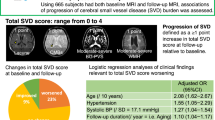
Abstract
Background
Intracranial artery stenosis (ICAS) and cerebral small vessel disease (CSVD) are associated with a heavy socioeconomic burden; however, their longitudinal changes remain controversial.
Methods
We conducted a longitudinal analysis on 756 participants of Shunyi Cohort who underwent both baseline and follow-up brain magnetic resonance imaging (MRI) and MR angiography in order to investigate the risk factors for ICAS and CSVD progression in community population. Incident ICAS was defined as new stenosis occurring in at least one artery or increased severity of the original artery stenosis. CSVD markers included lacunes, cerebral microbleeds (CMB), and white matter hyperintensities (WMH).
Results
After 5.58 ± 0.49 years of follow-up, 8.5% of the 756 participants (53.7 ± 8.0 years old, 65.1% women) had incident ICAS. Body mass index (BMI) (OR = 1.09, 95% CI = 1.01–1.17, p = 0.035) and diabetes mellitus (OR = 2.67, 95% CI = 1.44–4.93, p = 0.002) were independent risk factors for incident ICAS. Hypertension was an independent risk factor for incident lacunes (OR = 2.12, 95% CI = 1.20–3.77, p = 0.010) and CMB (OR = 2.32, 95% CI = 1.22–4.41, p = 0.011), while WMH progression was primarily affected by BMI (β = 0.108, SE = 0.006, p = 0.002). A higher LDL cholesterol level was found to independently protect against WMH progression (β = −0.076, SE = 0.027, p = 0.019).
Conclusions
Modifiable risk factor profiles exhibit different in patients with ICAS and CSVD progression. Controlling BMI and diabetes mellitus may help to prevent incident ICAS, and antihypertensive therapy may conduce to mitigate lacunes and CMB progression. LDL cholesterol may play an inverse role in large arteries and small vessels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Intracranial artery stenosis (ICAS) and cerebral small vessel disease (CSVD) are common causes of stroke worldwide and result in a heavy socioeconomic burden [1, 2]. They may have a long asymptomatic period, which can only be detected via vascular or structural brain imaging examinations [3]. Therefore, characterizing their progression and investigating their risk factors are clinically valuable.
Prior investigations have mainly estimated large arteries using computed tomography or ultrasonography once at baseline [4, 5]; therefore, a more precise and dynamic assessment of ICAS through magnetic resonance angiography (MRA) is meaningful. To date, studies investigating the deterioration of intracranial large arteries and small vessels in the general population, based on a longitudinal community cohort, are scarce [5].
Thus, based on the prospective Shunyi Cohort Study, we aimed to describe the five-year longitudinal changes and investigate the risk factors differences in progression of ICAS and CSVD.
Methods
Population
The present study is a longitudinal analysis of an ongoing community-based Shunyi Cohort Study in China [6]. All residents aged ≥ 35 years living in the five villages of Shunyi were invited to participate. Figure 1 presents a flowchart of the participant inclusion and exclusion. From June 2013 to April 2016, 1257 individuals finished baseline magnetic resonance imaging (MRI) examinations. After an average period of 5.58 years (standard deviation [SD] = 0.49, P25–P75:5.22–5.92, max = 7.20, min = 4.34), 756 stroke-free participants with both baseline and follow-up MRI and MRA were included in the final analysis. The research was conducted according to the Declaration of Helsinki. Written informed consent was obtained from all the participants. All study protocols were approved by the Ethical Committee of Peking Union Medical College Hospital (reference number: B-160).
Brain MRI and imaging analysis
MRI was performed using a single 3-T Siemens Skyra scanner (Siemens, Germany). All CSVD imaging markers were defined in accordance with the Standards for Reporting Vascular Changes in Neuroimaging [7]. In brief, lacunes were defined as focal fluid-filled cavities 3–15 mm in diameter situated in the basal ganglia, subcortical white matter, or brainstem. CMB were defined as small, round, or ovoid hypointense lesions observed on susceptibility-weighted imaging. White matter hyperintensities (WMH) were automatically segmented by the lesion growth algorithm as implemented in the lesion segmentation tool (LST) toolbox (http://www.statistical-modelling.de/lst.html) for Statistical Parametric Mapping at κ = 0.15 [8].
Arterial stenosis was assessed using MRI at the site with the most severe degree of stenosis on MRA [9]. ICAS was defined as any degree of stenosis in at least one of the following arteries: internal carotid artery, middle cerebral artery, anterior cerebral artery, intracranial segment of the vertebral artery, basilar artery, or posterior cerebral artery.
Incident lacunes and CMB were defined as one or more new lesions observed during follow-up imaging. WMH progression was described as the annualized volume change ([follow-up WMH volume − baseline WMH volume]/follow-up duration). Incident ICAS was defined as new stenosis occurring in at least one artery or an increase in the severity of the original arterial stenosis compared to that at baseline. Figure 2 showed illumination of incident ICAS. Trained physicians who were blinded to all clinical data independently evaluated the ICAS, lacunes, and CMB. Intra-rater agreements have been described elsewhere [10].
Illumination of incident intracranial artery stenosis (ICAS). Figure 2A shows a participant without ICAS at baseline and Fig. 2B shows incident stenosis in the bilateral posterior cerebral arteries at follow-up (arrow). Figure 2C shows a participant with stenosis in the left middle cerebral artery at baseline, while Fig. 2D shows worsened stenosis in the left middle cerebral artery at follow-up (arrowhead)
Assessments of covariates
Baseline demographics and vascular risk factors were estimated using structured interviews, physical examinations, and laboratory tests. Blood pressure was measured three times, and the mean value was used. Venous blood samples, routinely drawn after overnight fasting, were analyzed for plasma glucose and lipid levels. Hypertension was defined as self-reported hypertension, treatment with antihypertensive medication, systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg. Diabetes mellitus was defined as self-reported diabetes, use of oral hypoglycemic drugs or insulin, fasting serum glucose ≥ 7.0 mmol/L, or hemoglobin A1c ≥ 6.5%.
Statistical analysis
Data are presented as mean (SD), median (P25–P75), or number (percentage). We applied multivariable linear or logistic regression models to investigate the potential independent risk factors for incident ICAS and progressive CSVD markers, as appropriate. Based on medical knowledge, previous reports, and univariate analysis results, the following variables were considered candidate risk factors: age; sex; and conventional vascular risk factors, including body mass index, baseline hypertension, diabetes mellitus, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and current smoking. WMH progression was corrected to the baseline brain parenchymal fraction to quantify WMH volumetric changes over time. Statistical significance was set at a two-sided p < 0.05. All analyses were performed using SPSS version 19.0 (IBM Co., USA).
Results
Table 1 presents the baseline and follow-up characteristics of the study population. Altogether, 756 participants with a mean age of 53.7 years (SD = 8.0), of whom 492 were women (65.1%), were included in the analysis. Among the 492 females, 287 (58.3%) had gone through the menopause. Table S1 showed the demographics of participants with and without both the baseline and follow-up MRI. Compared with participants with both the baseline and follow-up MRI imaging (n = 784), people without both the baseline and follow-up MRI examination (n = 802) had more advanced age (59.5 vs. 53.8, p < 0.001); higher portion of men (44.3% vs. 35.6%, p < 0.001); and a higher prevalence of baseline hypertension (58.3% vs. 47.7%, p < 0.001), diabetes mellitus (20.9% vs. 14.0%, p < 0.001), and current smoker (26.8% vs. 21.2%, p < 0.011).
At baseline, 10.7% (81/756) of the participants had ICAS. The prevalence of ICAS increased from 3.4% (8/236) at 35–50 years to 11.6% (52/447) at 50–65 years, and further increased to 28.8% (21/73) at ≥ 65 years. In total, 11.6% (88/756) and 9.3% (70/765) of the participants had baseline lacunes and CMB, respectively. The baseline WMH volume was 2.72 (1.81–4.45) cm3. After an average of 5.58 years (SD = 0.49) of follow-up, 8.5% (64/756) of the participants had incident ICAS; therefore, 55 participants had new artery stenosis and 9 had increased severity of the original artery stenosis. The incident rate also significantly increased with age, which was 4.7% (11/236), 8.9% (40/447), 17.8% (13/73) for 35 ≤ age<50 years, 50 ≤ age<65, and age ≥ 65 years, respectively. A total of 10.3% (78/756) of the participants had incident lacunes, and 8.2% (62/756) had incident CMB. The mean follow-up WMH volume was 4.30 (2.73–7.13) cm3. The annual increase in the WMH volume increase was 0.44 (0.61) cm3/y. Detailed data on the progression of CSVD in different age groups are listed in Table S2.
Risk factors of incident ICAS
Table 2 presents the multivariable logistic regression results of incident ICAS, and all independent variables of the multiple logistic regression analysis, except for the follow-up time. Age (per year, OR = 1.05, 95% CI = 1.02–1.10, p = 0.004), BMI (per kg/m2, OR = 1.09, 95% CI = 1.01–1.17, p = 0.035), and diabetes mellitus (OR = 2.67, 95% CI = 1.44–4.93, p = 0.002) were independent risk factors for incident ICAS.
Risk factors of CSVD progression
Table 3 summarizes the multivariate logistic regression results for CSVD progression. Hypertension (OR = 2.12, 95% CI = 1.20–3.77, p = 0.010) and baseline lacunes burden (OR = 3.91, 95% CI = 2.09–7.31, p < 0.001) were independent risk factors for incident lacunes. Age (per year, OR = 1.04, 95% CI = 1.01–1.08, p = 0.029), hypertension (OR = 2.32, 95% CI = 1.22–4.41, p = 0.011), and baseline CMB (OR = 4.16, 95% CI = 2.13–8.14, p < 0.001) were risk factors for incident CMB. WMH progression was primarily influenced by age (β = 0.076, SE = 0.003, p = 0.046), BMI (per kg/m2, β = 0.108, SE = 0.006, p = 0.002), and baseline WMH volume (β = 0.553, SE = 0.031, p < 0.001). Interestingly, LDL cholesterol (per mmol/L, β = −0.076, SE = 0.027, p = 0.019) was identified as an independent protective factor for WMH progression. Hypertension was significantly associated with WMH progression.
Discussion
In this community-based longitudinal study, we investigated the progression of intracranial large artery and cerebral small-vessel disease, and their association. This study has three important findings. First, the progression of ICAS and MRI markers of CSVD was remarkable in this population. Second, incident ICAS and CSVD progression have different risk factor profiles. A higher BMI and the presence of diabetes mellitus increased the risk of incident ICAS, whereas hypertension elevated the risk of incident lacunes and CMB. Third, a higher LDL cholesterol level was identified as an independent protective factor against WMH progression.
The cross-sectional prevalence of ICAS in population-based studies has been reported as 6–13% [1]. The prevalence of this condition among East Asian people is higher than that among Western populations [1]; the prominent growth rate might be explained by the racial characteristics of Asians [11,12,13] and the poor management of vascular risk factors [12]. However, limited data are available on ICAS progression in community populations. Ryu et al. reported that in 12% (8/65) of Korean participants (mean age, 64 years) with asymptomatic ICAS, the condition progressed after a 5.7-year follow-up [14]. In the present longitudinal study, baseline ICAS progressed in 11% (9/81) of the participants. The incidence rates of ICAS in the population with baseline ICAS were similar.
The Rotterdam Study (mean age, 71 years) reported that 8% of patients aged 60–70 years had an incident lacunar infarction after a 3.4-year follow-up [15]. Compared to the corresponding age group, 20% (34/165) had incident lacunes in our study. The Rotterdam Study also reported that 7.6% of patients aged 60–70 years had incident CMB, [16] whereas the rate in our study was 16.4% (27/165). Although the follow-up duration was longer than that of the Rotterdam Study, the incidence rates of lacunes and CMB in the same age range were significantly higher. The Framingham Offspring Study revealed an annualized WMH volume change of 0.11 (0.22) cm3/y in patients aged <65 years and 0.42 (0.64) cm3/y in those aged ≥ 65 years [17]. Meanwhile, the annualized WMH volume change in our study was 0.49 (0.70) cm3/y in the 50 ≤ age<65 group and 0.74 (0.70) cm3/y in those aged ≥ 65. Overall, the progression of CSVD in the Shunyi Study was more remarkable than that in the Western population, which is consistent with previous research demonstrating that Asians have a higher risk of CSVD.
Previous cross-sectional studies have indicated that hypertension, diabetes mellitus, hypercholesterolemia, and smoking are common modifiable vascular risk factors for ICAS [1, 18]. A cross-sectional investigation revealed a similar conclusion that hypertension, diabetes mellitus, higher LDL cholesterol levels, and lower HDL cholesterol levels were associated with ICAS [10]. However, in this longitudinal study, BMI and diabetes mellitus were the only modifiable risk factors for incident ICAS, whereas hypertension and hypercholesterolemia were not. This confirmed the adverse effect of metabolic disturbance in intracranial large arteries, [19] implying that the influence of glucolipid metabolism on ICAS was prominent within 5 years, and highlighted that the primary prevention for this population should focus on the management of blood glucose and weight.
Our previous cross-sectional study on CSVD indicated that hypertension and diabetes mellitus were independently associated with lacunes, while no significant association was identified between vascular risk factors and CMB. In this follow-up study, hypertension was independently associated with the incidence of lacunes and CMB [20]. These longitudinal results were similar to those of the Rotterdam Study, which demonstrated that hypertension was the only independent vascular risk factor for incident CMB but no vascular risk factor for incident lacunes was determined [15, 16]. Previous longitudinal population-based studies have reported that hypertension or higher systolic blood pressure is significantly associated with WMH progression [15, 21,22,23]. The Framingham Offspring Study even observed that hypertension at midlife was associated with an annual WMH volume increase [17]. Hypertension was related to baseline WMH volume, but not to WMH progression in the Shunyi Study. In contrast, BMI was an independent risk factor for WMH progression. The influence of blood pressure on WMH may involve long-term accumulation, whereas obesity can aggravate WMH progression in the short term. Moreover, higher LDL cholesterol levels prevented WMH progression. Several cross-sectional studies have reported a similar conclusion [24, 25]. A longitudinal population-based Cardiovascular Health Study also identified a negative correlation between LDL levels and worsening WMH grade and even observed that the use of statins aggravated WMH progression during a 5-year follow-up [22]. LDL cholesterol plays an essential role in the development of the central nervous system and in the creation and maintenance of new synapses [26, 27]. Additionally, it may share some genetic burden with WMH [28, 29]. Although the detailed pathogenesis of LDL cholesterol and WMH merits further investigation, it indicates a different mechanism for small- and large-vessel diseases and warrants more cautious consideration for intensive lipid-lowering therapy.
According to the results of our investigation, the progression of ICAS and CSVD had different modifiable risk factor profiles, which led to inconsistent characteristics between the intracranial large arteries and small vessels. Distinct risk factor profiles were also observed among MRI markers of CSVD, which raised an alarm for nonspecific prophylactic treatment when CSVD was considered a disease entity.
This study has several limitations. First, bias may have been caused by participants without complete longitudinal imaging detection because they had a higher prevalence of vascular risk factors and worse status of both large and small vessels. Second, we did not include patients with extracranial carotid atherosclerosis in this analysis because the proportion of people with carotid stenosis was low (5.5%) in the Shunyi Study [30]. Although previous studies have demonstrated that the prevalence of extracranial atherosclerotic disease embolism is relatively uncommon in the study population and likely plays a minor causative role in the burden of lacunes, [31] it may provide an overall insight into cervico-cerebral large artery stenosis. Third, MRA could not be used to visualize the arterial wall. Therefore, lumen-based measurements may underestimate both the atherosclerotic burden and plaque-related outward remodeling.
Conclusions
The 5-year progression of ICAS and CSVD has different modifiable risk factor profiles. Large arteries may be vulnerable to glucolipid metabolism disorder, whereas small vessels are susceptible to hypertension. LDL cholesterol may play an opposite role in large arteries and small vessels. Further intensive lipid-lowering therapy should be targeted and refined.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICAS:
-
Intracranial artery stenosis
- CSVD:
-
Cerebral small vessel disease
- CMB:
-
Cerebral microbleeds
- WMH:
-
White matter hyperintensities
- MRI:
-
Magnetic resonance imaging
- MRA:
-
Magnetic resonance angiography
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- LST:
-
Lesion segmentation tool
References
Gutierrez J, Turan TN, Hoh BL, Chimowitz MI. Intracranial atherosclerotic stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2022;21(4):355–68.
Markus HS, van Der Flier WM, Smith EE, Bath P, Biessels GJ, Briceno E, Brodtman A, Chabriat H, Chen C, de Leeuw FE et al. Framework for clinical trials in Cerebral Small Vessel Disease (FINESSE): a review. JAMA Neurol 2022.
Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of Vascular Brain Injury: a systematic review and Meta-analysis. JAMA Neurol. 2019;76(1):81–94.
Kneihsl M, Hofer E, Enzinger C, Niederkorn K, Horner S, Pinter D, Fandler-Hofler S, Eppinger S, Haidegger M, Schmidt R, et al. Intracranial pulsatility in relation to Severity and Progression of Cerebral White Matter Hyperintensities. Stroke. 2020;51(11):3302–9.
Vinke EJ, Yilmaz P, van der Toorn JE, Fakhry R, Frenzen K, Dubost F, Licher S, de Bruijne M, Kavousi M, Ikram MA, et al. Intracranial arteriosclerosis is related to cerebral small vessel disease: a prospective cohort study. Neurobiol Aging. 2021;105:16–24.
Han F, Zhou LX, Ni J, Yao M, Zhai FF, Liu YT, Wu W, Xue HD, Li ML, Yang M, et al. Design of the Shunyi study on cardiovascular disease and age-related brain changes: a community-based, prospective, cohort study. Ann Transl Med. 2020;8(23):1579.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–38.
Schmidt P, Gaser C, Arsic M, Buck D, Forschler A, Berthele A, Hoshi M, Ilg R, Schmid VJ, Zimmer C, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. NeuroImage. 2012;59(4):3774–83.
Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643–6.
Zhai FF, Yan S, Li ML, Han F, Wang Q, Zhou LX, Ni J, Yao M, Zhang SY, Cui LY, et al. Intracranial arterial dolichoectasia and stenosis: risk factors and relation to Cerebral Small Vessel Disease. Stroke. 2018;49(5):1135–40.
Shitara S, Fujiyoshi A, Hisamatsu T, Torii S, Suzuki S, Ito T, Arima H, Shiino A, Nozaki K, Miura K, et al. Intracranial Artery Stenosis and its Association with conventional risk factors in a General Population of Japanese men. Stroke. 2019;50(10):2967–9.
Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, Wang Y, Zou X, Leung TW, Cai Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese intracranial atherosclerosis (CICAS) study. Stroke. 2014;45(3):663–9.
Kim YD, Choi HY, Cho HJ, Cha MJ, Nam CM, Han SW, Nam HS, Heo JH. Increasing frequency and burden of cerebral artery atherosclerosis in Korean stroke patients. Yonsei Med J. 2010;51(3):318–25.
Ryu WS, Park SS, Kim YS, Lee SH, Kang K, Kim C, Sohn CH, Lee SH, Yoon BW. Long-term natural history of intracranial arterial stenosis: an MRA follow-up study. Cerebrovasc Dis. 2014;38(4):290–6.
van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam scan study. Stroke. 2008;39(10):2712–9.
Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, Vernooij MW. Incidence of cerebral microbleeds in the general population: the Rotterdam scan study. Stroke. 2011;42(3):656–61.
Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–8.
Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12(11):1106–14.
Shu MJ, Zhai FF, Zhang DD, Han F, Zhou L, Ni J, Yao M, Zhang SY, Cui LY, Jin ZY, et al. Metabolic syndrome, intracranial arterial stenosis and cerebral small vessel disease in community-dwelling populations. Stroke Vasc Neurol. 2021;6(4):589–94.
Han F, Zhai FF, Wang Q, Zhou LX, Ni J, Yao M, Li ML, Zhang SY, Cui LY, Jin ZY, et al. Prevalence and risk factors of Cerebral Small Vessel Disease in a Chinese Population-based sample. J Stroke. 2018;20(2):239–46.
Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian stroke Prevention Study. Neurology. 1999;53(1):132–9.
Longstreth WT Jr., Arnold AM, Beauchamp NJ Jr., Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O’Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36(1):56–61.
Scharf EL, Graff-Radford J, Przybelski SA, Lesnick TG, Mielke MM, Knopman DS, Preboske GM, Schwarz CG, Senjem ML, Gunter JL, et al. Cardiometabolic Health and Longitudinal Progression of White Matter Hyperintensity: the Mayo Clinic Study of Aging. Stroke. 2019;50(11):3037–44.
Chung CP, Chou KH, Peng LN, Liu LK, Lee WJ, Chen LK, Lin CP, Wang PN. Associations between low circulatory low-density lipoprotein cholesterol level and brain health in non-stroke non-demented subjects. NeuroImage. 2018;181:627–34.
Jimenez-Conde J, Biffi A, Rahman R, Kanakis A, Butler C, Sonni S, Massasa E, Cloonan L, Gilson A, Capozzo K, et al. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke. 2010;41(3):437–42.
Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294(5545):1354–7.
Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29(2):190–201.
Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D’Agostino RB, DeCarli C. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 2004;35(7):1609–13.
Austin MA, Edwards KL, McNeely MJ, Chandler WL, Leonetti DL, Talmud PJ, Humphries SE, Fujimoto WY. Heritability of multivariate factors of the metabolic syndrome in nondiabetic Japanese americans. Diabetes. 2004;53(4):1166–9.
Han F, Zhang DD, Zhai FF, Xue J, Zhang JT, Yan S, Zhou LX, Ni J, Yao M, Yang M, et al. Association between large artery stenosis, cerebral small vessel disease and risk of ischemic stroke. Sci China Life Sci. 2021;64(9):1473–80.
Del Brutto OH, Mera RM, Espinosa V, Nader JA, Zambrano M, Simon LV, Parikh PR, Castillo PR, Matcha G. Distribution of Cervicocephalic Atherosclerotic Lesions and their correlation with Cardiovascular Risk factors in a Population of amerindians. The Atahualpa Project. J Stroke Cerebrovasc Dis. 2018;27(11):3356–64.
Acknowledgements
The authors are grateful to the study participants, and the staff of the Shunyi Study.
Funding
This study was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS #2021-I2M-1-025), National High Level Hospital Clinical Research Funding (2022-PUMCH-A-068) and National Natural Science Foundation of China (82271368).
Author information
Authors and Affiliations
Contributions
ZA.P.: conception, design of the work, acquisition, analysis, interpretation of data, drafted the work and substantively revised it; DD. Z.: acquisition, analysis, interpretation of data, drafted the work; ZY. L.: interpretation of data, new software used; MJ. S.: interpretation of data, new software used; FF Z.: interpretation of data, new software used; M. Y.: conception, interpretation of data; LX. Z.: conception, interpretation of data; J. N.: conception, interpretation of data; ZY. J.: conception, interpretation of data; SY. Z.: conception, interpretation of data; LY. C.: conception, interpretation of data; F. H.: conception, design of the work, acquisition, interpretation of data, substantively revised; YC. Z.: conception, design of the work, acquisition, interpretation of data, substantively revised.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from all the participants. All study protocols were approved by the Ethical Committee of Peking Union Medical College Hospital (reference number: B-160).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pan, ZA., Zhang, DD., Liu, ZY. et al. Risk factor differences in five-year progression of Intracranial artery stenosis and cerebral small vessel disease in general population. BMC Neurol 24, 328 (2024). https://doi.org/10.1186/s12883-024-03835-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-024-03835-5