Abstract
Background
Idiopathic CD4 lymphocytopenia (ICL) is an underdiagnosed immunodeficiency syndrome characterised by persistent low CD4 counts in the absence of HIV and other causes of lymphocytopenia. ICL patients are susceptible to opportunistic infections, with human papillomavirus, cryptococcal, and tuberculosis being the most common infections reported. Nocardiosis is rarely reported in patient with ICL.
Case Presentation
We herein discuss a 46-year-old female presented with complaints of weight loss, low grade fever and cough with expectoration from last four months. The patient was diagnosed with pulmonary nocardiosis and aspergillosis co-infection four years back; in addition she also had ICL. Subsequently, the patient was lost in follow-up and readmitted four years later. Bronchoalveolar lavage sample shows the presence of acid-fast bacilli in modified gram stain, which later identified as Nocardia otitidiscaviarum by metagenomic next-generation sequencing. Her CD4 counts were still found low (298 cells/mm3). After an initial improvement with trimethoprim-sulfamethoxazole (TMP-SMX), she was commenced on indefinite secondary prophylaxis.
Conclusions
Nocardiosis without usual risk factors should be evaluated for ICL. This case emphasize the importance of periodic follow-up with CD4 count monitoring and secondary prophylaxis therapy to prevent recurrence or the emergence of new infections in ICL.
Clinical trial number
Not applicable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isolated CD4 lymphocytopenia (ICL) is a rare immunodeficiency syndrome characterised by persistent low CD4 counts (< 300 cells/mm3, at least six weeks apart) in the absence of Human immunodeficiency virus (HIV) or other immunodeficiency disorders [1]. Individuals with ICL are at increasing risk of acquiring opportunistic infections such as bacterial, fungal, and tuberculosis [2]. Nocardiosis is an opportunistic infection predominantly associated with patients on immunosuppressants, malignancy, transplant recipients, and HIV [3]. Recurrence of nocardiosis is an uncommon occurrence usually seen in patients with solid organ transplant recipients [4]. Nocardiosis is rarely documented with ICL in the literature, with a few case reports so far [5,6,7]. We herein present a case of recurrent pulmonary nocardiosis in a diagnosed patient of ICL. To our knowledge, this is the first case documenting the recurrence of pulmonary nocardiosis in ICL after four years.
Case Presentation
A 46-year-old homemaker resident from Western India, presented with complaints of persistent cough with expectoration for the past four months and low-grade fever for the last month. She also had a history of decreased appetite and undocumented weight loss for the past three months. She has a history of pulmonary Nocardia spp. coinfection with invasive pulmonary Aspergillosis, diagnosed in 2019. Both sputum and bronchoalveolar lavage (BAL) modified ZN stain showed long filamentous acid-fast branching bacilli suggestive of Nocardiosis. The BAL galactomannan levels were positive, with a value of 1.8 (normal < 0.5). The CT thorax revealed multiple cavitary consolidations with centrilobular nodules and septal thickening. She was put on a weight-based regimen of tablet trimethoprim-sulfamethoxazole (TMP-SMX) for six months and tablet voriconazole for six weeks in view of co-infection. Repeat imaging at six weeks showed clinical and radiological improvement. The patient did not have any immunocompromised state at that time (negative HIV, no evidence of malignancy). Notwithstanding, her total CD4 count was detected low at six weeks apart (164 cells/mm3 and 178 cells/mm3 ). The patient subsequently lost to follow-up and did not receive secondary prophylaxis for nocardiosis despite having isolated CD4 lymphocytopenia.
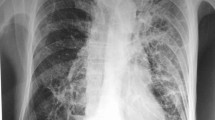
Now, at the current presentation, the patient was vitally stable. On respiratory examination, Left infra-scapular and infra-axillary crepitation were present. Laboratory investigations revealed elevated inflammatory markers (C-reactive protein: 67, normal < 5 mg/dL, erythrocyte sedimentation rate: 98, normal < 20 mm/hr). HRCT thorax showed features of old infective sequelae with multiple thick-walled cavitary nodules in the right upper lobe and left lower lobe (largest: 1.1*1.2 cm, Fig. 1). Patchy GGOs were seen in the bilateral lungs, predominantly in the left lower lobe and right upper lobes. Patchy consolidation was also noted in the left lower lobe (Fig. 1). Based on these findings, tuberculosis, nocardiosis, aspergillosis, and histoplasmosis were kept as differentials. Sputum and BAL analysis workups were negative for tuberculosis (negative Gene Xpert, and TB culture), histoplasmosis (negative culture and histoplasma antigen), cryptococcosis (negative culture and cryptococcal antigen) and malignancy (no malignant cell in cytology). However, sputum and BAL modified-ZN stain showed the presence of long beaded acid-fast bacilli, suggestive of Nocardia species (Fig. 2), with cultures pending. BAL galactomannan testing yielded a negative result (0.4). Considering the underlying isolated CD4 count lymphocytopenia, a repeat CD4 count was performed and found to be 298 cells/mm3. The patient was again evaluated for immunodeficiency disorders, which were negative. No evidence of malignancy like lymphoproliferative disorders or myeloma was found with normal protein electrophoresis. No history of immunosuppressant drug uses was found. Normal Immunoglobulin levels and NK cell activity ruled out primary immunodeficiency disorders. Serology for HIV and Epstein-Barr virus were also negative. BAL sample was sent for metagenomic next-generation sequencing (mNGS), and the isolate was identified as Nocardia otitidiscaviarum. Culture also grew similar species with susceptibility to trimethoprim-sulfamethoxazole, amikacin and linezolid and resistant to ceftriaxone and imipenem. To rule out CNS involvement, a CECT brain was performed, which showed no abnormalities. Given the extensive lung involvement and immunodeficiency state, the patient was advised trimethoprim-sulfamethoxazole (15 mg/kg trimethoprim component), with inj. Amikacin. At the time of discharge (at two weeks), the patient’s symptoms had improved. The patient was counselled about the importance of adherence and long-term follow-up. TMP-SMX therapy continued for the next six months. After that, she was put on life-long secondary prophylaxis for nocardiosis.
Non-contrast CT of the chest. (a, b) Axial high-resolution CT images in lung window showing cavitary lesions in right upper lobe (black arrows, a, c) along with bronchiectasis in right middle lobe, and multiple centrilobular nodules in bilateral lung fields. Patchy consolidation is also noted in left lower lobe on lung (star in b) and mediastinal window (d)
Discussion
Idiopathic CD4 T cell lymphocytopenia is a rare clinical syndrome which is often underdiagnosed. Patients with ICL may have a myriad of opportunistic infections, malignancies and autoimmune disorders [8]. Among the opportunistic infections, human papillomavirus, cryptococcosis, and non-tuberculous mycobacterium are the common infections documented in ICL [1]. Recurrent bacterial infections are uncommon in ICL unless patients have other risk factors like HIV or primary immunodeficiency disorders. We present a rare case of ICL who developed pulmonary nocardiosis and aspergillosis coinfection in the first episode; four years later, she was again diagnosed with Nocardia otitidiscaviarum lung infection. Nocardia species are aerobic gram-positive bacteria that can cause localised and systemic disease, especially in immunocompromising conditions like glucocorticoids/immunosuppressive medications, solid organ transplants, HIV infection, malignancy, and structural lung disease [9]. Lungs are the most commonly affected site in approximately 80% of cases of immunocompromised individuals and 50% of cases in structural lung diseases like bronchiectasis and chronic obstructive pulmonary disease [9]. More than half of individuals with pulmonary nocardiosis, especially in immunosuppressed settings, progress to disseminated nocardiosis with involvement of brain abscess [10]. Undetected disseminated diseases could be an important cause of disease recurrence. In our case, the patient did not have any features of disseminated disease.
Recurrence of nocardiosis is a rare occurrence, and most cases have been reported in solid organ transplant (SOT) recipients [4]. The recurrence rate of nocardiosis is around 5% in SOT recipients; secondary prophylaxis is indicated in these groups of patients. There are reports of recurrent Nocardia infection in iron overload conditions like sickle cell disease [11]. To our knowledge, this is the first case of recurrent Nocardia infection in a patient with ICL. There are a few case reports showing Nocardia infections in underlying ICL disease (summarised in Table 1) [5,6,7, 12,13,14]. Out of six cases, four patients had CNS involvement, whereas two patients had isolated pulmonary infection. Species identification was reported in five patients (Nocardia farcinica in 3, Nocardia abscessus and Nocardia otitidiscaviarum in one patient each). In the present case, Nocardia otitidiscaviarum species was identified, which is a rare cause of nocardiosis seen in only 0.3–2.9% of all cases [15].
This case has several key facets; at first presentation, the patient had co-infection of aspergillosis and nocardiosis, which is itself a rare occurrence. The patient had received voriconazole and TMP-SMX therapy and subsequently improved. Defective Cell-mediated immunity is a key risk factor for nocardiosis. However, the rarity of Nocardia infection in ICL is still puzzling compared to other opportunistic infections. The lack of suspicion for ICL in nocardiosis cases is also contributory to this. This case also highlights the role of long-term follow-up in patients with ICL, as the recurrence of previous infections may occur at any time. The role of secondary prophylaxis is debatable in such patients; data is sparse due to the rarity of diseases. In none of the previous cases, the patient received TMP-SMX prophylaxis. Since our patient had a recurrence, we emphasize lifelong secondary prophylaxis and follow-up in ICL patients. The role of mNGS is also evolving for the rapid diagnosis of nocardiosis, as highlighted in the current case. At first occurrence (2019), we could not identify the species; sometimes, this may lead to inadequate treatment if the drug regimen is resistant. However, antibiotic susceptibilities vary among different Nocardia species, and susceptibility-guided choice of antibiotic is always ideal to prevent resistance and better outcomes.
In conclusion, The presented case highlights a rare and unusual manifestation of recurrent pulmonary nocardiosis in the setting of isolated CD4 lymphocytopenia. This case underscores the importance of close follow-up, CD4 count monitoring and secondary prophylaxis in patients with ICL.
Data availability
Data can be provided by the corresponding author on reasonable request.
Abbreviations
- ICL:
-
Idiopathic CD4 lymphocytopenia
- TMP/SMX:
-
Trimethoprim-sulfamethoxazole
- HIV:
-
Human immunodeficiency virus
- BAL:
-
Bronchoalveolar lavage
- mNGS:
-
Metagenomic next-generation sequencing
- SOT:
-
Solid organ transplant
References
Lisco A, Ortega-Villa AM, Mystakelis H, Anderson MV, Mateja A, Laidlaw E, et al. Reappraisal of idiopathic CD4 Lymphocytopenia at 30 years. N Engl J Med. 2023;388(18):1680–91. https://doi.org/10.1056/NEJMoa2202348.
Luo L, Li T. Idiopathic CD4 lymphocytopenia and opportunistic infection–an update. FEMS Immunol Med Microbiol. 2008;54(3):283–9. https://doi.org/10.1111/j.1574-695X.2008.00490.x.
Margalit I, Goldberg E, Ben Ari Y, Ben-Zvi H, Shostak Y, Krause I, et al. Clinical correlates of nocardiosis. Sci Rep. 2020;10(1):14272. https://doi.org/10.1038/s41598-020-71214-4.
Yetmar ZA, Wilson JW, Beam E. Recurrent nocardiosis in solid organ transplant recipients: an evaluation of secondary prophylaxis. Transpl Infect Dis. 2021;23(6):e13753. https://doi.org/10.1111/tid.13753.
Göktay F, Mansur AT, Erşahin M, Adaleti R, Güneş P. Idiopathic CD4 + T lymphocytopenia with epidermodysplasia verruciformis-like skin eruption, Nocardia farcinica brain abscesses and pulmonary tuberculosis: a case report with fatal outcome. J Dermatol. 2011;38(9):930–3.
Jayaschandran V, Gjorgova-Gjeorgjievski S, Siddique H. Pulmonary nocardiosis in a patient with idiopathic CD4 T-lymphocytopenia. Respirol Case Rep. 2017;6(2):e00283. https://doi.org/10.1002/rcr2.283.
Tajima K, Terada T, Okuyama S, Akaneya D, Hori R, Abe S, et al. Nocardia otitidiscaviarum meningitis in a diffuse large B-cell lymphoma patient with CD4-positive lymphocytopenia and persistent oligoclonal CD8-positive lymphocytes in the peripheral blood. Int J Clin Exp Pathol. 2018;11(1):455–61.
Vijayakumar S, Viswanathan S, Aghoram R. Idiopathic CD4 Lymphocytopenia: current insights. Immunotargets Ther. 2020;9:79–93. https://doi.org/10.2147/ITT.S214139.
Duggal SD, Chugh TD. Nocardiosis: a neglected disease. Med Princ Pract. 2020;29(6):514–23. https://doi.org/10.1159/000508717.
Meena DS, Kumar D, Bohra GK, Midha N, Garg MK. Clinical characteristics and treatment outcome of Central Nervous System Nocardiosis: a systematic review of reported cases. Med Princ Pract. 2022;31(4):333–41. https://doi.org/10.1159/000525509.
Wessler JM, Adams DJ, Kunz AN, Babcock JG, Hartman KR. Recurrent Nocardia Sepsis in a patient with Sickle Cell Anemia receiving continuous deferoxamine. J Pediatr Infect Dis Soc. 2014;3(3):e35–7. https://doi.org/10.1093/jpids/pit038.
Yadav P, Kumar D, Meena DS, Bohra GK, Jain V, Garg P, et al. Clinical features, radiological findings, and treatment outcomes in patients with pulmonary nocardiosis: a retrospective analysis. Cureus. 2021;13(8):e17250. https://doi.org/10.7759/cureus.17250.
Adjamian N, Kikam A, Wessell KR, Casselman J, Toller-Artis E, Olasokan O, et al. Nocardia Brain Abscess and CD4(+) Lymphocytopenia in a previously healthy individual. Case Rep Immunol. 2015;374956. https://doi.org/10.1155/2015/374956.
Lafont E, Marciano BE, Mahlaoui N, Neven B, Bustamante J, Rodriguez-Nava V, et al. Nocardiosis Associated with primary immunodeficiencies (Nocar-DIP): an International Retrospective Study and Literature Review. J Clin Immunol. 2020;40(8):1144–55. https://doi.org/10.1007/s10875-020-00866-8.
Barry M, AlShehri S, Alguhani A, Barry M, Alhijji A, Binkhamis K, et al. A fatal case of disseminated nocardiosis due to Nocardia otitidiscaviarum resistant to trimethoprim-sulfamethoxazole: case report and literature review. Ann Clin Microbiol Antimicrob. 2022;21(1):17. https://doi.org/10.1186/s12941-022-00511-9.
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All Authors contributed to the study concept and design. TK, DSM, DK, NM, SK and TY drafted the manuscript and contributed to the literature search. SK and TK performed the microbiological culture and identification. TY analysed the radioimaging. DK, NM and DSM supervised the work, manuscript design and made suggestions to improve the manuscript. All authors read and approve the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent has been taken from the patient.
Consent for publication
The patient has given written consent for his personal and clinical details. The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given their consent for their images and other clinical information to be reported in the journal. The patient understand that her name and initials will not be published and due efforts will be made to conceal her identity.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kanagiri, T., Meena, D.S., Kumar, D. et al. Recurrent pulmonary nocardiosis due to Nocardia Otitidiscaviarum in a patient with isolated CD4 lymphocytopenia: a case report. BMC Infect Dis 24, 1033 (2024). https://doi.org/10.1186/s12879-024-09981-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09981-y