Abstract
Background
Corynebacterium striatum (C. striatum) is a gram-positive, anaerobic bacillus found both environmentally and in human skin and nasal mucosa flora. It is reportedly the etiologic agent of community-acquired and nosocomial diseases and is significantly associated with bacteremia and medical endovascular devices. This is the rare case of mitral valve native valve endocarditis (NVE) caused by C. striatum occurring in a young adult without underlying structural heart disease or indwelling cardiovascular medical devices successfully treated with multidisciplinary therapy.
Case presentation
The patient was a 28-year-old female with no medical history. She was transferred our hospital due to sudden onset of vertigo and vomit. A computed tomography on day 2 revealed the hydrocephalus due to the cerebellar infarction, and she underwent posterior fossa decompression for cerebellar infarction. An angiography on day 8 revealed a left vertebral artery dissection, which was suspected be the etiology. Afterwards, a sudden fever of 39 degrees developed on day 38. She was diagnosed with aspiration pneumonia and treated with ampicillin/sulbactam but was still febrile at the time of transfer for rehabilitation. Treatment continued with levofloxacin, the patient had no fever decline, and she was readmitted to our hospital. Readmission blood cultures (3/3 sets) revealed C. striatum, and an echocardiogram revealed an 11 mm long mitral valve vegetation, leading to NVE diagnosis. On the sixth illness day, cardiac failure symptoms manifested. Echocardiography revealed mitral valve rupture. She was transferred again on the 11th day of illness, during which time her mitral valve was replaced. C. striatum was detected in the vegetation. Following surgery, she returned to our hospital, and vancomycin administration continued. The patient was discharged after 31 total days of postoperative antimicrobial therapy. The patient experienced no exacerbations thereafter.
Conclusions
We report the rare case of C. striatum mitral valve NVE in a young adult without structural heart disease or indwelling cardiovascular devices.
Clinical trial number
Not applicable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The Corynebacterium striatum (C. striatum) is a gram-positive, rod-shaped, nonspore-forming, facultative anaerobic bacillus that is found in the environment, as well as in the flora of human skin and nasal mucosa [1,2,3]. It represents an emerging human pathogen that is largely underappreciated and has been associated with severe infections in immunocompetent and immunocompromised hosts [2, 4, 5]. Moreover, it has been reported to be the etiologic agent of diseases acquired both through the community and nosocomially [1, 6]. In recent years, there has been an increasing proportion of isolates of antimicrobial multidrug-resistant C. striatum strains related to various nosocomial diseases and/or outbreaks, with fatal invasive infections occurring in immunosuppressed and even immunocompetent patients [6]. On the other hand, it is conceivable that positive blood cultures for this pathogen are often regarded as a sign of contamination and that it is considered a rare pathogen in infective endocarditis (IE) [1, 5]. In addition, the studies available describe few cases of IE caused by species of Corynebacterium [7].
Here, we present the rare case of native valve endocarditis (NVE) in the mitral valve caused by C. striatum, occurring in a young adult with no underlying structural heart disease or indwelling cardiovascular medical devices and was successfully treated with multidisciplinary therapy. Due to the novelty of this case, we report it here, together with a brief review of the literature.
Case presentation
Our patient was a 28-year-old female. The patient had no particular past medical history including any history of thrombotic events. She did not receive hormonal estrogenic therapy. The patient reported no use of tobacco or illicit drugs, no habit of drinking alcohol, and no known allergies. She was single and lived alone. She was independent in her everyday life before the illness. She had undergone a regular medical check-up six months prior, and no abnormal results were found. She worked at a clerical job and had no family history of hereditary diseases or malignant diseases.
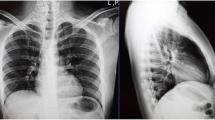
Her first chief complaint was vertigo and vomit. She was transferred our hospital due to sudden onset of symptoms as above. At the emergency department (ED), her vital signs were abnormal: her blood pressure was 100/70 mmHg, her heart rate was 78 regular beats/min, her body temperature was 36.3 °C, her oxygen saturation was 100% under ambient air, her respiratory rate was 24 breaths/min, and her Glasgow Coma Scale score was 14 points (E3V5M6). Upon physical examination, finger-nose-finger test together with diadochokinesis were awkward, on the other hand, no abnormal findings regarding to infection focus were detected. A magnetic resonance imaging revealed a high intensity area in her left cerebellum, and she was admitted on the diagnosis of cerebellar infarction (Fig. 1a). However, a computed tomography (CT) on day 2 revealed the hydrocephalus due to the cerebellar infarction, and the patient underwent posterior fossa decompression on day 3. An angiography on day 8 revealed a left vertebral artery dissection, which was possible cause of cerebellar infarction (Fig. 1b). Afterwards she had been stable, however sudden fever of 39 degrees developed on day 38. She underwent a routine laboratory examination including of complete blood count, biochemistry, urinalysis, and chest to abdominal CT. On the other hand, neither blood nor sputum culture was not taken at that time. The CT revealed a consolidation in S6 segment of the right lung and she was diagnosed with aspiration pneumonia (Fig. 1c). We treated with ampicillin/sulbactam 3 g x 4 /day for 7 days (Day 38–44) but was still febrile at the time of transfer for rehabilitation on day 50. Following her transfer, treatment with levofloxacin 500 mg x 1 /day for 5 days (Day 50–54) was continued, but her fever persisted. She was then readmitted to our hospital by ambulance.
a T2 weighted image of magnetic resonance imaging on emergency department. It revealed a high intensity area in her left cerebellum (red arrow). b Angiography on day 8. It revealed a left vertebral artery dissection (red arrow). c Computed tomography on day 38. It revealed consolidations in S6 segment of the right lung (red arrow)
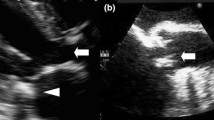
At the ED, her vital signs were abnormal: her blood pressure was 85/54 mmHg, her heart rate was 93 regular beats/min, her body temperature was 37.6 °C, her oxygen saturation was 96% under ambient air, her respiratory rate was 16 breaths/min, and her Glasgow Coma Scale score was 15 points (E4V5M6). Upon physical examination, a Levine III to IV holosystolic murmur was found at cardiac auscultation. In addition, a small, red, tender erythema on her right middle finger and a small, red, painless erythema on her left index finger were confirmed. No abnormal findings regarding to infection focus were detected upon physical examination, including skin and neurological findings. A routine laboratory examination taken upon arrival at the ED revealed increased values of white blood cells (14.5 × 103/µL), platelets, lactic acid dehydrogenase (241 U/L), alkaline phosphatase (154 U/L), gamma-glutamyl transpeptidase (95 IU/L), blood sugar and C-reactive protein (11.4 mg/dL); and decreased values of red blood cells and albumin. Urinalysis ruled out a urinary tract infection. On the other hand, other values, including procalcitonin (0.21 ng/mL), complete blood count, and biochemistry results, were normal. An electrocardiogram revealed no abnormal findings. Images from chest to abdominal computed tomography scans also revealed no abnormal findings. The two sets of blood culture were taken at once on readmission, and another set was additionally taken 12 h later. All 3/3 sets revealed C. striatum by the MALDI Biotyper® (Beckman Coulter, Inc.), a microbial identification system based on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Fig. 2), and the interval between the stroke admission and positive blood cultures for C. striatum was 53 days. The antibiotic susceptibility testing was performed by broth microdilution method, and the susceptibility interpretation was according to Clinical and Laboratory Standards Institute. The strain was susceptible to gentamicin (GM), vancomycin (VCM), daptomycin (DAP), linezolid (LZD), rifampicin, and sulfamethoxazole-trimethoprim. On the other hand, it was resistant to benzylpenicillin, imipenem/cilastatin, erythromycin, and clindamycin. At this point, we suspected her diagnosis to be IE. On the second hospital day, a transthoracic echocardiogram was performed, revealing an 11 mm long mobile vegetation at the mitral valve (Fig. 3a and b). Based on these findings, the patient met Duke criteria and we diagnosed this patient with NVE caused by the C. striatum.
The patient’s condition was stable, and she showed no symptoms of heart failure upon readmission. We started the combination therapy by intravenous administration, based on the drug sensitivity test. The details are as follows; 1 g x 2 /day of VCM and 6 mg/kg x 1 /day of GM. Their subsequent dosage was decided based on therapeutic drug monitoring (TDM). Fortunately, the side effects had not been confirmed and we completed GM treatment for 5 days. On the sixth hospital day, symptoms of cardiac failure manifested. Further echocardiography revealed a rupture of the mitral valve, so she was then transferred to another hospital on the 11th hospital day and underwent mitral valve replacement on the same day. The patient’s clinical course of the initial admission is shown in Fig. 4a. Intraoperative findings revealed a large vegetation on the mitral leaflets together with valve regurgitation (Fig. 5a). We assumed that presence of a cardiac murmur on readmission as well as concomitant severe valve regurgitation at the first echocardiography were associated with these intraoperative findings, and they were due to secondary to rupture of chorda tendinae or valve perforation. Based on these findings, a prosthetic valve was placed (Fig. 5b). The culture of vegetation revealed as C. striatum by the MALDI Biotyper®. Following surgery, the patient was transferred to our hospital. Our cardiac surgeon consulted antimicrobial stewardship team regarding the management of treatment on the first day of the second hospitalization. They managed with this patient since then. The treatment with VCM was continued and their doses were based on therapeutic drug monitoring, with targeted trough value of 15–20 µg/mL. She was discharged after a total of 31 days of postoperative antimicrobial therapy. We had followed up her 36 months after discharge and she experienced no further exacerbations thereafter. The patient’s clinical course of the readmission is shown in Fig. 4b.
Surgical findings on the 11th hospital day after mitral valve replacement. a Preplacement of the mitral valve. Vegetation was attached to the mitral valve, causing severe damage (blue arrow). Additionally, the infection spreads from the mitral valve to the papillary muscle, and the chordae tendineae are vulnerable. b Post replacement of the mitral valve. A prosthetic valve was placed
Discussion and conclusions
We present the rare case of NVE in the mitral valve caused by C. striatum occurring in a young adult without underlying structural heart disease or indwelling cardiovascular medical devices; this patient was successfully treated with multidisciplinary therapy. Consequently, there is value in reporting this event.
Regarding the etiology of stroke, the patient had a detailed examination by neurosurgeon. There, angiography on day 16 revealed a left vertebral artery dissection, which was suspected cause of the stroke. Vertebral artery dissection is responsible for 9–24% of strokes in the young adults, causing a high index of suspicion in this group and this case is compatible to previous report [8]. On the other hand, the patient did not have any predisposing factors of vertebral artery dissection, like congenital disorders which affect vascular connective tissue, like vascular Ehlers-Danlos syndrome, Marfan syndrome, and osteogenesis imperfecta, fibromuscular dysplasia, a non-atherosclerotic, non-inflammatory vascular diseases [8]. Furthermore, atrial fibrillation was not detected by electrocardiogram during hospitalization, therefore we denied the possibility of embolism as the etiology. In addition, the patient did neither have fever nor meet the Duke criteria at the onset of stroke, and she occurred a fever of 39 °C on day 38. Furthermore, we found neither article which mentioned the association stroke with C. striatum IE, nor ones which mentioned the association vertebral artery dissection with C. striatum IE. Bases on these, we considered that the stroke was not caused by C. striatum IE. On the other hand, we should have performed more detailed examination at the onset of fever.
Infections due to C. striatum tend to occur more commonly in males [4]. Upon examination of 30 cases of IE caused by Corynebacterium species, 21 patients (70%) were found to be elderly, and infection was associated with having a prosthetic heart valve [1]. Another literature review of IE caused by C. striatum revealed that the mean age of patients was 62.1 years (range 24–83), 19 patients (63%) were male, and 17 patients (57%) had heart disease [3]. The majority of these patients exhibited NVE (24/30, 80%), and the vast majority were associated with the left side of the heart; the plurality of cases was associated with the mitral valve (13/30, 43%), with the aortic valve being the second most common (9/30, 30%) [3]. A number of cases have also been associated with medical devices, namely, artificial valves or pacemaker leads [3]. In literature review of IE caused by C. striatum, only a single patient was found to have no comorbidities, confirming the enhanced virulence of the pathogen, particularly for patients who were hospitalized long term and had an underlying disease [9]. The valve most commonly affected was the mitral valve (14/27, 52%), with the aortic valve being the second most common (8/27, 30%) [9]. Regarding 27 patients backgrounds, 8 (29%) of the patients had diabetes, and 6 (22.2%) had chronic renal disease [9]. Additionally, 7 of patients (22%) had a prosthetic valve (hereinafter referred to as a pacemaker) and 1 patient (4%) a ventriculoatrial shunt for congenital hydrocephalus [9]. The two most significant factors that predispose patients to developing IE are underlying structural heart disease and prosthetic valves; however, other factors include the use of injected drugs, the presence of an intravascular catheter, large preexisting wounds, and recurring visits to healthcare institutions (most commonly for hemodialysis) [7]. Compared to the findings of previous reports, the patient in our case was the youngest yet recorded, and additionally lacked any underlying structural heart disease or indwelling cardiovascular medical devices.
To date, the major infection sites of C. striatum have included the bloodstream, lungs, and central nervous system, therefore the infection have been associated with bacteremia, central line infections, and occasionally cardiac device-associated IE accounting for the majority of cases [1, 2, 4]. From that perspective, C. striatum could be added to the list of typical IE pathogens in conjunction with an implanted coronary device. Of these, intracardiac electronic device (ICED) infections are further subdivided into two categories: pocket and systemic infections [2]. With regard to IE, the pathogen C. striatum should be considered for both native valve and prosthetic valve endocarditis, as well as device infections [1]. In this case, the etiology seems to have been a nosocomial peripheral venous catheter blood stream infection developing into IE.
Clinical detection of IE is critical, and it is vital to consider the risk in all patients with an unexplained low fever and nonspecific clinical symptoms; echocardiography should also be performed promptly [3]. In this case, the patient suggested a Janeway lesion, which should be counted as a minor criterion (vascular phenomenon). From that perspective, we could have performed an echocardiography based on the detailed physical examination on the readmission. For examinations, the primary tool is echocardiography, although molecular methods are recommended when a clinical suspicion of a rare infection leads to monitoring of the infection [3]. C. striatum is a commonly isolated Corynebacterium species in a clinical microbiology laboratory context, although relatively few C. striatum infections have been confirmed [3]. For diagnosing C. striatum IE, the traditional diagnostic methods, which make use of microbiology laboratory findings, are now mature and comparatively inexpensive [3]. In recent years, however, C. striatum infections have appeared to be increasingly common, and a likely contributing factor to this perception is the increased ease and accuracy of identifying Corynebacterium spp., including C. striatum, in clinical cultures [5].
The presence of heterogeneous clinical presentations and inconsistent diagnostic criteria and definitions, which can even vary among individuals in scientific societies, can make diagnosis challenging [2]. According to the 2015 European Society of Cardiology guidelines on IE, a diagnosis can be established according to the modified Duke criteria, which implies that a definite diagnosis does not necessarily require a positive echocardiogram [2]. In addition, the International Society for Cardiovascular Infectious Diseases (ISCVID) convened a multidisciplinary Working Group to update the diagnostic criteria for IE [10]. The resulting 2023 Duke-ISCVID IE criteria proposed significant changes, and C. striatum was added as a typical IE pathogen [10].
Regarding the management of IE caused by C. striatum, it is crucial to have a multidisciplinary team able to provide timely diagnosis and prompt, appropriate treatment [2, 7]. In certain cases, conservative long-term antibiotic therapy approaches may be a viable option, but this depends on effective, tolerable oral antibiotics [2]. Although initial studies suggested susceptibility of clinical isolates of C. striatum to a broad variety of antibiotics, increased multidrug resistance has been noted in more recent reports [2]. In addition, most of the C. striatum strains characterized had multidrug resistance to antimicrobial agents generally used to treat gram-positive organisms, including penicillin, ceftriaxone, meropenem, clindamycin, and tetracycline [1, 5, 9]. On the other hand, a number of reports have shown that the lowest minimum inhibitory concentration is that of VCM [2]. Therefore, the current drug of choice for empiric treatment of IE caused by C. striatum is intravenous VCM [2, 4, 9]. Regarding duration of antibiotics, 52% of patients were treated with VCM for four to six weeks [8]. One patient was switched to oral LZD after four weeks of VCM treatment for an additional 28 days [9]. Surgical intervention should never be neglected as an option to prevent fatal cardiovascular events through the course of conservative management [3]. A literature review of IE caused by C. striatum revealed that a high percentage of patients required surgical treatment (44%), 33% of whom required valve replacement, and 11% of whom underwent a procedure to remove leads or an entire pacemaker [9]. The decision to intervene surgically should be made in a timely manner when there is a valve failure causing hemodynamic effects, large vegetation that could give rise to septic embolisms, or cases proving resistant to medical treatment [1]. Regarding the mortality, 50% of patients underwent surgical intervention and the mortality rate reported as 13–20% among surgical patients; on the other hand, the overall mortality rate among all patients was 23% [1, 3, 9].
This case study has several limitations: it reviews only a single case report. Therefore, the actual situation and nature of the disease may differ from the results of the literature review as a result of reporting bias. Additional studies are needed to further evaluate the impact of clinical presentation, laboratory findings, microbiology, imaging, treatment patterns, and outcomes. In addition, microbiological examinations do not have 100% sensitivity or specificity, meaning that we cannot fully rule out the possibility of involvement of other organisms not identified through culture, which may have caused the patient’s deterioration during her hospitalization.
In conclusion, we present the rare case of NVE in the mitral valve caused by C. striatum, which occurred in a young adult without underlying structural heart disease or indwelling cardiovascular medical devices and was successfully treated with multidisciplinary therapy. On the other hand, the patients might have had IE during the first hospitalization, namely nosocomial acquisition of IE and we might have missed the diagnosis. We should have performed more detailed examination at the onset of fever. From this point of view, timely diagnosis and appropriate treatment are essential for the management of this disease.
Data availability
All relevant data are fully presented in the manuscript.
Abbreviations
- C. striatum:
-
Corynebacterium striatum
- IE:
-
Infective endocarditis
- NVE:
-
Native valve endocarditis
- ED:
-
Emergency department
- CT:
-
Computed tomography
- GM:
-
Gentamicin
- VCM:
-
Vancomycin
- DAP:
-
Daptomycin
- LZD:
-
Linezolid
- TDM:
-
Therapeutic drug monitoring
- ICED:
-
Intracardiac electronic device
- ISCVID:
-
International Society for Cardiovascular Infectious Diseases
References
Dilmen S, Kilic S, Torun A. A rare case of aggressive infective endocarditis due to Corynebacterium striatum. Cureus. 2023;15:e44903.
Melo N, Correia C, Gonçalves J, Dias M, Garcia RM, Palma P, Duro R. Corynebacterium striatum cardiac device-related endocarditis: a case report. IDCases. 2021;27:e01371.
Zheng MM, Shang LM, Du CK, Zhang L, Sun W, Wang ZP, Zhu YC, Tian Y. Corynebacterium striatum Endocarditis after renal transplantation confirmed by Metagenomic Next-Generation sequencing: Case Report and Literature Review. Infect Drug Resist. 2022;15:4899–906.
Lee PP, Ferguson DA Jr, Sarubbi FA. Corynebacterium striatum: an underappreciated community and nosocomial pathogen. J Infect. 2005;50:338–43.
McMullen AR, Anderson N, Wallace MA, Shupe A, Burnham CA. When good bugs go bad: Epidemiology and Antimicrobial Resistance profiles of Corynebacterium striatum, an emerging Multidrug-Resistant, Opportunistic Pathogen. Antimicrob Agents Chemother. 2017;61:e01111–17.
Silva-Santana G, Silva CMF, Olivella JGB, Silva IF, Fernandes LMO, Sued-Karam BR, Santos CS, Souza C, Mattos-Guaraldi AL. Worldwide survey of Corynebacterium striatum increasingly associated with human invasive infections, nosocomial outbreak, and antimicrobial multidrug-resistance, 1976–2020. Arch Microbiol. 2021;203:1863–80.
Cabanilla MG, Jones E, Norville SV, Santana A. A case series of Corynebacterium striatum native valve infective endocarditis. J Cardiol Cases. 2022;26:194–6.
Clark M, Unnam S, Ghosh S. A review of carotid and vertebral artery dissection. Br J Hosp Med (Lond). 2022;83:1–11.
Biscarini S, Colaneri M, Mariani B, Pieri TC, Bruno R, Seminari E. A case of Corynebacterium striatum endocarditis successfully treated with an early switch to oral antimicrobial therapy. Infez Med. 2021;29:138–44.
Fowler VG, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL, Dahl A, DiBernardo L, Durante-Mangoni E, Duval X, Fortes CQ, Fosbøl E, Hannan MM, Hasse B, Hoen B, Karchmer AW, Mestres CA, Petti CA, Pizzi MN, Preston SD, Roque A, Vandenesch F, van der Meer JTM, van der Vaart TW, Miro JM. The 2023 Duke-International Society for Cardiovascular Infectious diseases Criteria for Infective endocarditis: updating the modified Duke Criteria. Clin Infect Dis. 2023;77:518–26.
Acknowledgements
None.
Funding
This work was supported by JSPS KAKENHI (grant number JP24K15491).
Author information
Authors and Affiliations
Contributions
DU, YK, RO, and YK drafted the primary manuscript. DU, MK, YS, RS, TI, EN, ST, RS, KK, SM, RT, an MS revised the manuscript. DU, SS, YH, IO, RK, SI, KM, AK, KM, HT, TK, TN, and MS involved in the clinical management and follow-up of the patient. DU re-examined histopathology and reported the findings. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the ethics committee of Juntendo University Nerima Hospital. Written informed consent to participate in this study was provided by the patient.
Consent for publication
Written informed consent was obtained from the patient to publish this case report.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Usuda, D., Kojima, Y., Ono, R. et al. Native valve endocarditis caused by Corynebacterium striatum without underlying structural heart disease or indwelling cardiovascular medical devices: a case report. BMC Infect Dis 24, 939 (2024). https://doi.org/10.1186/s12879-024-09825-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09825-9