Abstract
Background
Incremental peritoneal dialysis (IPD) refers to the use of less than standard full-dose peritoneal dialysis (SPD) in end-stage renal disease patients. While the use of IPD is being reported in the literature, its safety and efficacy vs. SPD is unclear. We hereby performed a systematic review of studies comparing mortality, peritonitis, technique survival, anuria-free survival and residual renal function (RRF) between IPD and SPD.
Methods
All comparative studies published on PubMed, Embase, CENTRAL, Scopus, and Web of Science databases from inception to 5th September 2023 and reporting on given outcomes were eligible.
Results
Ten studies were included. Definitions of IPD were heterogenous and hence mostly a qualitative synthesis was undertaken. Majority of studies found no difference in patient survival between IPD and SPD. Meta-analysis of crude mortality data also presented no significant difference. Peritonitis and technique survival were also not significantly different between IPD and SPD in the majority of studies. Data on RRF was conflicting. Some studies showed that IPD was associated with the preservation of RRF while others found no such difference.
Conclusion
IPD may be a safe alternative to SPD in incident dialysis patients. There seems to be no difference in patient survival, peritonitis, and technique survival between the two modalities. However, the impact of IPD on RRF is still questionable. Evidence is heterogeneous and conflicting to derive firm conclusions.
Similar content being viewed by others
Introduction
End-stage kidney disease (ESKD) is a major public health problem affecting around 4–7 million individuals around the world [1]. The need for renal replacement therapy for survival imposes enormous socioeconomic pressure on both the patients as well as health care setups globally. In about 80% of patients, the initial choice is hemodialysis but is associated with a high risk of mortality and rapid loss of residual renal function (RRF) in the initial months of maintenance dialysis [2,3,4,5,6]. Contrastingly, peritoneal dialysis (PD) offers lower mortality rates owing to the maintenance of RRF while providing more or less equivalent long-term survival as compared to hemodialysis [HD] [7, 8]. Nevertheless, while the number of patients requiring renal replacement therapy has grown exponentially, those enrolled in PD have not correspondingly increased. Lack of patient education, risk of catheter-related complications, shortage of trained manpower, and lack of support for assisted PD are important factors limiting the uptake of PD [9].
The concept of incremental PD (IPD) was first reported in the year 1997 and consisted of starting maintenance dialysis with PD but with lower-dose prescription rather than the full dose i.e. standard PD (SPD) while maintaining individualized clearance goals [10]. The dosage is gradually increased as the RRF declines by changing the dwell time, volume and number of exchanges [11, 12]. IPD can be implemented in both continuous ambulatory PD (CAPD) and ambulatory PD (APD) achieving the therapeutic goals of renal replacement therapy at a reduced cost [12]. Recommendations by the International Society for Peritoneal Dialysis (ISPD) in 2020 recommended the usage of IPD for improving patient experience and providing high-quality, goal-directed PD [13]. The presumed advantages of IPD like longer preservation of RRF, reduced costs, and reduced time on PD have prompted its utilization in several countries worldwide. However, these advantages are still debatable and it is unclear if PD should be started at a reduced dosage or full-dose. Several comparative studies on IPD vs. SPD have been published in the past decade [14,15,16], however, there has not been an attempt to pool published evidence. We hereby attempted to perform a comprehensive systematic review and meta-analysis to analyze the efficacy and safety of IPD vs. SPD.
Materials and methods
Search protocol
The present study was reported by the guidelines of PRISMA [17] and registered on PROSPERO (CRD42023458440). All studies published on PubMed, Embase, CENTRAL, Scopus, and Web of Science databases were searched from inception to 5th September 2023. Only English-language studies were eligible. It was conducted by an experienced medical librarian along with one study reviewer. The search strategy included MeSH/Emtree terms and free keywords namely: “low dose”, “incremental”, “peritoneal dialysis”, “incremental dialysis”, “survival” “ and “mortality”. Details can be found in Supplementary Table 1. All search results were downloaded into EndNote X8 (Thompson ISI Research soft, Philadelphia, Pennsylvania), a reference manager software. Duplicate articles were identified and excluded. All remaining unique citations underwent screening by two reviewers. Full texts of articles found important were downloaded and further screened based on inclusion criteria.
Inclusion criteria
Both reviewers independently checked the eligibility of studies based on the following criteria:
-
1.
Any study design conducted on patients undergoing PD without any age restriction.
-
2.
Comparing outcomes of IPD with SPD. We did not predefine IPD and accepted the criteria mentioned in the included studies.
-
3.
Outcomes included all-cause mortality, peritonitis, technique survival, anuria-free survival, and RRF.
Studies comparing IPD with HD, not reporting any outcomes, on urgent-start PD, and single-arm studies were excluded.
All studies underwent final screening based on these criteria. The reviewers resolved any disagreements (if any) involving the study selection by discussion. In the end, one reviewer undertook a hand search of the reference list of included studies for any possible inclusions. In two studies were found to use the same database and had same or overlapping study duration, the study reporting the maximum outcomes was to be included.
Data extraction
A pre-defined data collection form was used by the reviewers to collect data. It included the author and publication information, location, IPD and SPD definition, duration of IPD, follow-up, sample size, age, gender details, diabetics, creatinine, baseline renal function, haemoglobin, albumin, baseline 24-hour urine output, and drug use. The second reviewer then cross-checked the data for correctness. All endpoint data based on the inclusion criteria was extracted. We did not predefine any endpoints and all definitions reported by the included studies were acceptable.
Study quality
The Newcastle-Ottawa Quality Assessment Scale (NOS) was selected for assessing individual study bias of observational studies [18]. The scale intends to rate selection bias, comparability of the exposed and unexposed groups, outcome assessment, and completeness of follow-up. Points are awarded based on pre-determined questions and the final score of each study can be 0 meaning the highest risk of bias up to 9 meaning the lowest risk of bias. The Cochrane risk of bias-2 tool was used to examine risk of bias in randomized controlled trials (RCT) [19]. Two reviewers were involved in the risk of bias analysis and disagreements were resolved by discussion.
Statistical analysis
Quantitative synthesis was planned if a minimum of three studies were available for each outcome. If outcomes were reported on different scales or without 95% confidence intervals (CI), meta-analysis was not conducted. Outcome data was extracted as a crude ratio or adjusted ratio. Both data were to be pooled separately. Crude dichotomous data was pooled to obtain.
RR and 95% CI. Subgroup analysis was conduct based on study design. A random-effects model was chosen because of anticipated heterogeneity. The chi-square test judged the heterogeneity between studies; the I2 statistic was also calculated. The I2 statistic gives the percentage of the variability in effect size based on heterogeneity rather than sampling error. Any value > 50% was considered substantial heterogeneity. “Review Manager” (RevMan, version 5.3; Nordic Cochrane Centre (Cochrane Collaboration), Copenhagen, Denmark; 2014) was the software used. Values of p < 0.05 were considered to be statistically significant. Owing to a low number of studies funnel plot was not generated for publication bias. GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) was used to assess certainty of evidence.
Results
Search results
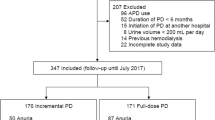
The final number of articles obtained from all databases was 2016. Unique articles amongst these were 992. The reviewers eliminated 973 studies based on initial screening. Nineteen underwent full-text analysis and ten were selected for the meta-analysis [14,15,16, 20,21,22,23,24,25,26] (Fig. 1).
Baseline details of studies
Study details and baseline data of participants at the start of PD are presented in Tables 1 and 2. All were retrospective studies and only one was an RCT. One study was a secondary analysis of an RCT. The studies published between 2013 and 2023 included patients from India, Italy, China, Korea, Australia, the USA and Portugal. The RCT of Yan et al [24] compared 3 vs. 4 exchanges/day of CAPD with a follow-up of two years. Hayat et al [20] compared < 56 L/week vs. ≥ 56 L/week of PD fluid exchange with a follow-up of about two years. In the retrospective studies, there was a wide variation in what was defined as IPD. Most studies however, considered ≤ 3 dwells of CAPD per day as IPD. Jeloka et al [22] reported IPD as one icodextrin exchange/day. Huang et al [14] defined IPD as CAPD < 8 L/day with no patient receiving it for < 7 days a week. SPD was defined as ≥ 4 exchanges on CAPD per day in four studies. In three studies, ≥ 3 exchanges/day was considered as SPD. Four studies did not report the duration of IPD. In the remaining studies, it ranged from one year to 2.5 years. A total of 1540 patients were on IPD while 1837 were on SPD. The mean age was above 40 years in both IPD and SPD groups across studies. Majority of studies had a male predominance in the sample. The percentage of diabetics was < 50% across studies. However, in the study of Jeloka et al [22] and Naljayan et al [21] > 50% of patients in both the IPD and SPD groups were diabetics. GFR was ≤ 10 ml/min/1.73m2 in most studies, both in IPD and SPD. GFR was significantly better in the IPD group vs. the SPD group in the study of Lee et al [15]. No such difference was noted in the remaining studies. The follow-up duration varied from 17 months to a maximum of 12 years. On examination of study quality of observational studies, the reviewers gave one study 5 points, three studies scored 6 points, while the remaining got 8 points. The study of Yan et al [24], being an RCT, was assessed by the Cochrane risk of bias tool. There were “some concerns” regarding the randomization process and missing outcome data, high risk of bias for outcome assessment, but low risk of bias for deviations from intended interventions, and selection of reported result. Overall the RCT was classified as having high risk of bias.
Mortality
Outcome data reported by studies is presented in Table 3. Much of the data was not amenable to a meta-analysis. Since crude mortality rates were reported by five studies, we decided to pool them together. No statistically significant difference was noted in mortality rates between IPD and SPD (RR: 0.84 95% CI: 0.46, 1.55 I2 = 28%). The results were non-significant for both the single RCT and non-RCTs (Fig. 2). GRADE assessment of evidence showed certainty of evidence was “very low” for both the RCT and non-RCTs (Supplementary Table 2). All-cause mortality adjusted for confounders was reported by Lui et al [16] (HR: 0.79 95% CI:0.58–1.09) and Lee et al [15] (HR: 0.504 95% CI: 0.230–1.104) but without any statistically significant difference between the two groups. Naljayan et al [21] also reported the incidence rate ratios (IRR) of mortality rates in both CAPD and APD groups. No statistically significant difference in mortality was noted between IPD and SPD for CAPD (IRR: 0.68; 95% CI: 0.43–1.06) and APD (IRR: 0.62; 95% CI: 0.34–1.14) groups. As noted in Table 3, except for the study of Jeloka et al [22] which noted better survival with IPD, the remaining studies also noted no statistically significant difference in patient survival between IPD and SPD.
Peritonitis
Peritonitis was reported as an outcome of eight studies. Lee et al [15] found a statistically significant lower risk of peritonitis in patients undergoing IPD as compared to SPD. The incidence of peritonitis was 1/18 patient-year in IPD patients and 1/7 patient-year in the SPD group (p = 0.002). However, all remaining studies noted no statistically significant difference in the risk of peritonitis with either modality.
Technique survival
Six of the ten studies reported on technique survival. In the study of Fernandez et al [26], 5/57 IPD patients and 8/30 patients SPD patients had technique failure. Mechanical complications with SPD (2/30) were also significantly higher as compared to IPD (0/57) in their study. Overall, the authors noted superior technique survival with IPD (p = 0.026). None of the remaining five studies noted a statistically significant difference in technique survival between the two modalities.
Anuria-free survival
Three studies reported on anuria-free survival. Yan et al [24] in their RCT noted no statistically significant difference between IPD and SPD for anuria-free survival. However, the two retrospective studies of Lui et al [16] and Lee et al [25] found statistically significant better anuria-free survival with IPD. Huang et al [14] also noted no statistically significant difference in residual urea and creatinine clearance at the end of follow-up between the two groups. On the other hand, Fernandez et al [26] found that renal urea and creatinine clearance were statistically significant higher with IPD in the first two years of follow-up. GFR was also found to be superior to IPD in the first two years. Sandrini et al [23] reported longer preservation of RRF with IPD as compared to SPD. Naljayan et al [21] found no difference in GFR values in the one-year follow-up between IPD and SPD with both CAPD and APD. Hayat et al [20] also noted no statistically significant difference in RRF and urine volumes between IPD and SPD.
Discussion
The use of IPD has been suggested as an alternative treatment strategy such that the combination of reduced dose and RRF leads to sufficient small solute clearance reducing the burden of uremic symptoms [12]. As the RRF declines with time, the dose of PD is also correspondingly increased to SPD. IPD offers an advantage of gentle prescription at PD initiation leading to an easier transition to full-dose by offering a more personalized and flexible treatment regimen. It also reduces the disruption faced by incident dialysis patients with a better quality of life [27]. However, whether is it as efficacious and safe as SPD is still unclear. Our systematic review and meta-analysis hereby present the first formal, updated, and comprehensive comparative evidence on the outcomes of IPD and SPD. A total of ten recent studies including both RCTs and non-RCTs were analyzed to generate exhaustive evidence on patient survival, technique survival, peritonitis, and preservation of RRF between the two groups. We did not limit ourselves to RCTs as first, there were just two in literature (of which one is a secondary analysis of an RCT); and second, it would not have generated all-encompassing evidence on the differences between IPD and SPD. Nevertheless, due to methodological variations, differences in IPD definitions, and varied presentation of data, much of the review involved a qualitative rather than a quantitative analysis. A pooled analysis was conducted only for mortality rates which showed no statistically significant difference between the two modalities. Our results concur with the prior systematic review of Garofalo et al [28] who pooled together one study on IPD and ten studies on incremental HD and compared it with standard dialysis in a single meta-analysis only to note no statistically significant difference in mortality. Their study also showed lower mean loss of RRF with incremental PD and HD when compared with standard dialysis but with only one study on PD. Similar results have been noted in a narrative review as well [29].
Despite IPD being an old concept from the 1990s, its definition is still unclear [10]. The same was noted in the included studies where none of the studies were homogenous in defining IPD. As per the recent ISPD guidelines3, IPD can be any dose of PD which is less than the full-dose while achieving the kidney clearance target and prescribed to increase the dose as and when RRF declines. However, what constitutes SPD is subject to regional variations and body habitus. In Asian populations, SPD may be considered as 3 exchanges of 2 L/day while in Western countries SPD consists of 4 exchanges of 2 L/day on CAPD or equivalent on APD with a long dwell [29,30,31]. Such variation was noted in the studies in our review with Yan et al [24] from China comparing 3 vs. 4 exchanges/day while Hayat et al [20] from Australia comparing based on total volume exchange/week. Thus, it is clear from the current literature that IPD cannot be based on a singular cut-off and is based on patient population, individual lifestyle and clearance requirements. Furthermore, even in a single region, IPD can be delivered in several ways depending on fill volume, number of exchanges, and dry periods for PD [27].
Patient survival assumes precedence in cases of ESKD. Initiating IPD can entail the hazard of under-dialysis and reduced small solute clearance. Also, IPD has a small margin of safety in terms of peritoneal clearance if RRF declines. Failure to increase the dose promptly based on RRF can increase the risk of complications, fluid overload and mortality. There can be “therapeutic inertia” on the part of the physician due to inadequate monitoring or due to the patient’s reluctance to get the PD prescription changed or increased from what they are used to [29, 32]. Nevertheless, this systematic review did not note any statistically significant difference in survival between IPD and SPD groups. Meta-analysis of crude mortality rates failed to show any statistically significant difference between the two groups. Also, qualitative synthesis of studies reporting adjusted data failed to show any statistically significant difference in patient survival. The only study of Jeloka et al [22] which noted better survival with IPD has important bias due to the small sample size and selection of participants.
The number of exchanges during PD is an important risk factor for peritonitis [33]. In CAPD, reduced exchanges and fewer connections can decrease the risk of contamination. Also, prolonged dwell time for a single bag of dialysate positively affects the peritoneal defence status [34]. Therefore, theoretically, IPD may reduce the risk of peritonitis. Indirectly, it may also prolong the utilization of PD as peritonitis is a major reason cited by PD drop outs [35]. Nevertheless, a quantitative analysis to assess peritonitis risk between the two modalities was not possible in this review as the risk is directly proportional to the number of exchanges and dwell times. The variations in the IPD definitions amongst the included studies were too critical for a meta-analysis. During qualitative synthesis, we noted that except for one study [15], no study reported a statistically significant difference in the risk of peritonitis between IPD and SPD. Likewise, technique failure did not show any statistically significant difference between IPD and SPD across most studies. The study of Lee et al [15] found a statistically significant lower risk of peritonitis in the IPD group. Despite being a propensity score matched analysis, their study could include only 39 patients in the IPD group, which is a major limitation in the interpretation of results. Also, technique survival and peritonitis can be influenced by catheter insertion techniques, patient education, and rigorous follow-up and care. These practices may not be similar across studies, especially in the case of retrospective studies.
Clearance obtained by RRF as compared to PD can have important clinical implications. A study by Bargman et al. evaluating the relative contribution of RRF on patient outcomes has shown that per 5 L/week per 1.73 m2 increment of GFR improves patient survival by 12% [36]. Maintaining RRF also has cardiovascular implications by improving fluid status, blood pressure control, and reducing left ventricular hypertrophy [29]. Han et al [37] have shown that preservation of RRF also reduces the risk of peritonitis. Liao et al [38] have found that the rate of decline of RRF is a strong marker for all-cause mortality and technique failure in PD. The length and number of dialysis sessions and intradialytic hypotension have been identified as markers of RRF in hemodialysis patients [39]. Observational studies have shown that twice-weekly or incremental HD leads to the preservation of RRF as compared to thrice-weekly or standard hemodialysis [40, 41]. Similarly, a major advantage postulated for IPD vs. SPD is the preservation of RRF. However, analysis of studies comparing IPD vs. SPD for RRF demonstrated mixed outcomes. The RCTs of Yan et al [24] and Hayat et al [20] noted no statistically significant difference in anuria-free survival and RRF respectively between IPD and SPD. However, several observational studies did note better RRF with IPD. Importantly, evidence from observational studies cannot be relied upon due to selection bias. Most of the studies did not use propensity score matching for adjusting baseline variables. It is plausible that physicians may have prescribed IPD to healthier patients leading to bias in the outcomes. Based on current data, this review cannot determine if IPD leads to the preservation of RRF and there is a need for further RCTs on this subject.
There are other limitations to the review as well. The overall number of studies was not very high. Too many methodological differences, especially regarding IPD definition, study type, study population, exclusion criteria, follow-up period, etc., prevented a comprehensive quantitative analysis. The endpoints reported by the studies were also not numerically amenable to quantitative synthesis due to variability in reporting. Most of the data was observational and from medical records, therefore prone to bias. The method of selection of IPD patients was also not mentioned in studies. We were unable to differentiate the outcomes of CAPD and APD due to a lack of sufficient data from the studies. Also, it was unclear what were the outcomes in PD patients who subsequently underwent hemodialysis or kidney transplantation, as separate data for such patients was not reported. Lastly, important outcomes like cardiovascular diseases and pulmonary infections could not be assessed due to lack of data from the included studies.
Nevertheless, this systematic review presents a comprehensive qualitative analysis of outcomes with IPD and SPD. It sheds light on the limitations of current literature while also presenting important evidence for physicians who can make informed decisions. We believe the current review will encourage further research on the efficacy and safety of IPD vs. SPD in a more rigorous study design. Future studies should include comparable equivalent groups of patients for whom the observations should start at the beginning of PD. Such studies should report all the outcomes included in this review after sufficiently long follow-up to provide robust evidence.
Conclusions
IPD may demonstrate comparable outcomes as SPD in incident dialysis patients. There seems to be no difference in patient survival, peritonitis, and technique survival between the two modalities. However, the impact of IPD on RRF is still questionable. Evidence is heterogeneous and conflicting to derive firm conclusions.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request. Our paper is a systematic review, the Clinical Trial Number is not required.
References
Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. https://doi.org/10.1007/978-981-13-8871-2_1.
Eckardt K-U, Gillespie IA, Kronenberg F, Richards S, Stenvinkel P, Anker SD, et al. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int. 2015;88:1117–25. https://doi.org/10.1038/ki.2015.117.
Thomas B, Wulf S, Bikbov B, Perico N, Cortinovis M, Courville de Vaccaro K, et al. Maintenance Dialysis throughout the World in Years 1990 and 2010. J Am Soc Nephrol. 2015;26:2621–33. https://doi.org/10.1681/ASN.2014101017.
Zareba W. Initiation of dialysis: trigger or cause of cardiovascular events? Kidney Int. 2015;88:942–4. https://doi.org/10.1038/ki.2015.271.
Yaprak B, Arslan N, Alataş H. Multiple factors influencing mortality in hemodialysis patients. Eur Rev Med Pharmacol Sci. 2023;27:1095–103. https://doi.org/10.26355/eurrev_202302_31212.
Arslan N. Association of cardiometabolic risks with body composition in hemodialysis patients. Eur Rev Med Pharmacol Sci. 2023;27:2469–76. https://doi.org/10.26355/eurrev_202303_31780.
Marrón B, Remón C, Pérez-Fontán M, Quirós P, Ortíz A. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl. 2008;S42–51. https://doi.org/10.1038/sj.ki.5002600.
Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transpl. 2012;27:3568–75. https://doi.org/10.1093/ndt/gfr674.
Teitelbaum I, Finkelstein FO. Why are we not getting more patients onto peritoneal Dialysis? Observations from the United States with Global implications. Kidney Int Rep. 2023;8:1917–23. https://doi.org/10.1016/j.ekir.2023.07.012.
PG B. Incremental peritoneal dialysis. Perit Dial Int. 2020;40. https://doi.org/10.1177/0896860819895362.
Guest S, Leypoldt JK, Cassin M, Schreiber M. Kinetic modeling of incremental ambulatory peritoneal Dialysis exchanges. Perit Dial Int. 2017;37:205–11. https://doi.org/10.3747/pdi.2016.00055.
Reddy YNV, Mendu ML. The role of incremental peritoneal Dialysis in the era of the advancing American kidney Health Initiative. Clin J Am Soc Nephrol. 2020;15:1835–7. https://doi.org/10.2215/CJN.03960320.
Brown EA, Blake PG, Boudville N, Davies S, de Arteaga J, Dong J, et al. International Society for Peritoneal Dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. 2020;40:244–53. https://doi.org/10.1177/0896860819895364.
Huang LL, Mah JY, Howard J, Roberts MA, McMahon LP. Incremental peritoneal dialysis is a safe and feasible prescription in incident patients with preserved residual kidney function. Nephrol (Carlton). 2022;27:74–81. https://doi.org/10.1111/nep.13962.
Lee SM, Min YS, Son YK, Kim SE, An WS. Comparison of clinical outcome between incremental peritoneal dialysis and conventional peritoneal dialysis: a propensity score matching study. Ren Fail. 2021;43:1222–8. https://doi.org/10.1080/0886022X.2021.1960564.
Liu R, Ye H, Peng Y, Yi C, Lin J, Wu H, et al. Incremental peritoneal dialysis and survival outcomes: a propensity-matched cohort study. J Nephrol. 2023;36:1907–19. https://doi.org/10.1007/s40620-023-01735-4.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. https://doi.org/10.1016/j.ijsu.2021.105906.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. Oct. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 30 2020.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6. Cochrane; 2019. https://doi.org/10.1002/9781119536604.
Hayat A, Cho Y, Hawley CM, Htay H, Krishnasamy R, Pascoe E, et al. Association of Incremental peritoneal dialysis with residual kidney function decline in patients on peritoneal dialysis: the balANZ trial. Perit Dial Int. 2023;43:374–82. https://doi.org/10.1177/08968608231175826.
Naljayan M, Hunt A, McKeon K, Marlowe G, Schreiber MJ, Brunelli SM, et al. Use of incremental peritoneal dialysis: impact on clinical outcomes and quality of life measure. J Nephrol. 2023;36:1897–905. https://doi.org/10.1007/s40620-023-01703-y.
Indian J Nephrol. 2013;23:276–9. https://doi.org/10.4103/0971-4065.114496.
Sandrini M, Vizzardi V, Valerio F, Ravera S, Manili L, Zubani R, et al. Incremental peritoneal dialysis: a 10 year single-centre experience. J Nephrol. 2016;29:871–9. https://doi.org/10.1007/s40620-016-0344-z.
Yan H, Fang W, Lin A, Cao L, Ni Z, Qian J. Three Versus 4 daily exchanges and residual kidney function decline in Incident CAPD patients: a Randomized Controlled Trial. Am J Kidney Dis. 2017;69:506–13. https://doi.org/10.1053/j.ajkd.2016.08.019.
Lee Y, Chung SW, Park S, Ryu H, Lee H, Kim DK, et al. Incremental peritoneal Dialysis may be beneficial for preserving residual renal function compared to full-dose peritoneal Dialysis. Sci Rep. 2019;9:10105. https://doi.org/10.1038/s41598-019-46654-2.
Fernandes A, Matias P, Branco P. Incremental peritoneal dialysis: is it better for preservation of residual kidney function and clinical outcomes? Clin Nephrol. 2023;99:11–7. https://doi.org/10.5414/CN110958.
Qureshi MA, Hamidi S, Auguste BL. Five things to know about incremental peritoneal Dialysis. Can J Kidney Heal Dis. 2023;10:20543581231192748. https://doi.org/10.1177/20543581231192748.
Garofalo C, Borrelli S, De Stefano T, Provenzano M, Andreucci M, Cabiddu G, et al. Incremental dialysis in ESRD: systematic review and meta-analysis. J Nephrol. 2019;32:823–36. https://doi.org/10.1007/s40620-018-00577-9.
Cheetham MS, Cho Y, Krishnasamy R, Jain AK, Boudville N, Johnson DW, et al. Incremental Versus Standard (Full-Dose) peritoneal Dialysis. Kidney Int Rep. 2022;7:165–76. https://doi.org/10.1016/j.ekir.2021.11.019.
Yu X, Chen J, Ni Z, Chen N, Chen M, Dong J, et al. Number of daily peritoneal Dialysis exchanges and mortality risk in a Chinese Population. Perit Dial Int. 2018;38(Suppl 2):S53–63. https://doi.org/10.3747/pdi.2017.00283.
Diaz-Buxo JA, Youngblood BP, Torres AM. Delivered Dialysis Dose with PD Plus Therapy. Am J Nephrol. 1998;18:520–4. https://doi.org/10.1159/000013398.
Auguste BL, Bargman JM. Incremental peritoneal dialysis: new ideas about an old approach. Semin Dial. 2018;31:445–8. https://doi.org/10.1111/sdi.12712.
Bieber SD, Burkart J, Golper TA, Teitelbaum I, Mehrotra R. Comparative outcomes between continuous ambulatory and automated peritoneal dialysis: a narrative review. Am J Kidney Dis. 2014;63:1027–37. https://doi.org/10.1053/j.ajkd.2013.11.025.
de Fijter CW, Verbrugh HA, Oe LP, Peters ED, van der Meulen J, Donker AJ, et al. Peritoneal defense in continuous ambulatory versus continuous cyclic peritoneal dialysis. Kidney Int. 1992;42:947–50. https://doi.org/10.1038/ki.1992.371.
Chaudhary K. Peritoneal Dialysis Drop-out: causes and Prevention Strategies. Int J Nephrol. 2011;2011:434608. https://doi.org/10.4061/2011/434608.
Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–62. https://doi.org/10.1681/ASN.V12102158.
Han SH, Lee SC, Ahn SV, Lee JE, Kim DK, Lee TH, et al. Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transpl. 2007;22:2653–8. https://doi.org/10.1093/ndt/gfm242.
Liao C-T, Chen Y-M, Shiao C-C, Hu F-C, Huang J-W, Kao T-W, et al. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transpl. 2009;24:2909–14. https://doi.org/10.1093/ndt/gfp056.
Wong J, Vilar E, Davenport A, Farrington K. Incremental haemodialysis. Nephrol Dial Transpl. 2015;30:1639–48. https://doi.org/10.1093/ndt/gfv231.
Zhang M, Wang M, Li H, Yu P, Yuan L, Hao C, et al. Association of initial twice-weekly hemodialysis treatment with preservation of residual kidney function in ESRD patients. Am J Nephrol. 2014;40:140–50. https://doi.org/10.1159/000365819.
Lin Y-F, Huang J-W, Wu M-S, Chu T-S, Lin S-L, Chen Y-M, et al. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrol (Carlton). 2009;14:59–64. https://doi.org/10.1111/j.1440-1797.2008.01016.x.
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
SX conceived and designed the study. SX, WW, and JC collected the data, performed the literature search, and analyzed the data. SX was involved in the writing of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, S., Wu, W. & Cheng, J. Comparison of outcomes of incremental vs. standard peritoneal dialysis: a systematic review and meta-analysis. BMC Nephrol 25, 308 (2024). https://doi.org/10.1186/s12882-024-03669-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03669-w