Abstract
Background
Early detection of outbreaks requires robust surveillance and reporting at both community and health facility levels. Uganda implements Integrated Disease Surveillance and Response (IDSR) for priority diseases and uses the national District Health Information System (DHIS2) for reporting. However, investigations after the first case in the 2022 Uganda Sudan virus outbreak was confirmed on September 20, 2022 revealed many community deaths among persons with Ebola-like symptoms as far back as August. Most had sought care at private facilities. We explored possible gaps in surveillance that may have resulted in late detection of the Sudan virus disease (SVD) outbreak in Uganda.
Methods
Using a standardized tool, we evaluated core surveillance capacities at public and private health facilities at the hospital level and below in three sub-counties reporting the earliest SVD cases in the outbreak. Key informant interviews (KIIs) were conducted with 12 purposively-selected participants from the district local government. Focus group discussions (FGDs) were conducted with community members from six villages where early probable SVD cases were identified. KIIs and FGDs focused on experiences with SVD and Viral Hemorrhagic Fever (VHF) surveillance in the district. Thematic data analysis was used for qualitative data.
Results
Forty-six (85%) of 54 health facilities surveyed were privately-owned, among which 42 (91%) did not report to DHIS2 and 39 (85%) had no health worker trained on IDSR; both metrics were 100% in the eight public facilities. Weak community-based surveillance, poor private facility engagement, low suspicion index for VHF among health workers, inability of facilities to analyze and utilize surveillance data, lack of knowledge about to whom to report, funding constraints for surveillance activities, lack of IDSR training, and lack of all-cause mortality surveillance were identified as gaps potentially contributing to delayed outbreak detection.
Conclusion
Both systemic and knowledge-related gaps in IDSR surveillance in SVD-affected districts contributed to the delayed detection of the 2022 Uganda SVD outbreak. Targeted interventions to address these gaps in both public and private facilities across Uganda could help avert similar situations in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early detection of disease outbreaks requires robust surveillance at the community and health facility levels [1, 2]. To achieve this, Uganda adopted the Integrated Disease Surveillance and Response (IDSR) strategy in 2001, which aims to establish effective systems for disease detection and response to priority diseases, conditions, and events using indicator and event based surveillance systems. IDSR ensures that the information flows bidirectionally through the surveillance system, informing all levels (community, health facilities, district, regional, and national) of potential outbreaks and response interventions in a timely manner [3, 4]. Complementing this framework, the District Health Information System 2 (DHIS2) software was adopted to facilitate collection, analysis, and dissemination of health-related data from communities through health facilities to the national level [5]. Efforts to promote the adoption of IDSR and the utilization of DHIS2 to strengthen disease surveillance and response have been particularly strong in Uganda [6, 7]. This commitment was underscored by the introduction of the third version of IDSR in specific regions of Uganda in 2021 [8], marking continued engagement in bolstering the country’s preparedness for disease outbreaks.
Even with these measures, some diseases, such as Ebola disease (EBOD), remain challenging to detect early. EBOD is a rare and deadly viral hemorrhagic fever (VHF) caused by four species of Ebolavirus (Zaire ebolavirus, Sudan ebolavirus, Taï Forest ebolavirus, and Bundibugyo ebolavirus); causes occasional outbreaks among humans [9]. For EBOD, the nonspecific early symptoms and the lack of widespread diagnostic tools means can lead to delays in outbreak detection [10, 11]. Gaps in detection and reporting allow outbreaks to propagate unchecked, leading to larger-scale crises than would not have occurred with early detection and response [11]. The outbreak of Ebolavirus disease in West Africa from 2013 to 2015 serves as a stark reminder of the consequences of delayed detection; the massive scale of the outbreak – nearly 30,000 cases and over 11,000 deaths– has been partially attributed to its delayed detection of at least 3 months [12].
To date, there have been seven Ebolavirus disease outbreaks in Uganda [13]. The first and largest outbreak was documented in 2000. Since then, Uganda has successfully contained subsequent ebolavirus disease outbreaks, some of which were limited to a single case-patient [14]. The second-largest, caused by Sudan virus (SUDV), occurred from September to November 2022 in Mubende District, with 164 recorded cases (142 confirmed, 22 probable) [13]. Although the first case was confirmed on September 19, subsequent investigations unveiled multiple deaths among persons with symptoms resembling those caused by SUDV (sudden death with hemorrhagic signs) dating back to at least early August 2022 [15, 16]. These previously-unreported cases were later epidemiologically linked to confirmed cases through chains of transmission. We investigated factors that contributed to the delayed detection of the 2022 SVD outbreak in Uganda.
Methods
Study design and setting
We used both qualitative and quantitative research methods within the three sub-counties in Mubende District that reported the first confirmed and probable SVD cases in the 2022 outbreak. Mubende District, located in north-central Uganda (150 km from Kampala, Uganda’s capital city), is predominantly rural with scattered urban centers. It is home to more than 500,000 people, most of whom constitute agricultural communities [17] (Fig. 1).
Study procedures
For the quantitative component, all health facilities (public and private), including clinics, Health Centre (HC) IIs, IIIs, IVs, and hospitals for which the facility in-charges consented to take part in the study from the 3 sub-counties were included. A semi-structured health facility assessment tool was administered to investigate core surveillance capacities existing before the outbreak declaration.
For the qualitative component, we conducted key informant interviews (KII) with individuals involved in surveillance from the district local government and health workers involved in the clinical management of probable SVD cases before outbreak detection. Focus group discussions (FGDs) were also held with village health team (VHT) members, survivors, household members of cases, and community leaders in villages with probable cases and the index case. We grouped FGDs based on participants’ age and gender to encourage information-sharing. KIIs and FGDs focused on experiences with SVD and Viral Hemorrhagic Fever (VHF) surveillance in the district. Interviews were conducted in the local language, audio-recorded, transcribed verbatim, and later translated to English before analysis. Data were collected until saturation.
Data analysis
Quantitative data were analyzed descriptively using STATA V14. We employed thematic analysis to analyze qualitative data. We thoroughly reviewed transcripts multiple times to gain a deeper understanding of the data before categorizing it based on predefined areas of analysis. Content analysis was employed to establish a coding framework and classify key themes and concepts present in the responses. As modifying factors emerged, they were systematically labeled, resulting in an organically developed coding scheme. A final coding structure was established and applied consistently across all transcripts, with two independent reviewers ensuring accuracy.
Ethical considerations
We sought permission to conduct the study from the Ministry of Health, Mubende District Health officer, health facility in-charges, as well as village leaders. We obtained verbal informed consent from the participants. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy. §.
§See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. § 241(d); 5 U.S.C. § 552a; 44 U.S.C. § 3501 et seq.
Results
Quantitative results
Forty-six (96%) of 48 private facilities and all (100%) eight public facilities in Madudu (n = 28 facilities), Kiruma (n = 10), and Butoologo (n = 16) sub-counties participated. More public than private facilities reported that they had VHF case definitions displayed before the outbreak began (63% vs. 20%) (p = 0.02). At the time of the survey, 80% of private and all public facilities had case definitions displayed (p = 0.33). All public facilities had outpatient (OPD) registers, while 50% of private facilities had them (p = 0.02). Among facilities with OPD registers, 88% of public facilities sourced them from the Ministry of Health (MoH), compared to 35% of private facilities (p = 0.015). All (100%) public facilities reported to DHIS2, while only 9% of private ones did so (p < 0.001). All public facilities had ≥ 1 health worker trained in IDSR, compared to 15% of private facilities (p < 0.001) (Table 1).
Qualitative results
We conducted 12 KIIs with the district, health sub-district, and sub-county surveillance officers; the regional epidemiologist; two health facility surveillance officers; and five health workers from public and private facilities linked to the outbreak. Six FGDs were held in six villages from which the index and probable cases were reported.
Seven major themes emerged from the qualitative interviews as gaps in surveillance that might have led to late detection of the outbreak: weak community-based surveillance, low suspicion index for VHF among health workers, lack of IDSR training, poor private sector involvement in surveillance, limited capacity for data analysis and utilization at the facility level, funding constraints, and lack of active mortality surveillance in the district.
-
1)
Weak community-based surveillance
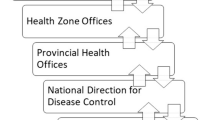
In Uganda, surveillance activities (including IDSR and DHIS2 implementation and utilization) at the district level are spearheaded by the district surveillance focal person (SFP), supported by surveillance officers and an epidemiologist at the regional referral hospital. The district SFP supervises the health sub-district (HSD) surveillance focal persons at the subdistrict level, health assistants (HA) at the subcounty level, facility surveillance focal person at HC IIIs, and village health teams (VHT) at village level (Fig. 2).
Community-based surveillance (CBS), which comprises all activities from the sub-county level downwards, may improve early detection and response to disease outbreaks by leveraging the capacity of community members to carry out surveillance activities within their communities. However, there were gaps across the district surveillance structure before the outbreak, leaving loopholes for delays in outbreak detection. Participants unanimously agreed that the community-based surveillance arm was non-functional (starting from sub-county level downwards) before the declaration of the outbreak. One participant noted that officers were not aware of their responsibilities in surveillance.
“The officers at subcounty level didn’t know it was their responsibility in regards to surveillance. How can you say you know what to do and not report multiple deaths in your catchment area yet you are even involved in burials? – KII participant.
The same participant also spoke about the reason that the responsible parties may have been unaware of their responsibilities, noting that trainings were not focused at the community level.
…Emphasis [for trainings on surveillance and reporting] had been put on HSD surveillance focal persons and not the HAs at sub-county level, yet facility surveillance focal persons who had an idea on what to do were not actively involved in surveillance activities at their level.
At the community level, FGD participants also pointed out that they were unaware that any specific events should be reported to the healthcare system. Additionally, there were numerous misconceptions about bleeding and multiple deaths within a family.
“We kept burying people in the village, but we didn’t report to anyone because we didn’t know how and where to report.” – FGD participant.
“We delayed to report these incidences because we thought one of our family members had brought witchcraft that killed our people for him to get riches…” – VHT and family member of affected family.
-
2)
Low suspicion index for VHFs among health workers
Passive surveillance in Uganda relies on the ability of health workers to suspect and detect diseases of public health interest among patients who seek care in their health facilities. Participants reported that the suspicion index for ebolaviruses was very low before the 2022 outbreak declaration, and that some health workers without previous exposure to VHFs were not sure what to look for outside of bleeding. Some health workers also had misconceptions about the illness, such as believing that all patients would bleed.
“This disease was a disaster because we had never had any VHF outbreak in our area. The presentation also didn’t show any sign of a viral [hemorrhagic fever] infection because there was no bleeding but rather persistent fevers and were not improving with treatment” – KII participant from a private health facility.
“…when fever persisted after antimalarial and antipyretic treatments, the health workers didn’t have the knowledge to suspect a VHF. Look at the first identified case, he had a diagnosis for malaria, typhoid, and gastro-intestinal infection before a VHF was suspected, not until the end stage [did his] signs and symptoms occur” – KII participant.
But even with a bleeding presentation, a VHF was not immediately suspected due to lack of prior exposure and VHF knowledge.
“This was actually not the first time we were receiving such patients that were bleeding so we took it lightly as a normal occurrence thinking we were treating other common conditions. I remember a patient we received at accident & emergency ward in July who was bleeding uncontrollably and we referred to Mulago. We didn’t suspect a VHF…but if the surveillance team was engaging us more, then maybe we would quickly suspect a VHF.” – KII participant from a public health facility.
Beyond the low index of suspicion for VHFs, Mubende District was having a malaria upsurge at the time of the outbreak, and most patients were testing positive for malaria. Such situations can make it easier to ignore or try to explain away increases in deaths.
“There was a malaria outbreak at the time that might have masked the outbreak, especially while still in the communities…patients tested positive for malaria though not improving on treatment.” – KII participant.
“Actually, in our first investigation report before confirmation, we thought it was drug-resistant malaria because of the way they were presenting. Most had malaria and bleeding was rare, with some bleeding at death; [ebolavirus] was only a second thought.” – KII participant.
-
3)
Lack of IDSR trainings
Through interactions, participants highlighted that the main contributor to the low suspicion index was the lack of training on surveillance; pointing out that if they had been routinely engaged on surveillance activities, they would know which diseases to report.
“IDSR 3rd edition was rolled out in the country, but Mubende had never been trained [on the 3rd edition]. By virtue that such trainings had not happened in Mubende region, it kept people ignorant about surveillance.” – KII participant.
“When surveillance trainings happen, regional referral staff are excluded from such trainings and only persons at the district are trained. Absence of these trainings at the RRH might have affected their suspicion index.” – KII participant.
“Lower-level facilities have received some surveillance training, mainly on vaccine-preventable diseases, but not integrated training on surveillance. VHTs too don’t receive enough trainings; as far as I can remember, VHTs have only been trained twice: during COVID-19 and in EVD so I don’t think they were knowledgeable on a condition like EVD or how it presents and that it should be reported.” – KII participant.
-
4)
Poor private sector involvement in surveillance
For multiple reasons, including the higher number of private than public facilities, patients often seek care from these facilities rather than a public facility. This was evident during the outbreak, with most cases seeking care from private health facilities before being detected. Surveillance in private facilities is critical to detecting health issues in a community early. However, participants reported that there was poor private sector engagement in surveillance.
“Private facilities aren’t aware of what diseases need to be reported on and of what importance it is to report these conditions for the greater good. They have not been engaged to make them appreciate the importance of good surveillance systems.” – KII participant.
“I have had no training on surveillance and have no knowledge about notifiable diseases, who to notify, utilization of data. Through my 5 years working here, I have never seen anyone from the district to tell me about such a thing as surveillance or notifiable disease so I don’t know what that is; what I know is that when we get complicated conditions that we can’t manage here at the facility, then we refer to Mubende [Regional Referral Hospital]” – KII participant from private health facility.
-
5)
Limited capacity for data analysis and utilization at facility level
Passive surveillance systems rely on data collected from hospital records which is analyzed and interpreted for appropriate and timely response. Participants felt the district neither analyzed nor utilized these data, in part due to an inability to do so.
“How do you know you have the true picture of the problem if you don’t know how many facilities should be reporting to you or how much data to expect? We aren’t even aware of the number of facilities (gov’t and private) in the district as some of the facilities aren’t registered” – KII participant.
“At my facility we don’t have the capacity to analyze surveillance data reported by VHTs and watch for any trends in disease patterns in the communities. We send the data to the district for analysis so we are blind in regards to community problems” – Facility surveillance focal person.
At the district, when data are analyzed, they are not utilized to influence service delivery, so the importance of data collection is lost to the one collecting it at the lower levels.
“Data analysis is done at district and regional referral level and compiled into a weekly bulletin that we share with stakeholders but I think the interest is not yet there because they have not yet seen the use of the data informing decisions. If people start to see that the data they collect informs decision making, then they will pick interest in it and own it as their data.” – KII participant.
-
6)
Funding constraints
For surveillance to be effective, there is a need for core activities like trainings, support supervision, and verification and response to alerts. These activities require funds that participants reported were not available at the district.
“Surveillance has no allocated budget, making it hard to execute duties in the district or even train responsible surveillance persons at the different levels. For example, we received IDSR trainings at the district, but we have no capacity to disseminate the information downwards” – KII participant.
“Sometimes you receive alerts or notice patterns in data, but there is no money for fuel to go and support…it took us a bit of time to respond to the Ebola situation due to lack of fuel.” – KII participant.
-
7)
No active mortality surveillance in the district
“There was delay in detecting changes in mortality rates in the communities because we didn’t have mortality surveillance in the district but it is good now that this has been established post-outbreak…we hope to capture all community deaths and observe for any changes.” – KII participant.
Discussion
Delays in detecting Ebola disease outbreaks can result in uncontrolled, widespread transmission, potentially escalating into larger-scale crises that could have been averted with earlier detection and response. Findings from this study highlight the challenges of detecting Ebola disease outbreaks, particularly in areas where diseases with similar presentations, such as malaria and typhoid fever, are prevalent, even in the presence of established surveillance structures and prior experience responding to similar outbreaks. This study revealedsystemic and knowledge gaps in surveillance activities which likely hampered early detection of the 2022 Sudan virus outbreak in Uganda. Despite a large majority of health facilities in the district being privately owned, private sector involvement in surveillance was negligible. Participation in surveillance activities was limited within the affected communities, with poor understanding of community-based surveillance or reporting responsibilities. Challenges to reporting EBOD-related symptoms were exacerbated by prevalent beliefs about the presentation of EBOD. There was a low suspicion index for VHFs among clinicians, insufficient training in surveillance responsibilities, and underutilization of surveillance data within healthcare facilities. The lack of active mortality surveillance also likely contributed to the outbreak’s late detection, as the district was unable to track changes in deaths. Documenting these gaps provides an opportunity to enact necessary changes and avoid similar delays in future.
Effective surveillance systems at all levels are essential for facilitating a rapid response to outbreaks [18]. However, community-based surveillance (CBS), which relies on community members to report unusual health events, was ineffective in the affected areas before the outbreak. Surveillance officers and community members were unaware of both opportunities to share information and their responsibility to report, resulting in delayed reporting at the community level. Previous research has demonstrated the pivotal role of effective CBS systems in the early detection and response to outbreaks such as measles and monkeypox in Cameroon [19]. These findings highlight the importance of implementing effective CBS systems to detect outbreaks early.
We found a low index of suspicion for VHFs among health workers, contributing to late detection of the outbreak. As this outbreak marked the first occurrence of EBOD in Mubende district, many health workers lacked prior exposure to VHFs. This was also a challenge early in the West African Ebolavirus disease outbreak [20]. Furthermore, not all patients exhibited bleeding, often considered a classical sign of EBOD. With the first clinical signs often resembling those of other endemic diseases like malaria or typhoid [21], it may not be surprising that some cases were missed. The absence of proper training in surveillance activities could also have contributed to the low suspicion index among health workers. At the time of the outbreak, the training on the updated IDSR guidelines (3rd edition) had not been fully implemented in the district, leaving health workers without refresher training on surveillance knowledge and skills [22]. Continued training on existing and updated guidelines and educational programs may help enhance the suspicion index among health workers for unusual but serious diseases such as EBOD.
A major challenge was the poor private sector involvement in surveillance. Although private facilities are often the first point of care in Uganda [23], only one in ten private facilities reported to DHIS2, and fewer than one in seven had at least one health worker trained in IDSR, resulting in many health workers in private facilities being unfamiliar with the reporting of notifiable diseases or the principles of surveillance. This finding aligns with observations in Uganda during the revitalized IDSR training, in which there was representation from more than 80% of the public health facilities in each district but few private facilities [6]. To bridge this gap, it is imperative to implement focused initiatives directed at enhancing awareness among personnel in private health facilities and fostering robust collaborations between the public and private sectors. This may involve providing non-monetary incentives, such as complimentary training credits towards Continuing Medical Education for consistent reporting, offering free medications, granting access to scholarly publications or books, or other non-monetary incentives [24].
The inability to analyze and use data at lower-level facilities was a frequently-reported problem; similar findings were reported in neighboring Tanzania [25]. Participants emphasized the importance of having appropriate data analytical capabilities across all levels. However, the absence of allocated funding for surveillance activities acts as a significant barrier to building such capacities. Vital surveillance tasks like training, support supervision, verification, and responding to alerts are often hindered by financial constraints [26, 27]. Addressing these funding gaps and securing resources for surveillance activities is imperative in fortifying the surveillance system.
Lastly, the absence of active mortality surveillance contributed to delays in recognizing the outbreak. Mortality surveillance helps identify trends and detect emerging health threats, prioritize healthcare services, and evaluate response effectiveness [28]. Historically, mortality surveillance has empowered countries to identify preventable causes of childhood mortality and formulate strategies to address these causes [29]. In a similar vein, the establishment of active mortality surveillance in Mubende District following the outbreak was regarded as a positive step toward comprehensively documenting all deaths and monitoring emerging trends.
Our study had limitations. We relied on self-reports to assess certain surveillance capacities, potentially leading to an overestimation of these capacities due to social desirability bias. Additionally, the survey was conducted in only three subcounties, which may limit the generalizability of our findings to the entire district and country.
In conclusion, systemic and knowledge-related gaps in the surveillance system contributed to the late detection of the SVD outbreak. Community engagements and support to village health teams to perform community-based surveillance may improve incident reporting and facilitate early outbreak detection. Targeted interventions like capacity building and improved mortality surveillance in both public and private facilities across Uganda could help avert similar situations in the future.
Data availability
The datasets and materials belong to the Uganda Public Health Fellowship Program and are not publicly available. However, these can be availed upon reasonable request from the corresponding author and with permission from the Uganda Public Health Fellowship Program.
Abbreviations
- IDSR:
-
Integrated Disease Surveillance and Response
- DHIS2:
-
District Health Information System 2
- SVD:
-
Sudan Virus Disease
- KII:
-
Key Informant Interview
- FGD:
-
Focus group discussion
- VHF:
-
Viral haemorrhagic fever
- WHO:
-
World Health Organization
- EBOD:
-
Ebola disease
- SUDV:
-
Sudan virus
- HC:
-
Health centre
- VHT:
-
Village health team
- OPD:
-
Outpatient department
- MoH:
-
Ministry of Health
- HA:
-
Health assistant
- SFP:
-
Surveillance focal person
- CBS:
-
Community-based surveillance
- U.S CDC:
-
United States Centers for Disease Control and Prevention
References
McGowan CR, et al. Community-based surveillance of infectious diseases: a systematic review of drivers of success. BMJ Global Health. 2022;7(8):e009934.
World Health Organization. Uganda’s Disease Surveillance System Proves to be Effective in Detecting and Follow up Ebola Contacts Suspected Cases. 2019.
Fall IS, et al. Integrated Disease Surveillance and Response (IDSR) strategy: current status, challenges and perspectives for the future in Africa. BMJ Global Health. 2019;4(4):e001427.
Uganda Ministry of Health. National Technical Guidelines for Integrated Disease Surveillance and Response, Third Edition. Kampala; 2021. p. iii.
Byrne E, Sæbø JI. Routine use of DHIS2 data: a scoping review. BMC Health Serv Res. 2022;22(1):1234.
Kihembo C, et al. The design and implementation of the re-vitalised integrated disease surveillance and response (IDSR) in Uganda, 2013–2016. BMC Public Health. 2018;18(1):1–11.
Kinkade C, et al. Extending and strengthening routine DHIS2 surveillance systems for COVID-19 responses in Sierra Leone, Sri Lanka, and Uganda. Emerg Infect Dis. 2022;28(Suppl 1):S42.
World Health Organization. Uganda Launches the Third Edition of the National Guidelines for Integrated Diseases Surveillance and Response. 2021.
Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377(9768):849–62.
World Health Organization. Clinical management of patients with viral haemorrhagic fever: a pocket guide for front-line health workers. Interim emergency guidance for Country adaption. World Health Organization; 2016.
Mbonye AK, et al. Ebola viral hemorrhagic disease outbreak in West Africa-lessons from Uganda. Afr Health Sci. 2014;14(3):495–501.
Baize S, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371(15):1418–25.
World Health Organization. Ebola disease caused by Sudan ebolavirus – Uganda. 2023.
Centers for Disease Control and Prevention. History of Ebola Disease Outbreaks. 2022.
Kiggundu T. Notes from the field: outbreak of Ebola Virus Disease caused by Sudan ebolavirus—Uganda, August–October 2022. MMWR. Morbidity and Mortality Weekly Report,; 2022. p. 71.
Honeyman DA, Notaras A. Resurgence of Sudan Virus Disease (SUVD) in Uganda 2022.
Uganda Bureau of Statistics - UBOS, I., Uganda Demographic and Health Survey. 2016, UBOS and ICF; 2018: Kampala, Uganda.
Allaranga Y et al. Lessons learned during active epidemiological surveillance of Ebola and Marburg viral hemorrhagic fever epidemics in Africa. East Afr J Public Health, 2010. 7(1).
Metuge A, et al. Humanitarian led community-based surveillance: case study in Ekondo-Titi. Cameroon Confl Health. 2021;15(1):1–12.
Mbonye AK, Joseph F. Issa Makumbi, and Jane Ruth Aceng. EVD virus hemorrhagic disease outbreak in West Africa-lessons from Uganda. Afr Health Sci. 2014;14(3):495–501. Nanyunja, AlexOpio.
Lado M, et al. Clinical features of patients isolated for suspected Ebola virus disease at Connaught Hospital, Freetown, Sierra Leone: a retrospective cohort study. Lancet Infect Dis. 2015;15(9):1024–33.
Organization WH. Integrated disease surveillance in the African region: a regional strategy for communicable diseases 1999–2003, in Integrated disease surveillance in the African Region: a regional strategy for communicable diseases 1999–2003. 1999. pp. 24–24.
Konde-Lule J, et al. Private and public health care in rural areas of Uganda. BMC Int Health Hum Rights. 2010;10:1–8.
Phalkey RK, et al. From habits of attrition to modes of inclusion: enhancing the role of private practitioners in routine disease surveillance. BMC Health Serv Res. 2017;17:1–15.
Mboera LE et al. Malaria surveillance and use of evidence in planning and decision making in Kilosa District, Tanzania. Tanzan J Health Res, 2017. 19(3).
Wu J. Integrated Disease Surveillance and Response (IDSR) in Malawi: implementation gaps and challenges for timely alert. PLoS ONE. 2018;13(11):e0200858.
Rumunu J et al. Evaluation of integrated disease surveillance and response (IDSR) and early warning and response network (EWARN) in South Sudan 2021. Pan Afr Med J, 2022. 42(6).
Africa CDC. Continental Framework on Strengthening Mortality Surveillance Systems in Africa. 2023.
Madewell ZJ, et al. Prioritising health-care strategies to reduce childhood mortality, insights from Child Health and Mortality Prevention Surveillance (CHAMPS): a longitudinal study. Lancet Global Health. 2022;10:S8.
Acknowledgements
The authors thank the staff of the Public Health Fellowship Program for the technical support and guidance offered during this assessment. The authors also extend their appreciation to Mubende district local government, health authorities, and community members for the support offered during the assessment.
Funding
This assessment was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) and the President’s Malaria Initiative (PMI) through US Centers for Disease Control and Prevention Cooperative Agreement number GH001353–01 through Makerere University School of Public Health to the Uganda Public Health Fellowship Program, Ministry of Health. The findings and conclusions in this report are exclusively the responsibility of the authors and do not essentially represent the official views of the US Centers for Disease Control and Prevention, the United States Agency for International Development, Makerere University School of Public Health, or the Ministry of Health, Uganda.
Author information
Authors and Affiliations
Contributions
JFZ took the lead in conceptualization, visualization, investigation, methodology, project administration, data collection and analysis, writing – original draft, writing – review & editing, HNN, EJN, RA, RB, MGZ, BNS, SNK, PCK, BA, MWW, ZK, MN, RZ, PK, and TK were involved in data collection, data analysis, and writing of the manuscript. HTN, DNG, IBK, LB, BK, DK, RM, ARA, and JRH were involved in conceptualizing the study idea and writing, editing, and reviewing the manuscript. All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This assessment was in response to a public health problem and was, therefore, determined to be non-research. The MoH gave the directive and approval to conduct this assessment. The Office of the Associate Director for Science, CDC/Uganda, also determined that this assessment was not human subject research, and its primary intent was public health practice or disease control activity (specifically, epidemic or endemic disease control activity). This assessment was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy .§ §See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. § 241(d); 5 U.S.C. § 552a; 44 U.S.C. § 3501 et seq. All experimental protocols, were approved by the US CDC human subjects review board and the MoH, and have been performed in accordance with the Declaration of Helsinki. Permission to collect data was obtained from Mubende District local government, hospital administrators and health facility in-charges. Consent was obtained from all participants before study procedure commencement. To protect the confidentiality of the respondents, each was assigned a unique identifier that was used instead of their names. All methods were carried out in accordance with relevant guidelines and regulations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zalwango, J.F., Naiga, H.N., Nsubuga, E.J. et al. Understanding the delay in identifying Sudan Virus Disease: gaps in integrated disease surveillance and response and community-based surveillance to detect viral hemorrhagic fever outbreaks in Uganda, September 2022. BMC Infect Dis 24, 754 (2024). https://doi.org/10.1186/s12879-024-09659-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09659-5