Abstract
Background
As a natural antioxidant, uric acid plays a protective role against neurodegenerative disorders, including Parkinson’s disease (PD). Therefore, the risk of PD has been found to be lower in people with hyperuricemia. In this article, we conducted a systematic review and meta-analysis to investigate whether gout affects the future risk of developing PD.
Methods
We searched PubMed, Scopus, the Web of Science, and Google Scholar to find relevant studies, up to March 16, 2022. Studies investigating the risk of PD, following a gout diagnosis, were included if they were cross-sectional, case–control or cohort studies. The Newcastle Ottawa Scale (NOS) checklist was used to assess the quality of all included studies. The meta-analysis was performed using STATA 17.0.
Results
Ten studies were included, which were comprised of three case-controls, six cohort studies and one nested case–control study. We found no significant association between gout and the risk of PD among both sexes (RR = 0.94, 95% CI: 0.86–1.04), although the association was significant for females (RR = 1.09; 95% CI: 1.02–1.17). Subgroup analysis also showed no significant findings by age group, whether they were receiving treatment for gout, study design, quality assessment score, and method of gout ascertainment. In contrast, the studies that defined PD according to the use of drugs showed significant results (RR = 0.82; 95% CI: 0.76–0.89). There was a significant publication bias on the association between gout and PD.
Conclusions
The presence of gout had no significant effect on the risk of subsequently developing PD. Further analyses are recommended to investigate the effects of demographic and behavioral risk factors.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a chronic, progressive disease primarily characterized by the degeneration of dopaminergic nigrostriatal neurons [1,2,3]. PD is the second most common neurodegenerative disease, after Alzheimer’s disease [4,5,6,7,8]. The prevalence of PD is 0.3% among the general populations of industrialized countries, 1% in the population over 60 years old and 4% in the population over 80 years old [4, 8]. Several factors are likely implied in the pathophysiology of PD and ultimately in dopaminergic neuron loss and alpha-synuclein pathology [6, 7]. Evidence suggests that oxidative stress and free radical production play a critical role in PD pathogenesis [1, 9,10,11,12,13]. Additional factors, such as mitochondrial dysfunction, neuro-inflammation, and excitatory toxicity, may also contribute to neuronal cell damage in PD [14,15,16]. Perturbation of mitochondria homeostasis and production of reactive oxygen species may result from environmental factors or mutations in specific genes, such as the Leucine-Rich Repeat Kinase 2 (LRRK2) gene, or a combination of the two [14, 17]. Dopamine plays a fundamental role in the modulation of motor control and output of smooth and balanced movements [18]. Reduction in dopamine levels in PD results in symptoms such as bradykinesia, rigidity, resting tremors, and postural imbalance, which become manifest when 70–80% of dopamine-producing neurons are lost [1, 19, 20].
The intracellular metabolism of dopamine is prone to the production of free radicals, therefore exposing dopaminergic cells to a greater risk of oxidative stress toxicity [21]. Urate or uric acid, as a metabolite of purine, is a powerful natural antioxidant that has an important role in eliminating free radicals [7, 14, 19, 22, 23]. With this in mind, it has long been debated whether people who have higher levels of urate are at a lower risk of developing PD [7, 14, 22,23,24,25]. It should be noted that, apart from a lower risk of PD, high levels of urate in serum and cerebrospinal fluid have been associated with a slower rate of clinical progression [7, 14, 24, 25]. However, definitive mechanisms of action for urate as a neuro-protection substance have yet to be determined [26].
Since gout is a chronic form of hyperuricemia, there is a possibility that gout may have a protective effect on the development of PD. Therefore, the present systematic review and meta-analysis aimed to evaluate whether an association exists between gout and the subsequent onset of PD.
Methods
We performed this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27].
Search strategy
In the present study, two authors independently searched the electronic databases, including PubMed, Scopus and the Web of Science to find relevant studies from the inception of the databases up to March 16, 2022. Furthermore, we manually searched the first 100 pages of the Google Scholar search engine to identify any eligible studies. There were no limitations or restrictions in the search fields, such as study type, date, or language. We also used backward and forward citation searches of the included studies, in order to find as many relevant articles as possible. Our search strategy included all terms related to Gout and PD, which is described in detail in Supplementary Table 1.
Study selection
Two researchers independently screened the title and abstract of all identified articles, based on the eligibility criteria. Following this, the same researchers reviewed the full texts of all selected articles. The inclusion criteria were as follows: 1) Cross-sectional studies investigating the development of PD in subjects with gout; 2) Case–control or cohort studies comparing the risk of PD in participants with and without gout; 3) odd ratio (OR), hazard ratio (HR), or relative risk (RR) with 95% confidence interval (CI) were reported or could be calculated. We excluded those studies which included patients diagnosed with PD before being diagnosed with gout. In addition, case reports, case series, editorials, commentaries, letters, review articles, notes, news, book chapters, meta-analyses, and the re-analysis of previously published articles were excluded. Any disagreements between the two researchers were resolved by discussion or consultation with a third reviewer.
Data extraction
We conducted the data extraction process using predefined Microsoft Office Excel forms. Two authors independently collected the following information from each eligible study: first author, study title, year of publication, country of study, sample size, study population, age range of participants, follow-up duration, smoking status, status of anti-gout therapy, using caffeine or diuretic agents or non-steroidal anti-inflammatory drugs (NSAIDs) or other drugs that can play a role in the treatment or augment the risk of gout disease (e.g., Allopurinol, Colchicine, Tacrolimus, Cyclosporine, Probenecid, beta blockers, angiotensin converting enzyme inhibitors), comorbidities, diet status, Charlson-Romano comorbidity score, gout diagnosis method, PD ascertainment, and the reported effect sizes (OR, HR, and RR), along with their 95% CIs that controlled for any potential confounders. Any discrepancies between the two researchers were resolved by discussion or consulting another reviewer.
Quality assessment
To assess the risk of bias and study eligibility of the selected articles, two authors independently used the Newcastle Ottawa Scale (NOS) to evaluate and score each included study according to the different parameters [28]. In brief, this scale appraised the quality assessment of each study across three domains: selection of the participants for each group; the comparability between the study groups; and the ascertainment of exposure in the case-control study or the outcome of interest in a cohort study. A study can be awarded a maximum of one star for each numbered item within the selection, and exposure and outcome categories, while a maximum of two stars can be given for comparability. A third investigator resolved any disagreements between the two authors.
Statistical analysis
The meta-analysis was performed using Stata 17.0 (Stata Corp, LLC, TX). We assessed statistical heterogeneity using the Q test and I2 statistics. I-square values above 50% represent significant heterogeneity. In the case of significant heterogeneity, we performed a random effect analysis, otherwise a fixed effect model was used [29, 30]. The reported HRs were considered to be equal to RRs [31]. ORs were considered to be equal to RRs, if the incidence rate in the included studies was low (< 10%) or the ORs were between 0.5 and 2.5. Otherwise we converted the ORs to RRs, based on the method proposed by Zhang et al. [32]. We aimed to use funnel plots for evaluating publication bias, if at least ten studies were included [33]. Subgroup analysis was performed according to age, gout treatment status, study design, methodological quality scoring, and disease definition. Moreover, the Egger’s test was used to evaluate publication bias [34]. A p-value of less than 0.5 was treated as statistically significant.
Results
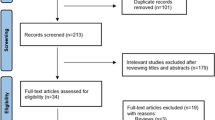
A total of 2,133 articles were identified from PubMed (n = 899), Scopus (n = 730), the Web of Science (n = 503), and Google Scholar (n = 1). Following the removal of 492 duplicate articles, the remaining 1,641 studies were screened and 75 records were selected for full text review. Sixty four publications were excluded because they did not report the outcome of interest. We could not access the full text of one article and thus it was also excluded [35]. The remaining ten records were used for qualitative and quantitative synthesis [36,37,38,39,40,41,42,43,44,45] (Fig. 1).
Study characteristics
Three studies were case-controls [38, 39, 45], one was a nested case–control [36], and six were retrospective cohort studies [37, 40,41,42,43,44]. The included articles were published between 2007 and 2022 and all were written in English. A total of 15.3 million participants were included in the meta-analysis, with 790,643 cases and about 14.5 million in the control group. Two studies reported data from the United Kingdom [36, 40], two from Taiwan [39, 43], and there was one study from each of the following countries: Canada [37], Denmark [38], Korea [44], Norway [41], the United States [42], and Spain [45]. Five studies reported that the cases used anti-gout therapies, like allopurinol, probenecid, and colchicine [36,37,38, 41, 44], but the level of urate and dietary patterns were not reported by any of the included articles. The comorbid conditions of participants are summarized in Supplementary Table 2. Gout diagnosis was based on clinical criteria in eight studies [36, 37, 39, 40, 42,43,44,45], while two studies based the diagnosis of gout on the concomitant use of anti-gout medications [38, 41]. Concerning diagnosis of PD, two studies diagnosed PD based on the use of therapeutic drugs (i.e. levodopa) [41, 45], while the rest used clinical criteria [36,37,38,39,40, 42,43,44]. Table 1 shows the characteristics of the included studies.
Overall analysis of the association between gout and PD
There was no significant association between gout and the subsequent development of PD (RR = 0.94, 95% CI: 0.86 to 1.04) (Fig. 2A). Moreover, the RRs of PD development in patients with gout were 0.92 (95% CI: 0.81 to 1.03) and 1.09 (95% CI: 1.02 to 1.17), among males and females respectively (Figs. 2B and Fig. 2C).
Subgroup analysis
Subgroup analysis was undertaken comparing age group, whether patients were receiving treatment for gout, study design, quality assessment scores, and type of gout and PD diagnoses. The pooled RRs for the age groups < 75 years old and ≥ 75 years of age were 0.95 (95% CI: 0.68 to 1.35) and 0.86 (95% CI: 0.67 to 1.09), respectively (Fig. 3A), and therefore not statistically significant. In terms of whether or not they were receiving treatment for gout, the treated patients had a higher RR than those who received no treatment (RR = 0.80; 95% CI: 0.62 to 1.02 vs. RR = 0.78; 95% CI: 0.61 to 1.00) (Fig. 3B), although this did not reach statistical significance. The subgroup analysis by study design showed no significant difference between the cohorts and case–control studies in the association between gout and PD (RR = 0.96; 95% CI: 0.86 to 1.08 for cohorts and RR = 0.92; 95% CI: 0.79 to 1.06 for case-controls) (Fig. 3C). However, studies with a quality assessment score of six (RR = 0.96; 95% CI: 0.86 to 1.06) had higher effect sizes than those with a score of seven (RR = 0.89; 95% CI: 0.70 to 1.14) (Fig. 3D), but this was not statistically significant. Studies which used clinical criteria for gout ascertainment had higher RRs than those which diagnosed gout based on the use of therapeutic drugs (RR = 0.95; 95% CI: 0.85 to 1.06 vs. RR = 0.92; 95% CI: 0.69 to 1.22, respectively), although both were non-significant (Fig. 3E). Finally, only two studies used drug administration to ascertain PD and these were statistically significant (RR = 0.82; 95% CI: 0.76 to 0.89) (Fig. 3F).
Risk of bias assessment
The mean risk of bias score was 6.3, which ranged from 6 to 7. Out of six cohort studies, three had an overall score of seven [37, 41, 43] and the remaining three had scores of six [40, 42, 44]. The adequacy of the follow-up duration and the assessment of outcomes were the lowest scoring criteria (Supplementary Table 3). All case–control studies had a quality assessment score of six. Failing to report non-response rates and poor representativeness of the cases received the lowest scores (Supplementary Table 4).
Publication bias
Egger’s test was statistically significant (p-value of 0.028), which indicates there was significant publication bias on the association between gout and PD. The funnel plot also shows an asymmetrical distribution of the included articles (Fig. 4).
Discussion
The present systematic review and meta-analysis was conducted with the aim of examining whether an association exists between gout and the subsequent development of PD. The ten publications included approximately 800 thousand gout patients and nearly 14.5 million non-gout cases. The study did not find an inverse association between a history of gout and PD in either sex, which is in contrast with previous evidence showing a lower risk of developing PD in males with gout [36], or in both sexes [37]. Interestingly, and unexpectedly, the present study found a higher risk of developing PD in females with gout, therefore gout does affect the risk of PD, at least in this subgroup. Furthermore, none of the subgroup analyses by study design, methodological quality scoring, and the definition of gout were able to link the incidence of PD with a history of gout. The only subgroup that was significant was an inverse association found between gout and PD when PD ascertainment was made according to therapeutic drug use.
A previous meta-analysis, conducted in 2015, included three case–control and two cohort studies and aimed to investigate the possible association between gout and PD [46]. This review made similar findings to our own, in that the risk of PD did not change in accordance with a prior history of gout in the overall sample (RR = 0.93, 95% CI 0.79 to 1.09), as well as for male participants (RR 0.89, 95% CI 0.57 to 1.39). However, they also found no association between gout and PD among females (RR 0.95, 95% CI, 0.76 to 1.19), which was in contrast with our pooled estimates [46]. This difference might be a result of the model used for the data analysis, since the previous review used a random-effect model for all estimates, while we used a fixed-effect model for the female subgroup, since the estimated heterogeneity did not exceed the required threshold. Therefore, understanding sex differences in the association between gout and the later development of PD requires further large-scale cohort studies.
Another controversial issue is the predictive value of uric acid levels in the subsequent risk of PD development. A meta-analysis on nearly five thousand participants was performed with the objective of comparing the levels of serum uric acid in PD patients with those of a control group. The study came to the conclusion that serum uric acid levels were substantially lower in patients with PD, compared to the control group (standardized mean difference [SMD] = -0.49, 95% CI -0.67 to -0.30), for both male (SMD = -0.66, 95% CI -0.87 to -0.44) and female (SMD = -0.53, 95% CI -0.70 to -0.35) cases [47]. Furthermore, multiple prospective cohort studies have reported that uric acid may have a protective role in the pathogenesis of PD and may also reduce the rate of disease progression [48,49,50]. It has also been demonstrated that oxidative stress is a major contributor to the degeneration of dopaminergic neurons and the further development of PD [1, 9]. On the other hand, a natural antioxidative feature of uric acid was beneficial in the brain tissue through the uptake of urate by Glute-9 transporters in dopaminergic neurons [51, 52]. Thus, the neuroprotective effect of uric acid may be due to the scavenging of reactive oxygen and nitrogen species [53,54,55]. According to our findings, gout and hyperuricemia did not decrease the risk of PD onset, regardless of sex, but instead increased the risk among female patients. Therefore, while a consensus has been reached regarding the protective role of uric acid, the debate remains regarding the association between gout and PD.
The following points may explain this inconsistency. Firstly, it could be hypothesized that the protective role of uric acid on the development of PD follows a dose-gradient, in which gradually increasing the level of serum uric acid up to the upper limit of the normal range represents an inverse association with PD onset, while above that level the association disappears overall and a positive association appears among female participants. Secondly, given that the majority of patients with hyperuricemia never develop gout, there may be a genetic predisposing factor in which uric acid might lose the neuroprotective properties when a patient presents with symptoms of gout. Nevertheless, it could be argued that there may be more than one genetic factor, either leading to hyperuricemia (without gout) and lowering the risk of PD, or causing hyperuricemia with gout and not protecting from PD. Another possibility is that there is one genetic factor that reduces the risk of developing PD and is collaterally associated with hyperuricemia, but that hyperuricemia is an epiphenomenon that has no direct protective effect on dopaminergic neurons. Thirdly, the condition of pro-inflammation and oxidative stress, due to the deposit of monosodium urate in gout attacks [56], might offset the antioxidative benefit of uric acid. Fourthly, urate-lowering agents are more frequently administrated for symptomatic patients with gout, compared to hyperuricemic asymptomatic patients. Thus, these medications may interfere with the protective effects of uric acid. Fifthly, gout is a well-documented risk factor for the development of cardiovascular diseases, diabetes, and chronic kidney disease [57]. As a result, gout sufferers are at increased risk of premature death, which may decrease the number of individuals reaching the peak ages for the development of PD. On the other hand, cardiovascular risk factors are associated with vascular parkinsonism, an extrapyramidal motor syndrome that mimics PD and could therefore be misdiagnosed. This highlights the importance of using accurate diagnostic criteria to select subjects and minimize biases of the analyses. In this respect, the only two studies that found a statistically significant lower risk of developing PD in patients with gout are those that ascertained PD according to the use of dopaminergic therapy: a good response to levodopa is a reliable predictor of PD diagnosis accuracy, meaning that these studies likely selected a good case population.
Apart from acting as an antioxidant molecule, a paradoxical pro-oxidative role has also been linked to uric acid [58]. By increasing the production of free radicals, uric acid has been proposed to induce inflammatory reactions [59]. The free radicals mainly target lipids and membranes, instead of other cellular components. The presence of unsaturated fatty acids in the lipid structure of neuronal membranes renders neurons highly vulnerable to lipid peroxidation. Therefore, it might be that high levels of uric acid have a dual-effect, depending on the time of exposure. In this regard, uric acid may exert its protective effect during the initial stages of the elevated levels, primarily due to its antioxidative features, but following long-term exposure to these elevated levels, after the diagnosis of gout, it may induce an inflammatory reaction that damages the brain tissue [60].
Under normal conditions, the leakage of uric acid molecules through the blood brain barrier is low. However, it has been shown that the concentration of uric acid is higher in the cerebrospinal fluid of males, than among females [61]. In addition, a recent investigation showed that uric acid might increase oxidative stress by increasing the production of reactive oxygen species, without affecting the scavenging process, particularly among females [62]. Therefore, female gout patients are less likely to benefit from the neuro-protective advantages of uric acid in the brain tissue and more likely to be exposed to the harmful oxidative metabolites of uric acid, compared to males. This results in the higher susceptibility of female patients to PD onset, as suggested by our findings.
7The present meta-analysis has several advantages over the earlier attempt [46]. Firstly, this is the most up-to-date meta-analysis examining the association between gout and the risk of subsequent PD development. Secondly, five large scale studies were included in our meta-analysis, which makes the final conclusions more robust. Thirdly, several subgroup analyses (i.e., based on age, status of gout treatment, study design, the methodological quality score, and disease definitions) were performed to more thoroughly understand any possible association. However, we acknowledge that the present study also has several limitations, which must be considered when interpreting our findings. Firstly, we could not conduct a subgroup analysis according to the type of urate lowering drug, due to a lack of data. Nevertheless, a study by Cortese and colleagues [41] found that patients with gout who were treated with allopurinol had a lower risk of developing PD. Therefore, future research is needed to evaluate the association between gout and PD, in the light of urate lowering drugs that gout patients may be using. Secondly, publication bias was identified in our study, according to the funnel plots and Egger’s test. However, the validity of these methods in samples consisting of ten or fewer studies has been called into question [63]. Thirdly, a high degree of heterogeneity was detected in our analysis, which might affect confidence in our findings. Fourthly, while we retrieved the effect sizes that controlled for several confounders, observational studies are always prone to residual biases that might not have been considered in the adjustment process. Fifthly, the majority of the included studies had medical record-based designs, which implies the possibility of data misclassification and limited the ability to evaluate the associations according to more disease characteristics and demographic factors. Sixthly, although comprehensive database searches were undertaken to identify all eligible publications, we cannot dismiss the probability of missing unpublished data. Seventhly, although some studies used the International Classification of Disease criteria for gout, there is currently no universally accepted diagnostic criteria for gout. Therefore, the studies included in our meta-analysis were mainly comprised of patients with gout that was defined by clinical manifestations or using anti gout medications, which should be considered when interpreting the findings. Similarly, while some studies used the International Classification of Disease criteria for defining PD, several studies based their definition on the use of relevant drugs or the opinions of expert neurologists. Therefore, subgroup analyses based on gout and PD ascertainment method were undertaken.
Conclusions
In contrast to several previous studies, which have found hyperuricemia to have a protective effect on the subsequent development of PD, our meta-analysis provides high-quality evidence that there is no overall association between gout and the risk of developing PD. In contrast, we found that females suffering from gout were more vulnerable to the subsequent onset of PD. Further observational investigations and meta-analyses, as well as more subgroup analyses of the demographic and behavioral risk factors (e.g. age, sex and smoking), are recommended in order to more clearly understand the association between gout and PD. In addition, the mechanism by which the sex differences occur, regarding the antioxidative and pro-oxidative features of uric acid, needs to be further investigated.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Duarte-Jurado AP, Gopar-Cuevas Y, Saucedo-Cardenas O, Loera-Arias MD, Montes-de-Oca-Luna R, Garcia-Garcia A, et al. Antioxidant Therapeutics in Parkinson's Disease: Current Challenges and Opportunities. Antioxidants. 2021;10(3).
Eusebi P, Franchini D, De Giorgi M, Abraha I, Montedori A, Casucci P, et al. Incidence and prevalence of Parkinson’s disease in the Italian region of Umbria: a population-based study using healthcare administrative databases. Neurol Sci. 2019;40(8):1709–12.
Schapira AH, Chaudhuri K, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(7):435–50.
De Rijk Md, Launer L, Berger K, Breteler M, Dartigues J, Baldereschi M, et al. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S21–3.
Rajput A. Frequency and cause of Parkinson’s disease. Can J Neurol Sci. 1992;19(S1):103–7.
Abbas MM, Xu ZY, Tan LC. Epidemiology of Parkinson’s Disease-East Versus West. Movement Disorders Clinical Practice. 2018;5(1):14–28.
Grażyńska A, Adamczewska K, Antoniuk S, Bień M, Toś M, Kufel J, et al. The Influence of Serum Uric Acid Level on Non-Motor Symptoms Occurrence and Severity in Patients with Idiopathic Parkinson's Disease and Atypical Parkinsonisms-A Systematic Review. Medicina (Kaunas). 2021;57(9).
Wirdefeldt K, Adami H-O, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26(1):1–58.
Jenner P. Oxidative stress in Parkinson’s disease. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 2003;53(S3):S26–38.
Chang KH, Chen CM. The Role of Oxidative Stress in Parkinson’s Disease. Antioxidants (Basel). 2020;9(7):597.
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, et al. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1994;36(3):348–55.
Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24(2):197-211.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nature Reviews Disease Primers. 2017;3(1):17013.
Crotty GF, Ascherio A, Schwarzschild MA. Targeting urate to reduce oxidative stress in Parkinson disease. Exp Neurol. 2017;298(Pt B):210–24.
Dorsey Ea, Constantinescu R, Thompson J, Biglan K, Holloway R, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384-6.
Kalia L, Lang A. Parkinson’s disease. Lancet Lond Engl. 2015;386:896–912.
Martin I, Kim JW, Dawson VL, Dawson TM. LRRK2 pathobiology in Parkinson’s disease. J Neurochem. 2014;131(5):554–65.
Kaur R, Mehan S, Singh S. Understanding multifactorial architecture of Parkinson’s disease: pathophysiology to management. Neurol Sci. 2019;40(1):13–23.
Ari BC, Tur EK, Domac FM, Kenangil GO. Uric acid: The role in the pathophysiology and the prediction in the diagnosis of Parkinson’s disease: A Turkish-based study. Ideggyogy Sz. 2022;75(1–02):51–9.
LANG AL A. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339(15):1044–53.
Przedborski S. Pathogenesis of nigral cell death in Parkinson’s disease. Parkinsonism Relat Disord. 2005;11:S3–7.
Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. The Lancet Neurology. 2016;15(12):1257–72.
Bakshi R, Logan R, Schwarzschild MA. Purines in Parkinson’s: Adenosine A 2A receptors and urate as targets for neuroprotection. The Adenosinergic System: Springer; 2015. p. 101–26.
Fernandez IH, Jesus S, Lojo JA, Gomez FJG, Redondo MC, Ruiz JMO, et al. Lower levels of uric acid and striatal dopamine in non-tremor dominant Parkinson’s disease subtype. Mov Disord. 2016;31:S250–1.
Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, Hare JM, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014;71(2):141–50.
Bakshi R, Macklin EA, Logan R, Zorlu MM, Xia N, Crotty GF, et al. Higher urate in LRRK2 mutation carriers resistant to Parkinson disease. Ann Neurol. 2019;85(4):593–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Deeks JJ, Higgins JP, Altman DG, Group obotCSM. Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions. 2019. p. 241–84.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Symons M, Moore D. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55(9):893–9.
Zhang J, Kai FY. What’s the relative risk?: A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Liu J, Dan X, Zhang H, Chan P. Serum uric acid and lifestyle factors associated with the risk of Parkinson’s disease. 2016;49:548–52.
Alonso A, Garcia Rodriguez LA, Logroscino G, Hernan MA. Gout and risk of Parkinson disease - A prospective study. Neurology. 2007;69(17):1696–700.
De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H. Gout and the risk of Parkinson’s disease: a cohort study. Arthritis Rheum. 2008;59(11):1549–54.
Schernhammer E, Qiu JH, Wermuth L, Lassen CF, Friis S, Ritz B. Gout and the risk of Parkinson’s disease in Denmark. Eur J Epidemiol. 2013;28(4):359–60.
Lai SW, Lin CH, Lin CL, Liao KF. Gout and Parkinson’s Disease in Older People: An Observation in Taiwan. Int J Gerontol. 2014;8(3):166–7.
Pakpoor J, Seminog OO, Ramagopalan SV, Pakpoor J, Seminog Goldacre MJ. Clinical associations between gout and multiple sclerosis, Parkinson's disease and motor neuron disease: record-linkage studies. BMC Neurol. 2015;15:16.
Cortese M, Riise T, Engeland A, Ascherio A, Bjørnevik K. Urate and the risk of Parkinson’s disease in men and women. Parkinsonism Relat Disord. 2018;52:76–82.
Singh JA, Cleveland JD. Gout and the risk of Parkinson's disease in older adults: a study of U.S. Medicare data. BMC Neurol. 2019;19(1):4.
Hu LY, Yang AC, Lee SC, You ZH, Tsai SJ, Hu CK, et al. Risk of Parkinson’s disease following gout: a population-based retrospective cohort study in Taiwan. BMC Neurol. 2020;20(1):338.
Kim JH, Choi IA, Kim A, Kang G. Clinical Association between Gout and Parkinson’s Disease: A Nationwide Population-Based Cohort Study in Korea. Medicina (Kaunas). 2021;57(12):1292.
Pou MA, Orfila F, Pagonabarraga J, Ferrer-Moret S, Corominas H, Diaz-Torne C. Risk of Parkinson’s disease in a gout Mediterranean population: A case-control study. Joint Bone Spine. 2022;89(6):105402.
Ungprasert P, Srivali N, Thongprayoon C. Gout is not associated with a lower risk of Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2015;21(10):1238–42.
Wen M, Zhou B, Chen Y-H, Ma Z-L, Gou Y, Zhang C-L, et al. Serum uric acid levels in patients with Parkinson’s disease: A meta-analysis. PLoS ONE. 2017;12(3):e0173731.
Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson’s disease in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2009;169(9):1064–9.
Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;70(1):268–75.
Yu Z, Zhang S, Wang D, Fan M, Gao F, Sun W, et al. The significance of uric acid in the diagnosis and treatment of Parkinson disease: An updated systemic review. Medicine (Baltimore). 2017;96(45):e8502-e.
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015;9:91.
Bi M, Jiao Q, Du X, Jiang H. Glut9-mediated urate uptake is responsible for its protective effects on dopaminergic neurons in Parkinson’s disease models. Front Mol Neurosci. 2018;11:21.
Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF. Uric acid as a CNS antioxidant. J Alzheimers Dis. 2010;19(4):1331–6.
Chen X, Burdett TC, Desjardins CA, Logan R, Cipriani S, Xu Y, et al. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci. 2013;110(1):300–5.
Huang T-T, Hao D-L, Wu B-N, Mao L-L, Zhang J. Uric acid demonstrates neuroprotective effect on Parkinson’s disease mice through Nrf2-ARE signaling pathway. Biochem Biophys Res Commun. 2017;493(4):1443–9.
Khalfina T, Maksudova A. AB0849 Oxidative Stress, Anti-Oxidant Activity in Patients with Gout. Ann Rheum Dis. 2014;73(Suppl 2):1083.
Stamp LK, Chapman PT. Gout and its comorbidities: implications for therapy. Rheumatology (Oxford). 2013;52(1):34–44.
Kang D-H, Ha S-K. Uric acid puzzle: dual role as anti-oxidantand pro-oxidant. Electrolytes & Blood Pressure. 2014;12(1):1–6.
Glantzounis G, Tsimoyiannis E, Kappas A, Galaris D. Uric acid and oxidative stress. Curr Pharm Des. 2005;11(32):4145–51.
Mijailovic NR, Vesic K, Borovcanin MM. The Influence of Serum Uric Acid on the Brain and Cognitive Dysfunction. Front Psychiatry. 2022;13:828476.
Reavis ZW, Mirjankar N, Sarangi S, Boyle SH, Kuhn CM, Matson WR, et al. Sex and race differences of cerebrospinal fluid metabolites in healthy individuals. Metabolomics. 2021;17(2):1–13.
Kurajoh M, Fukumoto S, Yoshida S, Akari S, Murase T, Nakamura T, et al. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci Rep. 2021;11(1):1–9.
Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–29.
Acknowledgements
None.
Funding
The present study was supported by the Tabriz University of Medical Sciences, Tabriz, Iran (Grant No. 69481).
Author information
Authors and Affiliations
Contributions
SAN, NJ, KG, AAK and SS conceptualized the topic; NJ searched the databases; AF and MZ performed screening and full-text review; AF and HA performed data extraction and quality assessment; NJ and MN performed statistical analysis; AF, MJMS, HA, SAN, MN, KG, AAK and SS prepared the first draft of the manuscript; SAN and MN critically revised and edited the manuscript; SS, KG, and AAK supervised this project. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not required.
Competing interests
No conflict of interest declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Search strategies for PubMed, Scopus, Web of Science, and Google scholar. Supplementary Table 2. Comorbid conditions of individuals who participated in the studies included in the meta-analysis. Supplementary Table 3. Risk of bias assessment for the included cohort studies. Supplementary Table4. Risk of bias assessments for the included case-control studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fazlollahi, A., Zahmatyar, M., Alizadeh, H. et al. Association between gout and the development of Parkinson’s disease: a systematic review and meta-analysis. BMC Neurol 22, 383 (2022). https://doi.org/10.1186/s12883-022-02874-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02874-0