.11%) conducted in 2015 among pregnant women in the same catchment population, with Sekisui OSOM® Trichomonas test, wet mount and in-house prepared culture [11]. This drop in prevalence is despite testing with molecular methods in a 92.8% antenatal population. The decline in this study can marginally be attributed to it being hospital-based and conducted during the COVID-19 pandemic lockdowns. However, a similar low prevalence of 1.96–2.8% has been reported in the hospital-based studies done between 2013 and 2023, in India among symptomatic women. [17,18,19].
In neighbouring, Sri Lanka, trichomoniasis is now a notifiable disease. A prevalence of 7.2% in 2012 [20] has now declined to 4.4% by molecular methods (2021) in Colombo city [9].
Despite a seemingly decreasing prevalence, the search for a rapid, cost-effective, and reliable diagnostic method continues. For the resource limited setting, the conventional wet mount is an extremely time-dependent option with a low sensitivity (50–60%) which further decreases with time beyond 10 min. Acridine orange, a fluorescence-based, non-specific, nucleic acid stain is an ideal alternative to wet mounts when a longer sample transport time is expected.
The sensitivity of acridine orange varies between 47 and 100% [17, 21, 22]. Khatoon et al. reported a sensitivity of 100% and specificity of 98% compared to wet mount [13] and sensitivity of 73.5% compared to culture [22]. The procedure is easy to perform, overcomes time dependency, but requires fluorescence microscopy and expertise to detect the organism.
The onsite inoculated culture is a sensitive and accurate method for the diagnosis of TV. However, culture requires microaerophilic conditions, ingredients that are poorly available and a quality check that is not possible in most diagnostic labs [10]. Further, daily microscopic examinations result in a high risk of media contamination. Thus, the sensitivity of culture ranges from 44 to 75% although specificity is 100% compared to molecular method [5]. The Biomed InPouch®TV (BioMed diagnostics, US) commercial Diamond’s culture media has shown a high sensitivity in the Indian setting. While Patil et al. in 2012 [23] reported the sensitivity as 73.33% and Singh et al., in 2020 [17] reported it as 100%.
It serves both as a transport and culture media, is stable at room temperature for up to 6 months and permits onsite inoculation by a health care worker and maintains a closed environment with a low risk of contamination. The greatest advantage, although requires some training, is that it couples as a wet mount that can be directly visualized under light microscope for motile trichomonads. The limit of detection is < 10 organisms/ml. The limitation, however, is its cost ($16.15) which makes it unaffordable for implementation in the high prevalence regions where screening is most required [23].
Molecular methods, offer the highest sensitivity and are the recommended diagnostic tool for TV detection.
Against various targets, PCR has proven superiority over the traditional gold standard culture, with sensitivities ranging from 90 to 100% [9, 14, 22].
The primers targets described in literature are Genus-specific TFR1/2, Species-specific Adhesin primers TVA 5/6 and AP65, repetitive DNA TVK3/7, Beta-tubulin BTUB 9/2, plus TV-E650 DNA repeat IP1/2, and the 18 S ribosomal gene primer TV1/2 (Table 5).
Genus-specific primer TFR1/2 described by Felleisen RS in 1997, was found to be 100% specific against human and animal trichomoniasis [30] and may be applied alongside or prior to the species detection.
Among the described species-specific molecular targets, TVK3/7, BTUB9/2 and AP65 had the highest reported sensitivities based on published diagnostic accuracies between 1992 and 2019.
Crucitti et al. (2003) have compared the performance of primers TVK3/7, BTUB9/2, IP1/2, and TVA 5/6, and reported TVK3/7 and BTUB9/2 with higher sensitivity and specificity [8] performance of TVK3/7 has been reported by independent researchers, in different populations with a sensitivity range of 77–100% and specificity of 97–100%.
During this study period, Herath et al. (2021) from Sri Lanka, further reported a 76.5% sensitivity and 100% specificity while from North India, Singh et al. (2020) found TVK3/7 100% sensitive and 99.5% specific [9, 17] and Dadwal et al., found it far superior to culture based methods [19]. In this study, TVK 3/7 primer is found equally sensitive and specific as InPouch®TV culture (p < 0.05) while Beta-tubulin 9/2 and Adhesin AP65 had sensitivities of 66.7% although they were 100% specific. The limit of detection was 0.2ng/µl while that of BTUB9/2 and AP65 was 0.7ng/µl.
This study further strengthens the ‘1994 Kenge et al TVK3/7 primer’, as the most sensitive species-specific molecular target currently described and globally evaluated.
Using TV as the STI denominator, co-infections among the TV positive cases were determined by Seegene’s Allplex RT-PCR. Ureaplasma parvum (66.6%) was the most common co-infection, followed by Mycoplasma hominis (55.5%). The triple coinfection of TV, M.hominis and U.parvum was seen in 3 cases (33.3%) and triple coinfection of TV, N.gonorrhoeae and U.parvum was seen in 1 case (11.1%) (Table 3). Coinfection rate of 71.4% with TV and M.hominis was reported in Kenya by Masha et al. [31], Becker et al. in Brazil demonstrated a rate of 56.7% [32] and Goo et al. in Korea 66.7% [33].
In a study done by Ginocchio et al., USA, co-infection prevalence of TV with C.trachomatis was 1.3% and with N.gonorrhoeae was 0.61% and the prevalence of triple co-infection with C. trachomatis, N. gonorrhoeae and TV was 0.24% [34].
Clinically, despite a low prevalence, the infections seen here were consistent with global findings; as mainly seen in the 35–44 years age group, seven of nine cases (77.8%) were symptomatic and the most common presentation was a foul-smelling discharge. These patients were treated with metronidazole 500 mg BD for 7 days and their partners with metronidazole 2 g OD. They were followed up telephonically for a period of eight months for treatment response and were found have recovered from their symptoms. At delivery, none had complications of pregnancy namely, premature rupture of membrane, preterm labor or low birth weight babies.
Conclusion
In rural Vellore, the current hospital-based prevalence of TV among women visiting an out-patient facility is 1.5% constituting 1.8% of the symptomatic patients. For a sustainable affordable molecular option, repetitive DNA TVK3/7 primer was found to date, to be the most sensitive, species-specific target.
Limitations
Prevalence was determined in the rural population during the COVID-19 pandemic lockdown restrictions. The poor treatment-seeking behaviour among non-pregnant rural women for genital symptoms, may have contributed to a low prevalence.
Data availability
Data is uploaded in related file.
Abbreviations
- BD:
-
Twice daily
- COVID:
-
19-Coronavirus disease-2019 (SARS-CoV2 pandemic)
- DNA:
-
Deoxyribonucleic acid
- DNA/RNase:
-
Deoxyribonucleic acid / Ribonuclease
- HIV:
-
Human Immunodeficiency Virus
- LR:
-
Likelihood Ratio
- NAAT:
-
Nucleic Acid Amplification Test
- NPV:
-
Negative Predictive Value
- OD:
-
Once Daily
- OG:
-
Obstetrics and Gynaecology
- PCR:
-
Polymerase Chain Reaction
- PPV:
-
Positive Predictive Value
- RT:
-
PCR-Real Time-Polymerase Chain Reaction
- RUHSA:
-
Rural Unit for Health and Social Affairs
- STI:
-
Sexually Transmitted Infection
- TV:
-
Trichomonas vaginalis
References
Taylor M, Alonso-González M, Gómez B, Korenromp E, Broutet N, Taylor M, et al. World health organization global health sector strategy on sexually transmitted infections: an evidence-to-action summary for Colombia. Rev Colomb Obstet Ginecol. 2017;68(3):193–201.
Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, et al. Chlamydia, Gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97(8):548–P562.
Wiringa AE, Ness RB, Darville T, Beigi RH, Haggerty CL. Trichomonas Vaginalis, endometritis and sequelae among women with clinically suspected pelvic inflammatory disease. Sex Transm Infect. 2020;96(6):436–8.
Sood S, Mohanty S, Kapil A, Tolosa J, Mittal S. InPouch TV culture for detection of Trichomonas vaginalis. Indian J Med Res. 2007;125(4):567–71.
Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I et al. Sexually Transmitted Infections Treatment Guidelines, 2021. 2021;70(4):192.
Kengne P, Veas F, Vidal N, Rey JL, Cuny G. Trichomonas vaginalis: repeated DNA target for highly sensitive and specific polymerase chain reaction diagnosis. Cell Mol Biol Noisy–Gd Fr. 1994;40(6):819–31.
Nabweyambo S, Kakaire O, Sowinski S, Okeng A, Ojiambo H, Kimeze J, et al. Very low sensitivity of wet mount microscopy compared to PCR against culture in the diagnosis of vaginal trichomoniasis in Uganda: a cross sectional study. BMC Res Notes. 2017;10(1):259.
Crucitti T, Van Dyck E, Tehe A, Abdellati S, Vuylsteke B, Buve A, et al. Comparison of culture and different PCR assays for detection of Trichomonas Vaginalis in self collected vaginal swab specimens. Sex Transm Infect. 2003;79(5):393–8.
Herath S, Balendran T, Herath A, Iddawela D, Wickramasinghe S. Comparison of diagnostic methods and analysis of socio-demographic factors associated with Trichomonas vaginalis infection in Sri Lanka. PLoS ONE. 2021;16(10):e0258556.
Madico G, Quinn TC, Rompalo A, McKee KT, Gaydos CA. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J Clin Microbiol. 1998;36(11):3205–10.
Pai NP, Daher J, Prashanth HR, Shetty A, Sahni RD, Kannangai R et al. Will an innovative connected AideSmart! app-based multiplex, point-of-care screening strategy for HIV and related coinfections affect timely quality antenatal screening of rural Indian women? Results from a cross-sectional study in India. Sex Transm Infect [Internet]. 2018 Oct 15 [cited 2021 Aug 25]; https://sti.bmj.com/content/early/2018/10/15/sextrans-2017-053491.
Domeika M, Zhurauskaya L, Savicheva A, Frigo N, Sokolovskiy E, Hallén A, et al. Guidelines for the laboratory diagnosis of trichomoniasis in East European countries. J Eur Acad Dermatol Venereol JEADV. 2010;24(10):1125–34.
Khatoon R. Evaluation of Different Staining Techniques in the Diagnosis of Trichomonas vaginalis Infection in Females of Reproductive Age Group. J Clin Diagn Res [Internet]. 2014 [cited 2019 Nov 9]; http://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2014&volume=8&issue=12&page=DC05&issn=0973-709x&id=5261
Arora BB, Maheshwari M, Devgan N, Arora DR. Prevalence of Trichomoniasis, vaginal candidiasis, genital herpes, Chlamydiasis, and actinomycosis among urban and rural women of Haryana, India. J Sex Transm Dis. 2014;2014:1–5.
Hobbs MM, Lapple DM, Lawing LF, Schwebke JR, Cohen MS, Swygard H, et al. Methods for detection of Trichomonas Vaginalis in the Male partners of Infected women: implications for control of Trichomoniasis. J Clin Microbiol. 2006;44(11):3994–9.
Roth AM, Williams JA, Ly R, Curd K, Brooks D, Arno J, et al. Changing sexually transmitted infection screening protocol will result in Improved Case finding for Trichomonas Vaginalis among High-Risk Female populations. Sex Transm Dis. 2011;38(5):398–400.
Singh S, Saha R, Suneja A, Das S. A hospital-based study on the prevalence of trichomoniasis and evaluation of accuracy of various diagnostic techniques. Trop Parasitol. 2020;10(2):124–9.
Vijaya Mn D, Umashankar K, Sudha null, Nagure AG, Kavitha G. Prevalence of the trichomonas vaginalis infection in a tertiary care hospital in rural bangalore, southern India. J Clin Diagn Res JCDR. 2013;7(7):1401–3.
Dadwal R, Sharma N, Kanaujia R, Malhotra S, Chaudhry H, Rathore S, et al. Prevalence of Trichomonas vaginalis by polymerase chain reaction-based molecular method among symptomatic women from Northern India. Indian J Sex Transm Dis AIDS. 2023;44(1):40–4.
Herath S, Fernando D, Jayasinge S. Risk factors of Trichomonas Vaginalis in women attending central sexually transmitted diseases Clinic Sri Lanka. Retrovirology. 2012;9(1):P42.
Radonjic IV, Dzamic AM, Mitrovic SM, Arsic Arsenijevic VS, Popadic DM, Kranjcic Zec IF. Diagnosis of Trichomonas vaginalis infection: the sensitivities and specificities of microscopy, culture and PCR assay. Eur J Obstet Gynecol Reprod Biol. 2006;126(1):116–20.
Khatoon R, Jahan N, Ahmad S, Khan HM, Rabbani T. Comparison of four diagnostic techniques for detection of Trichomonas vaginalis infection in females attending tertiary care hospital of North India. Indian J Pathol Microbiol. 2015;58(1):36–9.
Patil MJ, Nagamoti JM, Metgud SC. Diagnosis of Trichomonas Vaginalis from vaginal specimens by Wet Mount Microscopy, in Pouch TV Culture System, and PCR. J Glob Infect Dis. 2012;4(1):22–5.
Queza MIP, Rivera WL. Diagnosis and molecular characterization of Trichomonas Vaginalis in sex workers in the Philippines. Pathog Glob Health. 2013;107(3):136–40.
Paul H, Peter D, Pulimood SA, Abraham OC, Mathai E, Prasad JH, et al. Role of polymerase chain reaction in the diagnosis of Trichomonas vaginalis infection in human immunodeficiency virus-infected individuals from India (South). Indian J Dermatol Venereol Leprol. 2012;78(3):323–7.
Lawing LF, Hedges SR, Schwebke JR. Detection of trichomonosis in vaginal and urine specimens from women by Culture and PCR. J Clin Microbiol. 2000;38(10):3585–8.
Pillay A, Radebe F, Fehler G, Htun Y, Ballard RC. Comparison of a TaqMan-based real‐time polymerase chain reaction with conventional tests for the detection of Trichomonas vaginalis. Sex Transm Infect. 2007;83(2):126–9.
Wendel KA, Erbelding EJ, Gaydos CA, Rompalo AM. Trichomonas vaginalis polymerase chain reaction compared with Standard Diagnostic and Therapeutic Protocols for Detection and Treatment of Vaginal Trichomoniasis. Clin Infect Dis. 2002;35(5):576–80.
Mayta H, Gilman RH, Calderon MM, Gottlieb A, Soto G, Tuero I, et al. 18S ribosomal DNA-Based PCR for diagnosis of Trichomonas vaginalis. J Clin Microbiol. 2000;38(7):2683–7.
Felleisen RS. Comparative sequence analysis of 5.8S rRNA genes and internal transcribed spacer (ITS) regions of trichomonadid protozoa. Parasitology. 1997;115(Pt 2):111–9.
Masha SC, Wahome E, Vaneechoutte M, Cools P, Crucitti T, Sanders EJ. High prevalence of curable sexually transmitted infections among pregnant women in a rural county hospital in Kilifi, Kenya. PLoS ONE. 2017;12(3):e0175166.
da Luz Becker D, dos Santos O, Frasson AP, de Vargas Rigo G, Macedo AJ, Tasca T. High rates of double-stranded RNA viruses and Mycoplasma hominis in Trichomonas Vaginalis clinical isolates in South Brazil. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2015;34:181–7.
Goo YK, Shin WS, Yang HW, Joo SY, Song SM, Ryu JS, et al. Prevalence of Trichomonas Vaginalis in Women visiting 2 obstetrics and gynecology clinics in Daegu, South Korea. Korean J Parasitol. 2016;54(1):75–80.
Ginocchio CC, Chapin K, Smith JS, Aslanzadeh J, Snook J, Hill CS, et al. Prevalence of Trichomonas Vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima trichomonas Vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50(8):2601–8.
Acknowledgements
We acknowledge Biomed Diagnostics, Inc., USA, for their support of the InPouch®TV culture media kits amidst the peak of the COVID-19 pandemic.
Funding
The Internal Fluid research grant, Christian Medical College, Vellore, India.
Author information
Authors and Affiliations
Contributions
Conceptualization: NLS, RDS; Data collection: NLS, TS, SR, ID; Data curation: NLS, TS, RDS; Formal analysis: NLS, RDS; Methodology: NLS, RDS and SM; Writing – original draft: NLS, RDS; Writing – review & editing: TS, ID, SR, SM, RDS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was formally approved by Institutional Review Board, Christian Medical College, Vellore, IRB no: 12405 dated 02.12.2019. A written informed consent has been obtained from the women who participated in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Surya, N.L., Suji, T., Rani, S. et al. Trichomonas vaginalis: comparison of primers for implementation as an in-house PCR in rural Vellore, South India. BMC Infect Dis 24, 1039 (2024). https://doi.org/10.1186/s12879-024-09619-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09619-z