Abstract
Objective
We aimed to evaluate the heterogeneity of gestational diabetes mellitus (GDM) patients diagnosed with various screening criteria.
Methods
We stratified pregnant women using consecutive fasting plasma glucose (FPG) and 2-hour postprandial plasma glucose (2hPPG) intervals of 0.2 mmol/L. The incidence of abnormal neonatal birthweight and birth-related adverse outcomes was compared with that of pregnant women without GDM.
Results
The study included 39,988 pregnant women (18–45 years, mean [SD], 31.5 [4.7] years) in Ningbo, China. The means (SDs) of FPG and 2hPPG within 24–28 weeks of gestation were 4.5 (0.5) and 6.8 (1.3) mmol/L, respectively. A total of 3025 (7.6%) women had 5.1–6.9 mmol/L FPG and 4560 (11.4%) had 8.5–11.0 mmol/L 2hPPG. The incidence of GDM according to the two combination criteria was 17.3% (6908 cases). The relative risk (RR) for < 10th percentile birthweight (< 10th WT) was 0.82 (95% CI, 0.74–0.91, p < 0.001) by 5.1 mmol/L FPG criterion and 1.14 (95% CI, 1.06–1.23, p < 0.001) by 8.5 mmol/L 2hPPG criterion, while the RRs for > 90th percentile birthweight (> 90th WT) were 1.48 (95% CI, 1.35–1.63, p < 0.001) and 0.95 (95% CI, 0.86–1.04, p = 0.29) according to the corresponding criteria. The FPG criterion was more strongly associated with maternal hypertension than the 2hPPG criterion. Both criteria did not show a distinct association with other composite adverse outcomes.
Conclusion
High FPG is significantly associated with high birth weight, whereas high 2hPPG is slightly associated with low birth weight. Our findings highlight the heterogeneity of patients with GDM diagnosed by different criteria.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM) refers to glucose intolerance with onset or first recognition during pregnancy [1]. GDM is considered a risk factor for multiple short- and long-term adverse outcomes that affect both mothers and newborns, including increased birthweight, gestational hypertension, stillbirth and neonatal death, and future cardiometabolic disease in mothers and offspring [2, 3]. GDM is typically diagnosed at 24–28 weeks of gestation and affects 6–25% of pregnant women according to differential diagnostic criteria [4]. However, there is no scientific consensus for the preferred screening strategy or diagnostic glycemic thresholds for early GDM, as illustrated by several versions of diagnostic criteria that depend on fasting glucose (FPG), 1-hour, 2-hour, and 3-hour glucose levels from the oral glucose tolerance test (OGTT). [5] The decision to adopt the lower or higher glycemic criterion is also controversial [6]. The lower criterion adopts FPG of ≥ 5.1 mmol/L or 2-hour glucose of ≥ 8.5 mmol/L, [7, 8] whereas the higher criterion adopts an FPG of ≥ 5.8 mmol/L or 2-hour glucose of ≥ 9.2 mmol/L [9]. Consequently, inconsistent diagnostic criteria for GDM lead to a varied incidence of GDM as reported and make international comparisons difficult [10].
Given that various incidences of women with GDM were found using different GDM criteria, we hypothesized that the inconsistent incidence implied heterogeneity in GDM. Our study aimed to investigate the incremental impact of increasingly abnormal glucose levels on maternal and neonatal adverse outcomes via FPG and 2-hour postprandial plasma glucose (2hPPG) criteria, which would address the changing impact of various glucose levels on adverse gestational outcomes, as well as the most clinically relevant cut-off points for plasma glucose levels for the screening of GDM.
Materials and methods
Study design and participants
This was a population-based retrospective study involving pregnant women aged 18–45 years with at least one hospital birth in Ningbo, China, from 1 January 2016 to 31 December 2022. This study included patients from Level 2 and Level 3 hospitals that participated in the inter-laboratory quality parity to ensure the accuracy and consistency of blood glucose measurements. The study used 100% of pregnant women who underwent GDM screening using 75-g OGTT or FPG measurements within 24–28-week gestation in general hospitals, women’s hospitals, and community health service centers. The exclusion criteria were a history of T1DM or T2DM, an FPG of > 6.9, and a 2hPPG of > 11 mmol/L. The data sets of mothers and their newborns were linked through unique identifiers that were deidentified to protect privacy. The data included OGTT or FPG measurements and clinical features. All the data were archived at the Ningbo Municipal Health Information Centre, Zhejiang Province, China. The statistician had access to anonymized data, whereas the coauthors had access to summarized data. The study complied with the Declaration of Helsinki.
Handling of plasma glucose levels
All measurements of glucose were obtained from venous plasma samples. We used FPG and 2hPPG from the OGTT as GDM screening criteria. The FPG screening criterion of 5.1–6.9 mmol/L was divided into 11 intervals of < 5.1, 5.1–5.29, and up to ≥ 6.9 mmol/L with an interval of 0.2 mmol/L. The 2hPPG screening criterion of 8.5–10.9 mmol/L was divided into 14 intervals of < 8.5, 8.5–8.69, and up to ≥ 10.9 mmol/L at an interval of 0.2 mmol/L. Consecutive intervals of two criteria were applied to stratify pregnant women. One measurement at the middle of the study period was chosen if there was more than one measurement of FPG and 2hPPG for each delivery of pregnant women. If the number of events in a subgroup was under three, we combined the two neighboring subgroups to ensure reliable statistical results.
GDM diagnosis, adverse outcomes, and insulin treatment
We diagnosed GDM according to the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria in 2013 on the basis of an FPG of 5.1–6.9 mmol/L and 2hPPG of 8.5–11.0 mmol/L [11]. We used the following adverse outcomes to evaluate the impact of different glucose levels: overweight status of newborns defined as > 90th percentile birthweight (> 90th WT); underweight status of newborns defined as < 10th percentile birthweight (< 10th WT); gestational preeclampsia and hypertension diagnosed during pregnancy; and a composite of multiple adverse outcomes of 1-min and 5-min Apgar scores below 8, neonatal death, intrauterine distress, labor dystocia, and birth trauma. The percentage of insulin treatment and the mean age of pregnant women were computed for each subgroup.
Statistical analysis
Categorical variables including the incidence of adverse outcomes and insulin use were expressed as percentages and 95% confidence interval (95% CI). Continuous variables including glucose level and age were expressed as mean and standard deviation (SD). We first examined the outliers for continuous variables by graphing and sorting the data. The erroneous and illogical values were removed. As a selection bias might exist among pregnant women who underwent OGTT, we created a reference population from the whole population without GDM according to both the FPG and 2hPPG criteria during 24–28 weeks of gestation. This reference population was used for all subgroups that were classified by both the FPG and 2hPPG criteria. The incidence of adverse outcomes among subgroups classified by consecutive glucose levels was compared with that of the reference population to obtain the relative risk (RR). Since older age was an important confounding factor in the incidence of GDM, we performed multiple logistical regression using binary abnormal neonatal weights as outcomes, glucose levels as factors, and age as a covariate to assess the confounding effect of age. Statistical analysis was performed using R (version 4.3) with a two-tailed type 1 error rate of p < 0.05 indicating statistical significance.
Results
The present study included 67,369 pregnant women (aged 18–45 years; mean [SD], 31.6 [4.7] years) who had at least one measurement of FPG or 2hPPG during 24–28 weeks of gestation through a search of the local medical electronic record data. The mean (SD) birth weight was 3322 (447) g. The cut-off values for the < 10th WT and > 90th WT were 2800 g and 3885 g, respectively. We used these cut-off values as the threshold values to identify abnormal birth weights. Among the 67,369 women, 45,909 had < 5.1 mmol/L FPG and/or < 8.5 mmol/L 2hPPG and were designated the reference population without GDM (Fig. 1).
Moreover, 39,988 (age: mean [SD], 31.5 [4.7] years) women had both FPG (mean (SD), 4.5 [0.5] mmol/L) and 2hPPG (mean (SD), 6.8 [1.3] mmol/L) measurements, which were designated the study population and classified by two glucose levels to investigate the incidence of adverse gestational outcomes. Among these 39,988 women, 3025 (7.6%) had 5.1–6.9 mmol/L FPG (Fig. 1A) and 4560 (11.4%) had 8.5–11.0 mmol/L 2hPPG (Fig. 1B). The incidence of GDM according to the two combination criteria was 17.3% (6908 cases).
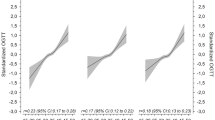
The notable finding was the distinct effects on < 10th WT and > 90th WT between higher FPG and higher 2hPPG identified by two GDM criteria. Overall, women with 5.1–6.9 mmol/L FPG were associated with a lower risk of < 10th WT (RR, 0.82; 95% CI, 0.74–0.91; p < 0.001) and with an increased risk of > 90th WT (RR, 1.48; 95% CI, 1.35–1.63; p < 0.001) (Fig. 1A). In contrast, women with 8.5–11.0 mmol/L 2hPPG were associated with an increased risk of < 10th WT (RR, 1.14; 95% CI, 1.06–1.23; p < 0.001) and with no increased risk of > 90th WT (RR, 0.95; 95% CI, 0.86–1.04; p = 0.29) (Fig. 1B). On the basis of the RRs obtained by the consecutive intervals of glucose measurements, we found several remarkable findings. There was a rapid increase in the RRs (from 1.29 to 2.17) of the > 90th WT from 5.1 to 5.9 mmol/L FPG intervals, and the RRs remained high (Fig. 1A). Our study clearly revealed an increasing trend in insulin use by the categorization of FPG levels, increasing from the lowest 0.4% below 5.1 mmol/L FPG to the highest 22.2% at 6.3 ~ mmol/L FPG (Fig. 1A). In contrast, the trend in insulin use among subgroups by 2hPPG was not as significant (Fig. 1B).
Figures 2 and 3 show the differential associations of the two GDM criteria with maternal hypertension and several other adverse outcomes. Among the study population of 39,988 women, 906 (2.3%) cases had hypertension and 1356 (3.4%) cases of composite adverse outcomes (Fig. 2A). Compared with the non-GDM reference population, 5.1–6.9 mmol/L FPG was much more strongly associated with an increased risk of hypertension (RR, 1.51; 95% CI, 1.24–1.83; p < 0.001) (Fig. 2A) than was 8.5–11.0 mmol/L 2hPPG (RR, 1.04; 95% CI, 0.85–1.26; p = 0.72) (Fig. 2B). Further analysis revealed that most FPG levels (6 levels > 5.2 mmol/L) were strongly associated with hypertension (RR, 1.7 to 3.94) (Fig. 2A), whereas only two levels of 2hPPG (> 10.5 mmol/L) had an evident association (RR, 1.56 and 1.68) with hypertension (Fig. 2B). With respect to the composite adverse outcomes, 5.1–6.9 mmol/L FPG slightly increased this risk (RR, 1.16; 95% CI, 0.97–1.39; p = 0.11) (Fig. 2A), and the same was true for the 8.5–11.0 mmol/L 2hPPG (RR, 1.04; 95% CI, 0.89–1.22; p = 0.61) (Fig. 2B).
Among the 1356 patient with composite adverse outcomes, 605 (1.5%) had 1-min and 5-min Apgar below 8 scores, 425 (1.1%) had breech presentations, 85 (0.2%) had neonatal deaths, and 241 (0.6%) had intrauterine distress (Fig. 3). Further investigation of associations with different types of adverse outcomes revealed that 5.1–6.9 mmol/FPG increased maternal hypertension (RR, 1.51; 95% CI, 1.24–1.83; p < 0.001) and breech presentation (RR, 1.47; 95% CI, 1.08–2.0; p < 0.05). Moreover, 8.5–11.0 mmol/L 2hPPG slightly increased the risk of breech presentation (RR, 1.26; 95% CI, 0.96–1.66; p = 0.10) and death (RR, 1.23; 95% CI, 0.66–2.3; p = 0.48), which were not statistically significant due to low occurrence. Both elevated FPG and 2hPPG were associated with a reduced risk of intrauterine distress.
As older age was an important confounding factor for the incidence of GDM, Next, we compared the mean age between the reference population and study population. The mean age of the reference population was 31.3 (SD, 4.6) years. Among the study population, the mean ages of the patients in the < 5.1 mmol/L and 5.1–6.9 mmol/L FPG subgroups were 31.4 (SD, 4.6) years and 32.5 (SD, 4.7) years, respectively (Fig. 1A). The mean age of the < 8.5 mmol/L and 8.5–11.0 mmol/L 2hPPG subgroups was 31.4 (SD, 4.6) years and 33.3 (SD, 4.5) years, respectively (Fig. 1B). These findings indicated that women with GDM diagnosed with 8.5–11.0 mmol/L 2hPPG were 0.8 years older than those diagnosed with 5.1–6.9 mmol/L FPG (p < 0.001). We performed multiple logistic regression using binary abnormal weights as outcomes, glucose levels as risk factors, and age as covariate (Table 1). The regression analysis revealed that after adjustment for age, 5.1–6.9 mmol/L FPG was more likely to lower the incidence of < 10th WT (RR, 0.94; 95% CI, 0.89–0.99; p < 0.001) and to increase the incidence of > 90th WT (RR, 1.36; 95% CI, 1.26–1.46; p < 0.001), whereas 8.5–11.0 mmol/L 2hPPG only increased the incidence of < 10th WT (RR, 1.14; 95% CI, 1.09–1.2; p < 0.001) with a noteworthy effect size. With respect to age, all the association sizes with < 10th WT and > 90th WT were small, ranging from 0.995 to 1.01, although the RR of 1.01 was statistically significant (p < 0.01) because of the large sample size. This finding implied that compared with the much larger effect sizes of increased FPG, the confounding effect of age was minor. These consistent findings after adjustment further supported our findings.
Discussion
Our main findings revealed that higher FPG was significantly associated with higher birthweight, whereas higher 2hPPG was more strongly associated with lower birthweight. This difference in the impact of the two GDM diagnosis criteria on neonatal birthweight was independent of the age of the women. GDM patients screened by the FPG criterion had a more significantly increased incidence of maternal hypertension than those diagnosed by the 2hPPG criterion.
Among the study population of our study, 7.6% of women were diagnosed with GDM according to the 5.1–6.9 mmol/L FPG criterion and 11.4% according to the 8.5–11.0 mmol/L 2hPPG via the OGTT during 24–28 weeks of gestation. The incidence of GDM according to the two combination criteria was 17.3% (6908 cases), which was close to that reported in a previous meta-analysis in which the incidence of GDM was 14.8% among 79,064 Chinese participants [12]. As different populations may have inconsistent plasma glucose levels, previous studies used 1 SD of glucose measurements to evaluate the impact size of elevated glucose. The seminal Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study included 25,505 women who underwent a 75 g OGTT at 24–32 weeks of gestation, and reported that 1 SD of elevated FPG (0.4 mmol/L) and 2hPPG (1.3 mmol/L) had a similar RR of 1.38 for birthweight > 90th WT. However, this strategy failed to detect an accurate trend in the impact of different glucose levels on gestation.
Our strategy was the application of finer consecutive 0.2 mmol/L intervals of FPG and 2hPPG to investigate the incremental impact of elevated glucose, which provided information about both the overall impact and its trend on adverse outcomes during gestation from elevated glucose. Our important finding was that 5.1–6.9 mmol/L FPG had a significantly positive association with > 90th WT (RR, 1.48; 95% CI, 1.35–1.63; p < 0.001) and with a lower risk of < 10th WT (RR, 0.82; 95% CI, 0.74–0.91; p < 0.001) (Fig. 1A), whereas 8.5–11.0 mmol/L 2hPPG had the opposite effect, i.e., it was associated with an increased risk of < 10th WT (RR, 1.14; 95% CI, 1.06–1.23; p < 0.001) and with no increased risk of > 90th WT (RR, 0.95; 95% CI, 0.86–1.04; p = 0.29) (Fig. 1B). This discrepancy was independent of age since the RR values adjusted for age led to identical conclusions (Table 1). We further investigated these characteristic associations by stratifying the population using a series of consecutive glucose levels of FPG and 2hPPG. The results revealed that the associations with > 90th WT according to the FPG criterion sharply increased from 5.1 to 5.9 with the strongest association (RR, 2.17) occurring at 5.7–5.9 mmol/L (Fig. 1A).
To our knowledge, no previous studies have reported an association between 2hPPG and low birthweight. In our study, the opposite action on < 10th WT between higher FPG and higher 2hPPG might have important clinical implications for the intervention of GDM regarding abnormally high and low birthweight. Currently, low birth weight has been increasingly found to be associated with elevated maternal glucose levels and is a risk factor for childhood and adult metabolic syndrome [13]. Previous research revealed that high birthweight predicted metabolic health in obese subjects with a higher whole-body insulin sensitivity index and lower trunk fat percentage than did low birthweight (P < 0.05) [14], whereas the recent study by Colaus reported that middle-aged adults born with low birthweight presented a greater incidence of diabetes and obesity than did those with a normal weight [15].
GDM is essentially characterized by a glucose tolerance disorder that may first appear during pregnancy or preexist before conception as a form of prediabetes. Research has shown that women with prior GDM have a 13.0-fold multivariable-adjusted risk (95% CI: 5.54–30.6) for diabetes and a 2.15-fold risk (95% CI: 1.76–2.62) for prediabetes compared with women without GDM [16]. According to statements by the American Diabetes Association (ADA), prediabetes includes impaired fasting tolerance (IFT) and impaired glucose tolerance (IGT) [17]. IFT is determined by elevated FPG and responds to hepatic glycogen metabolism. The IGT is determined by 2hPPG and responds to pancreatic problems [18]. Despite these disparate mechanisms for the two types of prediabetes, GDM is clinically diagnosed on the basis of a combination of FPG and 2hPPG criteria. Comparisons between cases that are diagnosed separately by two different criteria are rarely reported. This clinical context may partly explain our findings that GDM cases diagnosed by FPG and the 2hPPG criteria had distinct impacts on neonatal birth weight.
In addition to the different impacts on neonatal weight among women with GDM diagnosed by FPG and 2hPPG criteria, our study also revealed that women with GDM diagnosed by FPG were more likely to use insulin. There was an increasing trend in insulin use according to the categorization of FPG levels, increasing from the lowest 0.4% below 5.1 mmol/L FPG to the highest 22.2% at 6.3 ~ mmol/L FPG (Fig. 1A). In contrast, the trend in insulin use among subgroups by 2hPPG was not significant (Fig. 1B). The clinical meaning of these findings remains to be further clarified.
Another remarkable finding concerning the differences between the FPG and 2hPPG diagnostic criteria was the great disparity in the associations with maternal hypertension (Fig. 2). The findings indicated that elevated FPG was strongly associated with maternal hypertension (RR, 1.51; 95% CI, 1.24–1.83; p < 0.001) compared with elevated 2hPPG (RR, 1.04; 95% CI, 0.85–1.26; p = 0.72), only two levels (> 10.5 mmol/L) had an evident association (RR, 1.56 and 1.68) with hypertension (Fig. 2B). Concerning other adverse outcomes, only the incidence of breech presentation was increased among patients diagnosed by FPG and 2hPPG.
Finally, since high and low birth weights have differential clinical meanings, the distinctive associations of FPG and 2hPPG with birth weight deserve attention in future studies. Metabolically, an increase in 2hPPG is an indicator of abnormal insulin secretion in which the pancreas becomes insensitive to an increase in blood glucose and does not adequately secrete insulin, predicting declining beta cell function and worsening glucose tolerance over time [19]. Declining beta cell function also affects protein synthesis and leads to low birthweight, which explains our findings about the association between increased 2hPPG and low birth weight. This cause supports the evidence that low birth weight is a risk factor for future metabolic syndrome [20]. Therefore, based on our findings, future studies should include data on beta cell function to clarify the distinct associations with adverse outcomes among patients with GDM diagnosed by FPG or 2hPPG criteria.
The strengths of our study were the large sample size and the entire population of pregnant women. The application of consecutive intervals ensured a reliable description of the changing impacts of different maternal glucose levels on birth weight and adverse outcomes within each subgroup, which provided insight into the turning point and the trend of the effects. The limitation was that selection bias was likely among women who underwent or did not undergo the OGTT test, which was addressed using a common reference population and adjustment for age.
Conclusions
We obtained an incremental effect of increased FPG and 2hPPG from the OGTT on abnormal newborn birth weight, maternal hypertension, and a composite of adverse outcomes by stratifying large population-based pregnant women with consecutive intervals of glucose levels. Our study revealed that the FPG criterion was significantly associated with high birth weight, whereas 2hPPG was significantly associated with low birth weight. Our findings highlight the heterogeneity of patients with GDM diagnosed by different criteria.
Data availability
No datasets were generated or analysed during the current study.
References
Meloncelli NJL, Barnett AG, D’Emden M, De Jersey SJ. Effects of changing Diagnostic Criteria for Gestational Diabetes Mellitus in Queensland, Australia. Obstet Gynecol. 2020;135(5):1215–21.
Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, Brickman WJ, Clayton P, Ma RC, McCance D, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care. 2019;42(3):381–92.
Liang W, Sun FF. Does gestational diabetes mellitus increase the risk of cardiovascular disease? A mendelian randomization study. J Endocrinol Invest 2023.
Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M, Oats JJ, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the hyperglycemia and adverse pregnancy outcome (HAPO) study. Diabetes Care. 2012;35(3):526–8.
Gupta Y, Goyal A, Kalra S, Tandon N. Variation in the classification of hyperglycaemia in pregnancy and its implication. Lancet Diabetes Endocrinol. 2020;8(4):264–6.
Rhoads GG. Lower versus higher glycemic criteria for diagnosis of gestational diabetes. N Engl J Med. 2022;387(18):1719–20.
Weinert LS. International Association of Diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care. 2010;33(7):e97. author reply e98.
Diagnostic criteria and classification. Of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103(3):341–63.
American Diabetes A. 14. Management of diabetes in pregnancy: standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S183–92.
Saeedi M, Cao Y, Fadl H, Gustafson H, Simmons D. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;172:108642.
Sweeting A, Wong J, Murphy HR, Ross GP. A clinical update on gestational diabetes Mellitus. Endocr Rev. 2022;43(5):763–93.
Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. 2019;10(1):154–62.
Kanda T, Murai-Takeda A, Kawabe H, Itoh H. Low birth weight trends: possible impacts on the prevalences of hypertension and chronic kidney disease. Hypertens Res. 2020;43(9):859–68.
Bouhours-Nouet N, Dufresne S, de Casson FB, Mathieu E, Douay O, Gatelais F, Rouleau S, Coutant R. High birth weight and early postnatal weight gain protect obese children and adolescents from truncal adiposity and insulin resistance: metabolically healthy but obese subjects? Diabetes Care. 2008;31(5):1031–6.
Jornayvaz FR, Vollenweider P, Bochud M, Mooser V, Waeber G, Marques-Vidal P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: the CoLaus study. Cardiovasc Diabetol. 2016;15:73.
Shen Y, Wang P, Wang L, Zhang S, Liu H, Li W, Li N, Li W, Leng J, Wang J, et al. Gestational diabetes with diabetes and prediabetes risks: a large observational study. Eur J Endocrinol. 2018;179(1):51–8.
American Diabetes A. 2. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(Suppl 1):S13–22.
Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B, American Diabetes A. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–9.
Kramer CK, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, Retnakaran R. Delayed timing of post-challenge peak blood glucose predicts declining beta cell function and worsening glucose tolerance over time: insight from the first year postpartum. Diabetologia. 2015;58(6):1354–62.
Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93(446):26–33.
Funding
This study was funded by the Natural Science Foundation of Ningbo (No. 2019F1003) and the Zhejiang Provincial Medical and Health Science and Technology Plan Project (2021KY996).
Author information
Authors and Affiliations
Contributions
QS and XS contributed to the design, analysis and interpretation of the data, and wrote the manuscript. LL and HD contributed to the acquisition of data and revised the manuscript. QS and HD are the guarantors of this work, have full access to all study data, and assume responsibility for data integrity and analytical accuracy. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective observational study was approved by the Ethics Committee of the First Affiliated Hospital of Ningbo University (2023No.192RS-01). The need for informed consent to participate was waived by the Ethics Committee of the First Affiliated Hospital of Ningbo University.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, Q., Song, X., Li, L. et al. Fasting or 2-hour postprandial plasma glycemic criteria for gestational diabetes mellitus are aassociated with distinct adverse outcomes. BMC Pregnancy Childbirth 24, 570 (2024). https://doi.org/10.1186/s12884-024-06770-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06770-y