Abstract
Background
To estimate vaccine effectiveness(VE) against COVID-19-related hospitalization for inactivated vaccines during the Omicron BF.7-predominant epidemic wave in Beijing, China.
Methods
We recruited a cohort in Beijing on 17 and 18 December 2022, collected status of vaccination and COVID-19-related hospitalization since 1 November 2022 and prospectively followed until 9 January 2023. A Poisson regression model was used to estimate the VE.
Results
16(1.15%) COVID-19-related hospitalizations were reported in 1391 unvaccinated participants; 7(0.25%) in 2765 participants with two doses, resulting in a VE of 70.89%(95% confidence interval[CI] 26.25 to 87.73); 32(0.27%) in 11,846 participants with three doses, with a VE of 65.25%(95% CI 32.24 to 81.83). The VE of three doses remained above 64% at 1 year or more since the last dose. Elderly people aged ≥ 60 years had the highest hospitalization incidence(0.66%), VE for two doses was 74.11%(95%CI: − 18.42 to 94.34) and VE for three doses was 80.98%(95%CI:52.83 to 92.33). We estimated that vaccination had averted 65,007(95%CI: 12,817 to 97,757) COVID-19-related hospitalizations among people aged ≥ 60 years during the BF.7-predominant period in Beijing.
Conclusion
Inactivated COVID-19 vaccines were effective against COVID-19-related hospitalization, especially for the elderly population who have increased risk of severe disease owing to SARS-CoV-2 infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 pandemic has been ongoing for over 3 years and has caused a total of 6.9 million deaths, as of 27 August 2023 [1]. Although the World Health Organization (WHO) declared that COVID-19 no longer constitutes a public health emergency of international concern on 4 May 2023 [2], it remains a concerning disease with a heavy burden, especially for groups that continue to be at greater risk of severe disease and mortality [3]. The Omicron variant has been the dominant variant circulating globally since early 2022. Although vaccination coverage has increased worldwide, continuing mutation of SARS-CoV-2 has posed challenges to efforts for reduce disease incidence [4]. Since November 2022, the BF.7 (BA.5.2.1.7) Omicron subvariant has been circulating in Beijing, China [5]. In comparison with earlier Omicron strains, BF.7 contains nine mutations in the spike protein and an additional mutation in the receptor binding domain region [4], leading to a greater propensity to infect individuals who have had a previous SARS-CoV-2 infection, been vaccinated, or both [6].
The Omicron variant carries multiple spike mutations, with higher transmissibility and a greater ability than previous variants to evade immunity induced by either natural infection or vaccination [7]. However, vaccination remains the most effective means of SARS-CoV-2 infection prevention and control. The literature has generally reported that a primary series plus booster dose of mRNA vaccine shows modest to high vaccine effectiveness (VE) against severe COVID-19 outcomes, including hospitalization, the need for mechanical ventilation, and death [8, 9]. The WHO granted emergency use for three vaccines produced in China, two inactivated vaccines (BBIBP-CorV, Sinopharm; CoronaVac, Sinovac) and one adenovirus type 5 (Ad5)–vectored vaccine (Convidicea, CanSinoBIO), which have been widely used in many low-income countries (LICs). However, many LICs that commonly used inactivated COVID-19 vaccines experienced multiple epidemics before emergence of the Omicron variant, and there has been little opportunity to assess the effectiveness of these vaccines against the Omicron variant in the real world.
Since the SARS-CoV-2 emerged in Wuhan City, the dynamic Zero-COVID strategy has been adopted in China to curb the transmission of COVID-19 [10]. This strict, long-term approach is aimed to fully interrupt the transmission of SARS-CoV-2 and has kept most of the Chinese population free of infection [11, 12]. Upon conditional approval of BBIBP-CorV and CoronaVac, a territory-wide vaccination program was started in December 2020 to boost population immunity to the virus in mainland China. In March 2021, older adults aged 60 years and above were encouraged to be vaccinated, then expanding to adolescents aged 12–17 years in July 2021, and to children 3–11 years in October 2021. Vaccination coverage had reached 70% by the end of August 2021 [13]. Booster doses were authorized in October 2021 for adults who received their primary series at least 6 months earlier. By November 2022, the cumulative number of administered COVID-19 vaccines had increased to 3.4 billion doses, with more than 90% of the population fully vaccinated [14]. Beijing, the capital of China, with a population of 21.8 million in 2022, initiated its vaccine roll-out campaign in 2020. According to Immunization Information Management System data, as of 1 November 2022, approximately 63.52 million vaccine doses were administered in Beijing, and 99.55% of these doses were inactivated vaccine. The mRNA COVID-19 vaccines were not used during the study period, since the first mRNA vaccine(SYS6006) was authorized for emergency use in mainland China in March 2023.
On the basis of the high coverage of COVID-19 vaccines, accumulated experience in prevention and treatment, and the virus characteristics of high transmissibility and less virulence, the Chinese government announced 20 measures on 11 November and a further 10 measures on 7 December 2022 [15], which included restricting of testing coverage, reducing the quarantine period for close contacts or inbound travelers, suspending tracing of secondary contacts, stopping region-wide mass screening, and allowing home isolation or quarantine [16]. From early December 2022 to January 2023, a major COVID-19 epidemic rapidly emerged in major cities of China, including Beijing [17]. As estimated in a dynamics model, Beijing underwent a large COVID-19 epidemic wave with a cumulative infection attack rate of 75.7% in November–December 2022 [18], primarily caused by the Omicron variant sublineage BF.7 [5]. Most people in Beijing may not have experienced COVID-19-related infection before this wave.
Previous studies [16, 19, 20] have explored the VE of inactivated vaccines against Omicron and found that they could provide high levels of protection against severe or critical COVID-19, i.e., infection with Omicron BA.2 (VE: 58%–92% for the primary series, 90%–99% for the booster dose). However, the VE of inactivated vaccines against severe illness caused by the Omicron BF.7 variant remains unclear. Although the BF.7 variant is no longer the main epidemic strain, vaccination remains a long-term process [3]. A timely summary and analysis of the VE against different epidemic strains can provide valuable evidence for optimizing the vaccination program. Therefore, we conducted a cohort study during the peak of the BF.7 epidemic in Beijing to evaluate the VE of inactivated COVID-19 vaccines against hospitalization caused by the Omicron variant.
Methods
Study design and participants
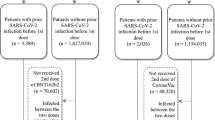
The study was designed as an ambispective cohort study. Considering that COVID-19 vaccination has not yet been expanded to children under the age of 3 years in China, we recruited a cohort of participants aged ≥ 3 years in Beijing on 17 and 18 December 2022. The study cohort was recruited using a multistage random sampling method. Namely, for each of the 17 districts of Beijing, we randomly selected five streets or townships in each district and two to eight communities in each of the selected streets or townships. Within each selected community, 10 households were recruited via random sampling and contacted via a phone number recorded by the community. Households that did not respond or refused to participate were omitted and the household next door or closest household was contacted. All members aged ≥ 3 years in the household were eligible for inclusion in the study. We excluded individuals who met the following criteria: individuals reporting a previous SARS-CoV-2 infection; those reporting COVID-19 vaccination after 1 November 2022; those who received one to two vaccine doses, with the latest dose < 14 days or three doses with the latest dose < 7 days before SARS-CoV-2 infection; and individuals who were hospitalized before 1 November 2022, to exclude recurrent hospitalization (Fig. 1).
A standardized questionnaire (Supplementary Material 1) was administered on the date of cohort entry by community health workers trained by the Beijing Center for Disease Control and Prevention to retrospectively collect baseline characteristics from participants, including sex, age, history of COVID-19 vaccination, previous SARS-CoV-2 infection (defined as positive reverse transcription polymerase chain reaction [RT-PCR] assay or antigen testing results before 1 November 2022), hospital admissions and laboratory testing from 1 November 2022 to the recruitment date. Then, from the recruitment date until 9 January 2023, participants were followed up prospectively once a week via telephone to collect information on COVID-19-related hospitalization and vaccination. Each participant was called up to three times on different days between 8:00 AM and 8:00 PM before they were classified as lost to follow-up with no response. Before 18 December 2022, citywide free SARS-CoV-2 RT-PCR testing [21] using oropharyngeal swab samples was performed for residents once every 3 days in Beijing City. After 18 December 2022, citywide SARS-CoV-2 RT-PCR screening was stopped and was only performed on request. A self-paid rapid antigen test (RAT) was made widely available during the study period. RT-PCR assay results and COVID-19 vaccination records were stored a public deposit Health Kit (Jiankang Bao) mini-program in WeChat or in the Alipay search bar, which could be accessed and validated with a user name and ID.
All participants in this study provided verbal informed consent before enrollment. Children aged < 14 years required consent from a parent or guardian.
Outcome
The primary outcome of the study was COVID-19-related hospitalization, defined as reported hospital admission that was specifically owing to SARS-CoV-2 infection, with any positive RT-PCR or positive RAT result for SARS-CoV-2, and reporting at least one of six symptoms (fever, sore throat, headache, myalgia, ageusia/loss or change in sense of taste, anosmia/loss or change in sense of smell) during the study period.
Exposures
Participants were required to self-check vaccination records using the Health Kit mini-program to avoid recall bias regarding vaccination information. For COVID-19 vaccination status, the primary series was defined as two doses of inactivated vaccine, and booster was defined as three doses of inactivated vaccine. In this study, we classified participants into four groups: those who were unvaccinated, those who were partially immunized (≥ 14 days after receipt of the first vaccine dose and before receipt of the second dose), those who completed the primary series ≥ 14 days after receipt of the second dose, and those who received a booster regimen ≥ 7 days after receipt of the third dose.
Statistical analysis
Cohen’s w effect size was used to estimate the degree of discrepancy between the distribution of our cohort and the population of Beijing for assessing sample representativeness. Population census data in 2022 were collected from the Beijing Municipal Bureau of Statistics. Cohen’s w was calculated as:
where P0i represents the proportion in cell i posited by the population overall according to the Population Census (Beijing Census and Statistics Department), P1i represents the proportion in cell i posited by the cohort, and n is the number of cells. A value of w = 0.1 represents weak discrepancy, w = 0.3 represents medium discrepancy, and w = 0.5 represents large discrepancy [22].
The association between vaccination and the risk of COVID-19-related hospitalization was estimated using a modified Poisson regression model. We controlled for several characteristics that could confound the association between vaccination and outcomes, including age, sex, and residential region. We estimated VE as 1 minus the relative risk for one vaccine dose, two doses, and three doses compared with the unvaccinated group. Moreover, our VE analysis was stratified by age group (children/adolescents aged 3–17 years, adults aged 18–59 years, and adults aged ≥ 60 years), by vaccination status (i.e., partially immunized, primary series, and booster), and by intervals since the last dose (< 180 days, 180–364 days, 365 or more days).
The difference between the estimated number of outcomes with versus without vaccination was calculated as the burden averted by vaccination. We took the age-specific hospitalization rate of the unvaccinated group in the survey, the estimated VE, and the coverage as of 1 November 2022 to estimate the number of people hospitalized in Beijing during the epidemic wave under the condition of vaccination in the real world. The burden averted by vaccination was calculated as follows [23]:
VCi and VEi represent the coverage and the VE of dose (i), respectively. We calculated the averted fraction as the number of hospitalizations averted owing to vaccination divided by the number of hospitalizations without vaccination.
We used chi-square or Fisher's exact tests to analyze categorical variables and the Wilcoxon rank-sum test to analyze continuous variables, as appropriate. A two-sided P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Results
Characteristics of participants
Among 16,201 participants aged 3 years and older included in the VE analysis, 8569 (52.89%) were female individuals, 11,392 (70.40%) were aged 18–59 years, 3169 (19.58%) were aged ≥ 60 years, and 8517 (52.57%) were urban residents. The observed differential distribution of sex, age, and residential region was largely consistent with territory-wide figures for the population of Beijing (w = 0.08–0.13) (Table 1). Most individuals in this study (11,846 of 16,201 [73.12%]) had received three doses of vaccine, 1391 (8.59%) were unvaccinated, 199 (1.23%) had received only one dose of vaccine, and 2765 (17.07%) had received two doses. Among 14,810 individuals who received a vaccine, 345 (2.33%) had received the latest dose < 3 months before testing, 1212 within 6 months (8.18%), 7832 between 6 months to 1 year (52.88%), and 5618 at 1 year or longer (37.93%) (Table 1). Figure 2 shows the coverage for different age groups in this cohort. The proportion of unvaccinated people in all age groups ranged from 6.81% to 35.58%, and this proportion was significantly increased among adults aged ≥ 60 years (13.44%), especially among those aged ≥ 80 years (35.58%).
Population composition with different COVID-19 vaccine doses in the study cohort, by age group. Partially immunized was defined as ≥ 14 days after receipt of the first vaccine dose and before receipt of the second dose. The primary series was defined as ≥ 14 days after receipt of the second dose. Booster was defined as ≥ 7 days after receipt of the third dose. The X axis represents age groups (years), and the Y axis represents the proportion of people with different immunity status
Occurrence of COVID-19-related hospitalization
During the study period, 55 (0.34%) COVID-19-related hospitalizations were reported. The risk of COVID-19-related hospitalization during this period was significantly different according to age group, residence, and vaccination status (P < 0.05). There was no significant difference between COVID-19-related hospitalizations among participants aged 3–17 years (n = 5, 0.31%) and that of adults (n = 27, 0.24%; P = 0.78), which were both lower than hospitalizations among elderly adults (n = 21, 0.66%; P < 0.001). Hospitalizations among adults over 70 years old (n = 13, 1.24%) were significantly greater than those among adults aged 60–69 years (n = 8, 0.38%; P = 0.005) (Table 1). COVID-19-related hospitalizations in the two-dose (n = 7, 0.25%) and three-dose (32, 0.27%) vaccine groups were significantly lower than those in the unvaccinated group (n = 16, 1.15%; P < 0.001) (Table 2).
Estimated VE
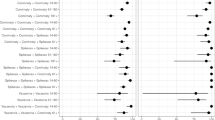
Using unvaccinated participants as the reference group, the primary series provided a VE of 70.89% (95% credible interval [CI] 26.25 to 87.73) against COVID-19-related hospitalization, and booster vaccine yielded a VE of 65.25% (95% CI 32.24 to 81.83). VE among elderly adults was 74.11% (95% CI − 18.42 to 94.34) for the primary series and 80.98% (95% CI 52.83 to 92.33) for booster vaccination. Within 180 days after vaccination. the estimate VE was 77.60% for the primary series and 71.56% for booster vaccination, but both were not significant. Within 180 days to 364 days after vaccination, VE was 66.19%(95% CI 9.35 to 87.40) for the primary series and 64.49%(95% CI 26.45 to 82.86) for booster vaccination The VE for booster vaccination remained above 64% at ≥ 1 year since the last dose (Table 2).
Estimated hospitalizations averted with inactivated COVID-19 vaccines
We estimated that inactivated COVID-19 vaccines averted 65,007 (95% CI 12,817 to 97,757) COVID-19-related hospitalizations among adults aged ≥ 60 years during the 2-month BF.7-predominant epidemic wave in Beijing, corresponding to an averted fraction of 67.51% (95% CI 37.74% to 86.67%).
Discussion
With a large infection-naive population and COVID-19 vaccines in widespread use, Beijing represents a unique environment for monitoring VE against the Omicron subvariant BF.7. In this study, we evaluated the real-world VE of inactivated virus COVID-19 vaccines in China. The results showed when given according to the recommended schedule of a two-dose primary series followed by a booster dose 6 months later, the VE against Omicron BF.7-related hospitalization was 70.89% and 65.25%, respectively. Greater effectiveness was observed among adults aged 60 years or older with booster vaccination. No reduction was observed in VE of booster starting from 1 year after the latest dose of vaccine.
A hospitalization incidence of 0.34% was observed in participants aged ≥ 3 years throughout the study period. Two similar studies of Omicron BA.2 infections in New England [24] and China [16], in which the study population had similar vaccination coverage levels to ours (New England: 9.8% unvaccinated, 3.5% partially vaccinated, 21% received the primary series, 66% received the booster dose; China: 6.6%, 2.5%, 41%, 50%, respectively) found that 0.4% of BA.2-infected patients required admission to the intensive care unit, and 0.15% had severe/critical SARS-CoV-2 infection with BA.2, respectively. The proportion of elderly adults who were hospitalized (0.66%) in this study was higher than that among participants aged 3–17 years (0.31%) and adults (0.24%), and this difference across age groups is consistent with the proportions for hospitalization owing to infection with the Omicron and Delta strains in the United Kingdom [25]. Omicron and its sublineages (including BA.1, BA.2, BA.4, BA.5, XBB, and BQ.1) have become the dominant circulating variants worldwide [3]. However, older adults and people with comorbidities continue to be at greatest risk of severe disease and mortality [3].
Our findings highlight the importance of the primary series and booster in reducing the risk of COVID-19-related hospitalization. Our results are consistent with other evaluations of inactivated vaccines produced in China against Omicron-caused infection (BA.5 or BA.2) in infection-naive populations (VE: 58%–92% with the primary series, 90%–99% with the booster dose) [16, 19, 20, 26]. Compared with age-specific studies of inactivated vaccines, our VE point estimates (44.66% after two doses) in the pediatric population during the Omicron-predominant wave were generally similar to the results of studies in Brazil [27] and Chile [28] with VE estimates after two doses of 40%–60%. The older adults in our study had a VE against hospitalization of 74% and 81% after two and three inactivated doses, respectively, results that were similar to those of a study in Hong Kong [20] with VE of CoronaVac against severe or fatal disease of 69.9% for adults aged 60 years or older at a median 125 days after two doses and additional benefit at a median of 61 days after the third dose. The existing mRNA vaccines showed cross-immunity in children, adolescents, and adults (VE 70%–90%) during the Omicron epidemic wave [8, 9, 20, 29]. Our findings reinforce the high VE of inactivated vaccine against COVID-19 related hospitalization owing to BF.7 variants. Although the Omicron BF.7 subvariant carries multiple spike mutations, the prototype vaccine retained some cross-immunity against mutant strains, which indirectly suggested potential benefit from cellular immunity. Although the point estimate of vaccine effectiveness shows VE in the booster group(65.25%) is lower than primary series group(70.89%), but judging from the 95% confidence interval, relative VE of 2 doses as compared to 3 doses is supposed to be non-significant. In contrast, VE among elderly adults was higher in the booster group (80.98%). However, comparisons of these point estimates do not represent objective facts, these differences between different groups needs to be further clarified by studying in the molecular and cellular immunology. VE of booster vaccination with narrow confidence intervals and statistical significance was obtained for the elderly population, who have a relatively high hospitalization rate, and we estimated that inactivated COVID-19 vaccines averted approximately 0.06 million (95% CI 0.01 to 0.10 million) COVID-19-related hospitalizations among adults aged ≥ 60 years in Beijing, which helped to relieve hospital overload.
The length of time since the latest vaccination had a minimal waning effect on VE against COVID-19 related hospitalization. In our study, the point estimate VE decreased 4.57%–7.07% after 6 months of vaccination compared with VE within 6 months (77.60%,71.56%), but VEs within 6 months were not significantly. Within 6 months to one year after vaccination, VE was 64.49% for booster vaccination and remained above 64% at more than 1 year. Although humoral immunity mediated by antibodies blocks SARS-CoV-2 from entering host cells, thereby preventing infection [30], SARS-CoV-2-specific CD4 + and CD8 + T cells appear to be responsible for limiting disease severity [31]. Despite a rapid reduction in serum antibody titers, memory T cells are more durable and may contribute to protection from severe disease. In one case–control study in Hong Kong, VE against hospitalization during the Omicron-dominant period was maintained for at least 6 months after the second dose of both CoronaVac (74.0%, 95% CI 71.8% to 75.8%) and BNT162b2 (77.4%, 95% CI 75.5% to 79.0%) vaccines; the booster dose was capable of restoring VE and maintaining protection over time [32]. Other previous studies have also demonstrated sustained protection against severe outcomes despite waning protection against infection in the general population [20, 33, 34].
In comparison with other countries, China initiated its vaccine roll-out campaign relatively earlier [35]. According to the surveyed coverage, the proportion of the population receiving at least one COVID-19 vaccine dose has exceeded 90%. Beijing had already raised the primary series coverage to a high level before the Omicron epidemic wave and quickly carried out the booster program for people over 18 years old. Therefore, higher coverage among elderly adults effectively averted 67.51% of hospitalizations among adults aged 60 years and above. Despite vaccination showing a relatively good VE against COVID-19-related hospitalization, 40% of adults aged 80 years and older remained unvaccinated or partially vaccinated after this Omicron epidemic wave in Beijing. This is consistent with reports of other coverage investigated in China [36], for the oldest age group, with higher proportion functional dependency or chronic diseases, more common in vaccination hesitancy, more lack of knowledge about vaccines may be the reasons for the highest percentage of unvaccinated individuals. Older adults were still listed as the highest priority groups in the COVID-19 vaccination guidance [37]. To prevent excessive disease severity and mortality owing to vaccination hesitancy [36], devoting greater effort toward vaccinating the whole population, especially the elderly population, is warranted given the uncertainty regarding future epidemic waves with frequent emergence of virus variants.
A strength of this study involves evaluation of the protective effect of the prototype inactivated vaccine against hospitalization associated with BF.7 strains in a largely SARS-CoV-2 infection-naive population. It is inevitable that, in the context of multiple epidemics in countries around the world, most people have experienced one or more infections during an epidemic wave involving the Omicron variant strains. In evaluating the protective effect of vaccination against Omicron, the confounding effects of previous infection with SARS-CoV-2 variants cannot be excluded.
Our study had several limitations. First, to improve compliance with the telephone survey, the survey content was simplified and the number of vaccine doses were collected without distinguishing the types of vaccines. Considering that 0.45% of individuals received the Ad5 COVID-19 vaccine, protein subunit COVID-19 vaccine, or heterologous booster following the primary series, the VE of inactivated vaccines could be fully evaluated in this study. Second, because most people in Beijing had received three doses of COVID-19 vaccine before the investigation, the sample of people who received zero, one, and two doses in the cohort was relatively small, and thus the possibility of bias was addressed in stratified analysis. Third, owing to the low proportion of hospitalizations among children, adolescents, and adults aged 18–59 years, the confidence intervals were too wide to reliably estimate VE. Fourth, information about the underlying medical conditions were not collected, it could affect the outcome of COVID-19 and individual COVID-19 vaccination intentions, which should be a confounder in VE analysis. Last, physicians might have different criteria to hospitalize COVID-19 patients, if some physicians requiring more hospitalization of non-vaccinated patients because they are more likely to get severe diseases, the occurrence of outcomes may change between the vaccinated and non-vaccinated populations.
In conclusion, two or three doses of inactivated vaccine were effective in preventing COVID-19-related hospitalizations, especially three doses in elderly adults, during the Omicron BF.7 variant-predominant epidemic wave in Beijing, China. Our findings highlight the protective effect of inactivated vaccines against COVID-19 related hospitalization. Considering their rapid development, higher yield, and good stability during storage, inactivated vaccines can be considered for use as reserve vaccines for use during the early stage of any future outbreaks involving other emerging infectious diseases.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization. Weekly epidemiological update on COVID-19 - 1 September 2023, https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---1-september-2023; 2023. Accessed 9 Sept 2023.
World Health Organization. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic, https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic; 2023. Accessed 5 May 2023.
World Health Organization. WHO SAGE roadmap on uses of COVID-19 vaccines in the context of OMICRON and substantial population immunity (Latest update: 30 March 2023). https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Roadmap. Accessed 20 May 2023.
Singh JK, Anand S, Srivastava SK, et al. Is BF.7 more infectious than other Omicron subtypes: Insights from structural and simulation studies of BF.7 spike RBD variant. Int J Biol Macromol. 2023;238:124–54.
Pan Y, Wang L, Feng Z, et al. Characterisation of SARS-CoV-2 variants in Beijing during 2022: an epidemiological and phylogenetic analysis. Lancet (London, England). 2023;401(10377):664–72.
Qu P, Evans JP, Faraone JN, et al. Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe 2023; 31(1),9–17.e13.
World Health Organization. Tracking SARS-CoV-2 variants. https://www.who.int/activities/tracking-SARS-CoV-2-variants/; 2023. Accessed 5 May 2023.
Chemaitelly H, Ayoub HH. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13(1):3082.
Tenforde MW, Self WH, Gaglani M, et al. Effectiveness of mRNA Vaccination in Preventing COVID-19-Associated Invasive Mechanical Ventilation and Death - United States, March 2021-January 2022. MMWR. 2022;71(12):459–65.
Li Z, Guan X, Mao N, Luo H, et al. Antibody seroprevalence in the epicenter Wuhan, Hubei, and six selected provinces after containment of the first epidemic wave of COVID-19 in China. Lancet Reg Health West Pac. 2021;8: 100094.
Mallapaty S. China is relaxing its zero-COVID policy - here’s what scientists think. Nature. 2022;612(7940):383–4.
He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–5.
National Health Commission of the People's Republic of China. Make every effort to prevent and control the COVID-19 epidemic, http://www.nhc.gov.cn/ ; 2022. Accessed 5 May 2023.
Chinese Center for Disease Control and Prevention. Epidemic situation of novel coronavirus infection in China, https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202302/t20230201_263576.html, 2022. Accessed 5 Feb 2023.
The State Council PRC. "New Tens" for the Control and Prevention of COVID-19, December 7, 2022, http://www.nhc.gov.cn/xcs/gzzcwj/202212/8278e7a7aee34e5bb378f0e0fc94e0f0.shtml;2022. Accessed 7 Apr 2023.
Tang L, Wang FZ, Rodewald LE, et al. Real-world effectiveness of primary series and booster doses of inactivated COVID-19 vaccine against Omicron BA.2 variant infection in China: a retrospective cohort study. J Infect Dis. 2023;228(3):261–9.
Liu J, Ding F, Wu Y, et al. Trends of SARS-CoV-2 Infection in Sentinel Community-Based Surveillance After the Optimization of Prevention and Control Measures - China, December 2022-January 2023. China CDC weekly. 2023;5(7):159–64.
Leung K, Lau EHY, Wong CKH. Estimating the transmission dynamics of SARS-CoV-2 Omicron BF.7 in Beijing after adjustment of the zero-COVID policy in November-December 2022. Nat Med, 2023;29(3):579–582.
Huang Z, Xu S, Liu J, et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA.2 variant infection, severe illness, and death. BMC Med. 2022;20(1):400.
McMenamin ME, Nealon J, Lin Y, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–43.
Feng Z, Zhang Y, Pan Y, et al. Mass screening is a key component to fight against SARS-CoV-2 and return to normalcy. Medical Review. 2022;2(2):197–212.
Tsang NNY, So HC, Cowling BJ, et al. Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study. Lancet Infect Dis. 2023;23(4):421–34.
Kostova D, Reed C, Finelli L, et al. Influenza illness and hospitalizations averted by influenza vaccination in the United States, 2005–2011. PLoS ONE. 2013;8(6): e66312.
Strasser ZH, Greifer N, Hadavand A, et al. Estimates of SARS-CoV-2 Omicron BA.2 Subvariant Severity in New England. JAMA network open 2022;5(10):e2238354. https://doi.org/10.1001/jamanetworkopen.2022.38354.
Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet (London, England) 2022;399(10332):1303–1312.
Yan VKC, Cheng FWT. Effectiveness of BNT162b2 and CoronaVac vaccines in preventing SARS-CoV-2 Omicron infections, hospitalizations, and severe complications in the pediatric population in Hong Kong: a case-control study. 2023;12(1):2185455.
Florentino PTV, Alves FJO. Vaccine effectiveness of CoronaVac against COVID-19 among children in Brazil during the Omicron period. Nat Commun. 2022;13(1):4756.
Jara A, Undurraga EA. Effectiveness of CoronaVac in children 3–5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat Med. 2022;28(7):1377–80.
Tan SHX, Cook AR, Heng D, et al. Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N Engl J Med. 2022;387(6):525–32.
Wherry EJ, Barouch DH. T cell immunity to COVID-19 vaccines. Science. 2022;377(6608):821.
Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183(4):996-1012.e1019.
Wei Y, Jia KM, Zhao S, et al. Estimation of Vaccine Effectiveness of CoronaVac and BNT162b2 Against Severe Outcomes Over Time Among Patients With SARS-CoV-2 Omicron. JAMA Netw Open. 2023;6(2):e2254777.
Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. MMWR. 2022;71(7):255–63.
Suah JL, Husin M, Tok PSK, et al. Waning COVID-19 Vaccine Effectiveness for BNT162b2 and CoronaVac in Malaysia: An Observational Study. Int J Infect Dis. 2022;119:69–76.
Zhang J, Yang H, Yang M, et al. The role of vaccines in COVID-19 control strategies in Singapore and China. Health Policy Technol. 2022;11(2): 100620.
Wang G, Yao Y, Wang Y, et al. Determinants of COVID-19 vaccination status and hesitancy among older adults in China. Nat Med. 2023;29(3):623–31.
World Health Organization. SAGE updates COVID-19 vaccination guidance, https://worldhealthorganization.createsend1.com/t/d-e-zklkult-ijidulchl-y/, 2023. Accessed 28 Mar 2023.
Acknowledgements
Not applicable.
Funding
This study was supported by Beijing Natural Science Foundation-Haidian District Joint Fund [number L212056] and the High Level Public Health Technical Talent Training Plan [Xuekedaitouren-01–03].
Author information
Authors and Affiliations
Contributions
Study design and supervision: Peng Yang, Daitao Zhang, and Luodan Suo. Project administration and data collection: Ying Sun, Jiaojiao Zhang, Jiaxin Ma and Jia Li. Analysis and interpretation of data: Dan Zhao, Juan Li, Xiaomei LI, Ying Ma, and Zhiqiang Cao. Writing of original manuscript draft: Dan Zhao and Ying Sun. Review and editing of manuscript: Peng Yang, Daitao Zhang, Quanyi Wang and Luodan Suo. All contributing authors approved the submitted manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board and Human Research Ethics Committee of the Beijing Center for Disease Prevention and Control (the ethics code is 2022–01). This study was performed in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, D., Sun, Y., Li, J. et al. Effectiveness of inactivated COVID-19 vaccines in preventing COVID-19-related hospitalization during the Omicron BF.7-predominant epidemic wave in Beijing, China: a cohort study. BMC Infect Dis 24, 991 (2024). https://doi.org/10.1186/s12879-024-09889-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09889-7