Abstract
Background
In addition to antibiotic resistance, persistence is another cause of treatment failure in bacterial infections, representing a significant public health concern. Due to a lack of adequate data on clinical isolates, this study was initiated to investigate persistence in clinical isolates in Burkina Faso.
Methods
Eighty (80) clinical isolates, including 32 Pseudomonas aeruginosa, 41 Staphylococcus aureus, and 7 Salmonella sp. obtained from clinical laboratories in Burkina Faso, were analyzed to assess their susceptibility to ciprofloxacin and gentamicin, as well as to determine the presence of persistence genes. The effects of ciprofloxacin and gentamicin on persister formation were evaluated by conducting colony counts at 1, 3, 5, 7, and 20 h after exposing the bacteria to high concentrations of these antibiotics.
Results
Results showed high sensitivity to both antibiotics (72.5% for ciprofloxacin and 82.5% for gentamicin). Persister formation occurred in Staphylococcus aureus with gentamicin and in Salmonella sp. with ciprofloxacin, while Pseudomonas aeruginosa did not form persisters. The mazF gene was found in 28.13% of P. aeruginosa and 2.44% of S. aureus isolates, and the hipA gene in 28.57% of Salmonella sp. None of the relE1 or relE2 genes were detected.
Conclusions
The study revealed high sensitivity in clinical bacterial isolates to ciprofloxacin and gentamicin. Staphylococcus aureus and Salmonella sp. showed persister formation under antibiotic stress, with low frequencies of the studied persistence genes. These findings enhance understanding of clinical bacterial behavior and inform strategies against antibiotic-resistant infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial infections pose a global health threat, driving widespread antibiotic use [1]. Under antibiotic pressure, bacteria have evolved intricate survival mechanisms, leading to treatment failures [2, 3]. While antibiotic-resistant mutants are well-known causes of treatment failure [4], a small subpopulation (0.001–1%) of bacteria, even when sensitive to antibiotics, transiently evade antibiotic action, known as persisters [5, 6].
First described in 1944 by Joseph Bigger [7], who noted the survival of a subset of Staphylococcus under lethal penicillin doses, persistence refers to bacteria’s ability to survive exposure to bactericidal drugs [8]. These bacteria do not grow in the antibiotic’s presence but resume growth once the stress is removed [9, 10]. Studies found recurrent infections like Salmonella and Streptococcus pyogenes were often caused by the same genovar, suggesting persistent bacteria drive these infections, leading to increased antibiotic use [11, 12]. Bacterial persistence is a significant public health concern as infections from persisters lead to antibiotic failure [13] and contribute to antibiotic resistance [11, 14]. This poses a real problem; especially as current trends are to reduce the use of antibiotics. The study of bacterial persistence is therefore of great importance to public health.
In vitro, bacteria exhibit various phenotypes and destruction kinetics under antibiotic treatment, such as resistant, persistent, tolerant, and susceptible bacteria. Persistent bacteria exhibit a characteristic biphasic killing curve, where most susceptible cells are rapidly eradicated by a high antibiotic concentration, while a small proportion survives longer due to the presence of a mixed bacterial population [15, 16]. To form persisters, bacteria utilize various mechanisms, including toxin-antitoxin (TA) systems [17]. There are currently eight types (I to VIII) of TA systems found in nearly all bacterial strains [18]. TA systems consist of a toxin that inhibits growth and an antitoxin that neutralizes the toxin’s effects [19]. Among these, Type II is extensively studied, widely distributed, and heavily implicated in bacterial persistence [20, 21].
Unfortunately, despite the clinical significance of persistent cells in bacterial infections, there’s a scarcity of studies on this phenomenon among clinical bacterial isolates, especially in Africa. Most research has focused on laboratory strains. Hence, this study was initiated to evaluate antibiotic susceptibility and assess persister cell formation in clinical isolates of Pseudomonas aeruginosa, Staphylococcus aureus, and Salmonella sp. after exposure to ciprofloxacin and gentamicin. Additionally, due to the potential involvement of TA systems in bacterial persistence, we investigated four type II TA system genes (mazF, hipA, relE1, relE2) in the studied isolates.
Materials and methods
Ethical considerations
Approval for the present study protocol was obtained from the Institutional Ethics Committee of the CERBA/LABIOGENE in its deliberation N° 2022-25/09–015 of 5 September 2022. All study participants or their guardians provided their free and informed consent in accordance with the Helsinki Declaration.
Type and study period
This study was a descriptive cross-sectional investigation focusing on bacteria collected from the laboratories of Hopital Saint Camille de Ouagadougou (HOSCO) and Centre de Recherche Biomoléculaire Pietro Annigoni (CERBA) in Ouagadougou. The collection of bacterial strains occurred between October 2022 and February 2023.
Bacterial strains
Eighty (80) bacterial isolates, comprising 41 Staphylococcus aureus, 32 Pseudomonas aeruginosa, and 07 Salmonella sp., were obtained from diverse human clinical samples. Bacterial suspensions of these isolates in Luria-Bertani (LB) broth supplemented with 20% glycerol were frozen and stored at the Laboratoire de Biologie et de Génétique Moléculaire (LABIOGENE), Université Joseph KI-ZERBO, for subsequent analysis.
Antibiotic susceptibility testing
Antibiotic susceptibility testing was conducted following the manual of procedures for performing antibiograms in Burkina Faso [22], which aligns with the 2015 recommendations of the Antibiogram Committee of the French Microbiology Society and the European Society of Clinical Microbiology and Infectious Diseases (CA-SFM/EUCAST-2015) [23]. The disk diffusion method (Kirby-Bauer) using Mueller-Hinton (MH) agar was employed. A bacterial suspension with a turbidity matching that of the 0.5 McFarland standard was prepared from a bacterial culture obtained after 24 h incubation. MH agar plates were inoculated, and gentamicin and ciprofloxacin antibiotic disks were applied. The plates were then incubated at 37 °C, and readings were taken after 24 h. Inhibition diameters were measured using vernier calipers, and the results were used to classify isolates as susceptible or resistant based on CA-SFM/EUCAST-2015 recommendations [23]. For sensitive strains, the higher critical concentrations of these antibiotics were considered.
Formation of persisters
For persistence testing, 5 bacterial isolates per species were selected. Only isolates sensitive to both gentamicin and ciprofloxacin were included. A random selection of 5 isolates per species was made to identify those used for this purpose. To assess the presence of persisters, bacterial strains were reactivated and plated on MH agar. Existing protocols were adjusted and customized for this study [24,25,26,27,28,29]. Bacterial suspensions in LB broth were incubated at 37 °C on a shaker-incubator (New Brunswick Innova® 44) set at 200 rpm until the optical density at 600 nm, measured using Biomate 3 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), reached a range between 0.2 and 0.3. Two tubes of bacterial suspension were prepared for each isolate. Gentamicin was added to one tube and ciprofloxacin to the other, both at very high concentrations. The entire set was then incubated at 37 °C. The antibiotics used for the study were Gentamicin 80 mg/2 mL (Panpharma, France) and Ciprofloxacin (Cipronat® 200 IV) 200 mg/100 mL (Dafra Pharma GmbH, Switzerland). Concentrations used were 100 times the upper critical concentration as follows: Ciprofloxacin at 0.1 mg/mL and Gentamicin at 0.1 mg/mL for Staphylococcus aureus, Ciprofloxacin at 0.1 mg/mL and Gentamicin at 0.4 mg/mL for Pseudomonas aeruginosa and Ciprofloxacin at 0.1 mg/mL and Gentamicin at 0.4 mg/mL for Salmonella sp.
At 1, 3-, 5-, 7-, and 20-hours post-antibiotic addition, the culture underwent two washes with 0.85% sterile saline to eliminate the antibiotic. Washing involved removing 100 µL of the antibiotic-containing bacterial suspension, adding 500 µL of sterile 0.85% saline, centrifuging and discarding the supernatant. Two serial dilutions (1/100) were then performed, with the final dilution being inoculated onto MH agar plates. The plates were incubated at 37 °C for 24 h. Subsequently, colonies on the agar plates were counted using the APD Colony App Lite cell phone application [30]. Time-kill curves for each isolate were plotted to analyze the typical biphasic curve. An antibiotic-free bacterial culture of each species served as a control.
Detection of bacterial persistence genes
Bacterial DNA extraction was conducted via heat shock following a protocol previously outlined [31]. Bacterial colonies were suspended in 1 mL of distilled water and subsequently boiled in a water bath for 10 min. Centrifugation was carried out at 1000 rpm for 5 min, and the resulting supernatant was collected in Eppendorf tubes. The quantity and purity of DNA were assessed using a BioDrop spectrophotometer (BioDrop, Cambridge, UK).
PCR was used to detect the presence of TA type II system genes (mazF, hipA, relE1, and relE2) using specific primers. Amplification was conducted in GeneAmp® PCR System 9700 thermal cycler (Applied Biosystems, USA) with a program comprising an initial denaturation step at 94 °C for 4 min, followed by 35 cycles of denaturation at 95 °C for 45 s, annealing at 60 °C for 45 s, and extension at 72 °C for 30 s. A final extension step at 72 °C for 5 min was included. Each PCR reaction had a final volume of 25 µl, containing 4 µl of master mix (FIREPol® Master Mix Ready to Load, 5X, Solis BioDyne), 0.5 µl of each primer (10 µM), 1 µl of DNA extract, and sterile PCR water to reach the final reaction volume.
PCR products were separated by electrophoresis (Mupid® One) on a 1.5% agarose gel (Cleaver Scientific, UK) containing ethidium bromide. Electrophoresis was conducted in 1X Tris-Acetate-EDTA buffer for 40 min at 100 volts alongside a 100 bp molecular weight marker (100 bp Ladder Ready to Load, Solis BioDyne). After migration, amplicons were visualized under UV light using the Trans-illuminator E-BOX and photographed. The primers used for the genes are listed in Table 1.
Statistical analysis
Data were entered into Excel 2019. Statistical analyses and the elaboration of figures were carried out using STATA software version 14.
Results
Our study population consisted of 80 bacterial isolates, including 32 Pseudomonas aeruginosa, 41 Staphylococcus aureus and 7 Salmonella sp. The bacteria were isolated from diverse biological samples, with most isolates coming from pus (48/80) as indicated in Table 2.
Susceptibility of bacterial strains to the antibiotics tested
Our results revealed high sensitivities of clinical isolates to the two antibiotics used: ciprofloxacin (72.5%) and gentamicin (82.5%). These findings are detailed in Table 3. It’s noteworthy that 53 isolates, specifically 27 P. aeruginosa, 21 S. aureus, and 5 Salmonella sp., exhibited sensitivity to both ciprofloxacin and gentamicin.
Bacterial persistence under antibiotic stress
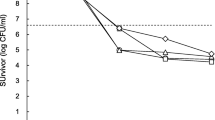
In Pseudomonas aeruginosa, no persistent phenotype was observed (Fig. 1). Among the isolates, P.a_54 and P.a_60 were sensitive to ciprofloxacin, whereas P.a_13, P.a_47, and P.a_79 displayed distinctive biphasic curves, indicating initial destruction followed by a gradual increase in Colony Forming Unit (CFU) (Fig. 1A). Regarding gentamicin, only P.a_54 was sensitive, while the remaining four isolates exhibited resistance (Fig. 1B).
In S. aureus, we present the results of testing on four strains. At the end of the manipulations, we realized that the fifth had been contaminated and the results were not usable. Persistent cells were observed in the presence of gentamicin, as evidenced by the biphasic curves (S.a_9 and S.a_84) in Fig. 2B, indicating initial destruction followed by a consistent or gradually decreasing number of CFUs. Additionally, two isolates (S.a_9 in Fig. 2A and S.a_77 in Fig. 2B) displayed distinctive biphasic curves similar to those described for P. aeruginosa. The remaining isolates exhibited a susceptible phenotype.
In Salmonella sp., persistent cells were observed in the presence of ciprofloxacin, as evidenced by the biphasic curve (Sal_20) in Fig. 3A, showing initial destruction followed by a gradual decrease in CFUs. The remaining four isolates displayed a susceptible phenotype. Additionally, all five isolates were sensitive to gentamicin, as shown in Fig. 3B.
Persistence genes
The outcomes of the persistence gene search are compiled in Table 4. None of the bacteria harbored more than one of the targeted genes. The gel images displaying the amplicons of persistence genes are depicted in Fig. 4.
Discussion
Despite the public health problem caused by bacterial persistence, few studies have addressed this phenomenon among clinical bacterial isolates, particularly in Africa. Our objective was to assess the susceptibility profile of bacteria in our study population to two commonly used antibiotics in Burkina Faso. Subsequently, we aimed to demonstrate the formation of bacterial persistence after exposing these isolates to the antibiotics. Lastly, we sought to identify four genes (mazF, hipA, relE1, relE2) of the type II TA system associated with this phenomenon in these clinical isolates.
More than half of the 80 bacterial strains in our study originated from pus samples. This is consistent with the fact that our study population primarily comprised P. aeruginosa and S. aureus, which are pyogenic bacteria commonly found in such samples. This observation is also reflected in the various laboratory antimicrobial resistance surveillance reports in Burkina Faso, which have been published annually since 2018 [22, 32,33,34]. Ciprofloxacin and gentamicin exhibited favorable activity against all bacterial strains in the current study, with rates of 72.5% and 82.5%, respectively. These antibiotics are recognized as broad-spectrum agents renowned for their potent bactericidal effects on both Gram-negative and Gram-positive bacteria. Consequently, they are extensively utilized in Burkina Faso for treating bacterial infections.
Our findings regarding Salmonella sensitivity (71.4% to ciprofloxacin and 85.7% to gentamicin) are similar to national data. The latest 2022 report on national surveillance of antimicrobial resistance in Burkina Faso [34] revealed sensitivities of 75.76% and 87.10% to ciprofloxacin and gentamicin, respectively. Similarly, national sensitivities for gentamicin in P. aeruginosa (96.7%) and S. aureus (70.7%) closely mirrored our results, with national figures at 71.79% for P. aeruginosa and 78.20% for S. aureus. However, notable differences were observed in ciprofloxacin sensitivities. National data indicated low sensitivities for P. aeruginosa (19.38%) and S. aureus (18.21%), whereas our study found much higher sensitivities at 84.4% for P. aeruginosa and 63.4% for S. aureus. Other studies have also reported low sensitivities of P. aeruginosa, such as 28.57% to gentamicin and 33.57% to ciprofloxacin [35]. These findings highlight the presence of still sensitive strains and emphasize the need to intensify efforts to curb the spread of resistance genes. The high antibiotic sensitivities observed in bacterial isolates during our study led to a significant number of strains being sensitive to both ciprofloxacin and gentamicin (53 strains in total, comprising 27 P. aeruginosa, 21 S. aureus, and 5 Salmonella sp.). This enabled us to employ random sampling to select five strains of each species for investigating bacterial persistence.
P. aeruginosa, S. aureus, Salmonella and many other bacteria have been recognized as harboring persistent cells [6]. To examine biphasic killing curves, we counted CFUs up to 20 h after antibiotic exposure in exponential growth phases. Out of the 5 Pseudomonas aeruginosa isolates tested with ciprofloxacin, 2 demonstrated susceptibility. The remaining 3 displayed distinctive biphasic curves, indicating rapid destruction followed by a progressively increasing number of CFUs, suggesting the proliferation of resistant bacteria. This phenomenon could be indicative of heteroresistance, characterized by a heterogeneous behavior where a subset of bacterial cells can multiply in the presence of antibiotic concentrations lethal to most of the population. Since the population primarily consists of susceptible bacteria, the Minimum Inhibitory Concentration (MIC) of the overall population is comparable to that of a susceptible strain, which might not have been detected by the initial susceptibility test. These curves exhibited similar characteristics to those developed by Gollan et al [36]. , outlining the heterogeneous behavior of a resistant bacterial subpopulation surviving in the presence of antibiotics at concentrations fatal to the rest of the population. Considering the experiments conducted by Mlynarcik and Kolar [37], this could also indicate acquired resistance to ciprofloxacin, as they also observed this phenomenon while testing P. aeruginosa with tobramycin. On the contrary, in gentamicin tests, only one strain exhibited a sensitive phenotype, while the remaining four displayed a resistant phenotype, despite their initial sensitivity. This may suggest resistance developed through exposure to antibiotics or acquired resistance via resistance genes. In summary, our findings suggest that ciprofloxacin and gentamicin did not induce persistence formation in the tested P. aeruginosa isolates. However, other studies have shown persistence formation in P. aeruginosa in the presence of ciprofloxacin [17]. A larger-scale study would have provided more insight into identifying persistent cells.
Out of the planned 5 isolates of Staphylococcus aureus, only 4 were tested. Among these, 3 isolates exhibited a phenotypically sensitive response to ciprofloxacin, indicating the efficacy of this antibiotic against S. aureus strains. However, one isolate displayed a phenotype suggestive of heteroresistance when exposed to ciprofloxacin, similar to the observed behavior in P. aeruginosa. Interestingly, ciprofloxacin did not induce persistence in S. aureus. Conversely, in the presence of gentamicin, 2 isolates demonstrated persistence phenotypes. Our findings revealed that gentamicin could induce bacterial persistence in S. aureus. This aligns with other studies that have also reported the formation of persistence in S. aureus when exposed to gentamicin [38]. Our findings are inconsistent with certain studies that have highlighted antibiotic-dependent persistence. For instance, a study examining 10 clinical isolates of Staphylococcus revealed the formation of persisters in over half of the isolates when exposed to vancomycin, oxacillin, ciprofloxacin, and penicillin. However, fewer isolates displayed persistence in the presence of gentamicin [39].
All 5 strains of Salmonella exhibited a gentamicin-sensitive phenotype. However, in the presence of ciprofloxacin, only one strain displayed a biphasic curve indicative of a bacterial persistence phenotype, while the remaining four showed sensitive phenotypes. Our findings highlighted the superior activity of gentamicin against the tested Salmonella strains. Some authors suggest that the persistence of these bacteria may occur within spleen and liver macrophages [40, 41]. Contrary to expectations, our results demonstrated that ciprofloxacin was capable of inducing persistence in Salmonella. Interestingly, ciprofloxacin is commonly prescribed as the first-line treatment for salmonellosis in Sub-Saharan Africa, including Burkina Faso, where these infections are prevalent. This observation might contribute to the high frequency of recurrent infections with these bacteria and the alarming rise in Salmonella resistance to fluoroquinolones, a concerning global trend.
The mazEF system is a well-studied type II TA module found in numerous bacteria [6, 42, 43]. However, in our study, the mazF gene was not detected in Salmonella sp., possibly due to the limited number (7) of isolates examined. We did identify the mazF gene in a small percentage of P. aeruginosa (28.13%) and S. aureus (2.44%) isolates. Our findings contrast with previous literature, which often reports a higher prevalence of the mazF gene in clinical isolates. For instance, a study in Iran [35] found the gene in 85.71% of 140 clinical P. aeruginosa strains, while in Turkey, it was present in 89.1% of 148 Staphylococcus isolates [44]. Additionally, another study reported universal mazF gene presence across all strains examined (78 S. aureus and 42 P. aeruginosa) [45]. A study reported a very low frequency (1.42%) of the hipA gene in 140 clinical strains of P. aeruginosa [35], a finding that aligns with our results as we did not detect this gene in any P. aeruginosa isolates. With a larger sample size, we might have identified at least one isolate with the hipA gene. Similarly, although some studies have identified the hipA gene in Salmonella [21], we only detected it in 2 Salmonella isolates in our study. Despite being the first gene associated with bacterial persistence [46], hipA does not appear to be prevalent in clinical isolates.
In our study, we did not detect the presence of relE1 or relE2 genes. However, these genes are recognized to play a role in bacterial persistence, particularly in clinical strains of P. aeruginosa as noted by Fernández-García et al. [42]. Additionally, studies have reported the presence of these genes in clinical isolates of S. aureus [38] and Klebsiella pneumoniae [47].
Conclusion
The threat of antibiotic resistance is well-documented in Burkina Faso, but bacterial persistence remains less explored. Our study aimed to fill this gap by investigating persistence in clinical isolates. Our findings demonstrate that these isolates can form persister cells when exposed to commonly used antibiotics like ciprofloxacin and gentamicin, and they possess type II TA system genes associated with persistence. Understanding bacterial persistence is crucial for addressing treatment failures, particularly in the context of recurrent infections. Further research is warranted to comprehensively understand and tackle this phenomenon in bacterial infections.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jung SH, Ryu CM, Kim JS. Bacterial persistence: fundamentals and clinical importance. J Microbiol. 2019;57(10):829–35. https://doi.org/10.1007/s12275-019-9218-0.
Harms A, Maisonneuve E, Gerdes K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. 2016;354(6318). https://doi.org/10.1126/science.aaf4268.
Michiels JE, Van den Bergh B, Verstraeten N, Michiels J. Molecular mechanisms and clinical implications of bacterial persistence. Drug Resist Updat. 2016;29:76–89. https://doi.org/10.1016/j.drup.2016.10.002.
Wilmaerts D, Windels EM, Verstraeten N, Michiels J. General mechanisms leading to Persister formation and awakening. Trends Genet. 2019;35(6):401–11. https://doi.org/10.1016/j.tig.2019.03.007.
Helaine S, Kugelberg E. Bacterial persisters: formation, eradication, and experimental systems. Trends Microbiol. 2014;22(7):417–24. https://doi.org/10.1016/j.tim.2014.03.008.
Van den Bergh B, Fauvart M, Michiels J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol Rev. 2017;41(3):219–51. https://doi.org/10.1093/femsre/fux001.
Bigger J. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet.497–500.
Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, et al. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol. 2019;17(7):441–8. https://doi.org/10.1038/s41579-019-0196-3.
Keren I, Shah D, Spoering A, Kaldalu N, Lewis K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J Bacteriol. 2004;186(24):8172–80. https://doi.org/10.1128/jb.186.24.8172-8180.2004.
Shah D, Zhang Z, Khodursky A, Kaldalu N, Kurg K, Lewis K. Persisters: a distinct physiological state of E. Coli. BMC Microbiol. 2006;6:53. https://doi.org/10.1186/1471-2180-6-53.
Windels EM, Michiels JE, Fauvart M, Wenseleers T, Van den Bergh B, Michiels J. Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates. Isme j. 2019;13(5):1239–51. https://doi.org/10.1038/s41396-019-0344-9.
Bingen E, Denamur E, Lambert-Zechovsky N, Braimi N, el Lakany M, Elion J. DNA restriction fragment length polymorphism differentiates recurrence from relapse in treatment failures of Streptococcus pyogenes pharyngitis. J Med Microbiol. 1992;37(3):162–4. https://doi.org/10.1099/00222615-37-3-162.
Kint CI, Verstraeten N, Fauvart M, Michiels J. New-found fundamentals of bacterial persistence. Trends Microbiol. 2012;20(12):577–85. https://doi.org/10.1016/j.tim.2012.08.009.
Bakkeren E, Diard M, Hardt WD. Evolutionary causes and consequences of bacterial antibiotic persistence. Nat Rev Microbiol. 2020;18(9):479–90. https://doi.org/10.1038/s41579-020-0378-z.
Lewis K. Persister cells. Annu Rev Microbiol. 2010;64(64, 2010):357–72. https://doi.org/10.1146/annurev.micro.112408.134306.
Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 2016;14(5):320–30. https://doi.org/10.1038/nrmicro.2016.34.
Golmoradi Zadeh R, Mirshekar M, Sadeghi Kalani B, Pourghader J, Barati M, Masjedian Jazi F. The expression of type II TA system genes following persister cell formation in Pseudomonas aeruginosa isolates in the exponential and stationary phases. Arch Microbiol. 2022;204(8):451. https://doi.org/10.1007/s00203-022-03038-x.
Song S, Wood TK. Toxin/Antitoxin system paradigms: toxins bound to antitoxins are not likely activated by Preferential Antitoxin Degradation. Adv Biosyst. 2020;4(3):e1900290. https://doi.org/10.1002/adbi.201900290.
Singh G, Yadav M, Ghosh C, Rathore JS. Bacterial toxin-antitoxin modules: classification, functions, and association with persistence. Curr Res Microb Sci. 2021;2:100047. https://doi.org/10.1016/j.crmicr.2021.100047.
Xie Y, Wei Y, Shen Y, Li X, Zhou H, Tai C, et al. TADB 2.0: an updated database of bacterial type II toxin-antitoxin loci. Nucleic Acids Res. 2018;46(D1):D749–53. https://doi.org/10.1093/nar/gkx1033.
Równicki M, Lasek R, Trylska J, Bartosik D. Targeting type II toxin-antitoxin systems as antibacterial strategies. Toxins (Basel). 2020;12(9). https://doi.org/10.3390/toxins12090568.
Ministère de la Santé. Secrétariat général, Direction des laboratoires de biologie médicale, Direction générale de l’accès aux produits de santé. Rapport synthèse de la surveillance de la résistance aux antimicrobiens au laboratoire. Ministère de la Santé du Burkina Faso. 2018. https://drive.google.com/file/d/1QWQWIkowD7FRnj5PeGTbzUE-7_6fz84i/view. Accessed 04 Apr 2024.
CA-SFM/EUCAST. Comité de l’antibiogramme de la Société Française de Microbiologie - Recommandation 2015 V.2.0. 2015. https://www.sfm-microbiologie.org/wp-content/uploads/2019/02/CASFMV2.juillet2015.pdf. Accessed 03 Apr 2024.
Cañas-Duarte SJ, Restrepo S, Pedraza JM. Novel protocol for persister cells isolation. PLoS ONE. 2014;9(2):e88660. https://doi.org/10.1371/journal.pone.0088660.
Conlon BP, Rowe SE, Gandt AB, Nuxoll AS, Donegan NP, Zalis EA, et al. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat Microbiol. 2016. https://doi.org/10.1038/nmicrobiol.2016.51. 1; doi.
Rowe SE, Conlon BP, Keren I, Lewis K. Persisters: methods for isolation and identifying contributing Factors–A review. Methods Mol Biol. 2016;1333:17–28. https://doi.org/10.1007/978-1-4939-2854-5_2.
Amraei F, Narimisa N, Sadeghi Kalani B, Lohrasbi V, Masjedian Jazi F. Persister cells formation and expression of type II toxin-antitoxin system genes in Brucella melitensis (16 M) and Brucella abortus (B19). Iran J Pathol. 2020;15(2):127–33. https://doi.org/10.30699/ijp.2020.118902.2294.
Kaldalu N, Hauryliuk V, Turnbull KJ, La Mensa A, Putrinš M, Tenson T. Vitro studies of Persister cells. Microbiol Mol Biol Rev. 2020;84(4). https://doi.org/10.1128/mmbr.00070-20.
Zadeh RG, Kalani BS, Ari MM, Talebi M, Razavi S, Jazi FM. Isolation of persister cells within the biofilm and relative gene expression analysis of type II toxin/antitoxin system in Pseudomonas aeruginosa isolates in exponential and stationary phases. J Glob Antimicrob Resist. 2022;28:30–7. https://doi.org/10.1016/j.jgar.2021.11.009.
Wong C-F, Yeo JY, Gan SK-E. Republication–APD colony counter app: using watershed algorithm for improved colony counting. Sci Phone Apps Mob Devices. 2019;5(5). https://doi.org/10.1186/2019/c23122019.
Dashti AA, Jadaon MM, Abdulsamad AM, Dashti HM. Heat treatment of bacteria: a simple method of DNA extraction for molecular techniques. Kuwait Med J. 2009;41(2):117–22.
Ministère de la Santé. Secrétariat général, Direction des laboratoires de biologie médicale, Direction générale de l’accès aux produits de santé. Rapport synthèse de la surveillance de la résistance aux antimicrobiens au laboratoire. Ministère de la Santé du Burkina Faso. 2019. https://drive.google.com/file/d/1UT6u9kSDGs1W3cl_td1IMLazAQ5yUVa_/view. Accessed 04 Apr 2024.
Ministère de la Santé. Secrétariat général, Direction des laboratoires de biologie médicale, Direction générale de l’accès aux produits de santé. Rapport synthèse de la surveillance de la résistance aux antimicrobiens au laboratoire. Ministère de la Santé du Burkina Faso. 2021. https://drive.google.com/file/d/1dWEoxqgSjVe_edY3whBnVnPPzntPqdHz/view?usp=drive_link. Accessed 04 Apr 2024.
Ministère de la Santé. Secrétariat général, Direction des laboratoires de biologie médicale, Direction générale de l’accès aux produits de santé. Rapport synthèse de la surveillance de la résistance aux antimicrobiens au laboratoire. Ministère de la Santé du Burkina Faso. 2022. https://drive.google.com/file/d/1hf_1WsKnNN3jqQecomW0CQvb3V8Wzlc5/view?usp=drive_link. Accessed 04 Apr 2024.
Hemati S, Azizi-Jalilian F, Pakzad I, Taherikalani M, Maleki A, Karimi S, et al. The correlation between the presence of quorum sensing, toxin-antitoxin system genes and MIC values with ability of biofilm formation in clinical isolates of Pseudomonas aeruginosa. Iran J Microbiol. 2014;6(3):133–9.
Gollan B, Grabe G, Michaux C, Helaine S. Bacterial persisters and infection: past, Present, and progressing. Annu Rev Microbiol. 2019;73. https://doi.org/10.1146/annurev-micro-020518-115650. :359 – 85; doi.
Mlynarcik P, Kolar M. Starvation- and antibiotics-induced formation of persister cells in Pseudomonas aeruginosa. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(1):58–67. https://doi.org/10.5507/bp.2016.057.
Karimaei S, Kazem Aghamir SM, Foroushani AR, Pourmand MR. Antibiotic tolerance in biofilm persister cells of Staphylococcus aureus and expression of toxin-antitoxin system genes. Microb Pathog. 2021;159:105126. https://doi.org/10.1016/j.micpath.2021.105126.
Manandhar S, Singh A, Varma A, Pandey S, Shrivastava N. High level of persister frequency in clinical staphylococcal isolates. BMC Microbiol. 2022;22(1):109. https://doi.org/10.1186/s12866-022-02529-7.
Trastoy R, Manso T, Fernández-García L, Blasco L, Ambroa A, Pérez D, Molino ML, et al. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin Microbiol Rev. 2018;31(4). https://doi.org/10.1128/cmr.00023-18.
Foster N, Tang Y, Berchieri A, Geng S, Jiao X, Barrow P. Revisiting Persistent Salmonella infection and the Carrier State. What Do We Know? Pathogens. 2021;10(10). https://doi.org/10.3390/pathogens10101299.
Fernández-García L, Blasco L, Lopez M, Bou G, García-Contreras R, Wood T, et al. Toxin-antitoxin systems in clinical pathogens. Toxins (Basel). 2016;8(7). https://doi.org/10.3390/toxins8070227.
Lobato-Márquez D, Díaz-Orejas R, García-Del Portillo F. Toxin-antitoxins and bacterial virulence. FEMS Microbiol Rev. 2016;40(5):592–609. https://doi.org/10.1093/femsre/fuw022.
Coskun USS, Cicek AC, Kilinc C, Guckan R, Dagcioglu Y, Demir O, et al. Effect of mazEF, higBA and relBE toxin-antitoxin systems on antibiotic resistance in Pseudomonas aeruginosa and Staphylococcus isolates. Malawi Med J. 2018;30(2):67–72. https://doi.org/10.4314/mmj.v30i2.3.
Williams JJ, Halvorsen EM, Dwyer EM, DiFazio RM, Hergenrother PJ. Toxin-antitoxin (TA) systems are prevalent and transcribed in clinical isolates of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus. FEMS Microbiol Lett. 2011;322(1):41–50. https://doi.org/10.1111/j.1574-6968.2011.02330.x.
Moyed HS, Bertrand KP. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol. 1983;155(2):768–75. https://doi.org/10.1128/jb.155.2.768-775.1983.
Narimisa N, Amraei F, Kalani BS, Mohammadzadeh R, Jazi FM. Effects of sub-inhibitory concentrations of antibiotics and oxidative stress on the expression of type II toxin-antitoxin system genes in Klebsiella pneumoniae. J Glob Antimicrob Resist. 2020;21:51–6. https://doi.org/10.1016/j.jgar.2019.09.005.
Acknowledgements
The authors wish to thank all participants in this study. A deep gratitude to all the staff of LABIOGENE and Centre de Recherche Biomoléculaire Pietro Annigoni (CERBA) for technical support.
Funding
The authors declare that this study did not receive any funding.
Author information
Authors and Affiliations
Contributions
Study concept and design: AKO, AMD and JS. Sampling and Laboratory analysis: AK, AKO and AMO. Statistical analysis and interpretation of data AK, AKO, AMD and JS. Drafting of the manuscript: AK. Critical revision of the manuscript for important intellectual content: AK, AKO, AMD, and JS. Administrative, technical, and material support: AKO, AMD and JS. Study supervision: AKO, AMD and JS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures met the ethical standards of the responsible committee and were approved by Institutional Ethics Committee of CERBA/LABIOGENE in its deliberation N° 2022-25/09–015 of 5 September 2022. All study participants or their guardians provided their free and informed consent in accordance with the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Konkobo, A., Ouattara, A.K., Mètuor Dabiré, A. et al. Exploring antibiotic-induced persister formation and bacterial persistence genes in clinical isolates from Burkina Faso. BMC Infect Dis 24, 994 (2024). https://doi.org/10.1186/s12879-024-09906-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09906-9