Abstract
Background
Multiple marker screening is offered to pregnant individuals in many jurisdictions to screen for trisomies 21 and 18. On occasion, the result is ‘double-positive’—a screening result that is unexpectedly positive for both aneuploidies. Although this occurs rarely, the paucity of available evidence about the outcomes of these pregnancies hinders patient counselling. This study aimed to investigate the association of double-positive results with preterm birth and other adverse perinatal outcomes.
Methods
We conducted a population-based retrospective cohort study of pregnancies with an estimated date of delivery from September 1, 2016, to March 31, 2021, using province-wide perinatal registry data in Ontario, Canada. Pregnancies with double-positive screening results where trisomies 21 and 18 were ruled-out were compared to pregnancies with screen negative results for both aneuploidies. We used modified Poisson regression models with robust variance estimation to examine the association of double positive results with preterm birth and secondary outcomes.
Results
From 429 540 pregnancies with multiple marker screening, 863 (0.2%) had a double-positive result; trisomies 21 and 18 were ruled out in 374 pregnancies, 203 of which resulted in a live birth. Among the pregnancies in the double-positive group resulting in a live birth, the risk of preterm birth was increased compared to pregnancies with a screen negative result: adjusted risk ratio (aRR) 2.6 (95%CI 2.0-3.6), adjusted risk difference (aRD) 10.5% (95%CI 5.4–15.7). In a sensitivity analysis excluding all diagnosed chromosomal abnormalities, the risk of preterm birth remained elevated to a similar degree: aRR 2.6 (95%CI 1.9–3.7), aRD 10.0% (95%CI 4.8–15.3). The risk of other adverse perinatal outcomes was also higher, including the risk of chromosomal abnormalities other than trisomies 21 and 18: aRR 81.1 (95%CI 69.4–94.8), aRD 34.0% (95%CI 29.2–38.8). Pregnancies with double-positive results were also less likely to result in a live birth, even when excluding all diagnosed chromosomal abnormalities; and at increased risk of adverse perinatal outcomes for those resulting in a live birth.
Conclusion
Although rare, double-positive multiple marker screening results are associated with an increased risk of preterm birth and other adverse perinatal outcomes, even when excluding all identified chromosomal abnormalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Prenatal screening for trisomies 21 and 18 has been offered to pregnant individuals since the 1980s in the form of multiple marker screening [1]. Although the advent of cell-free DNA (cfDNA) screening technologies has considerably changed many prenatal screening programs, most still rely on multiple marker screening as the main screening modality and offer cfDNA screening in a contingent funding model or as a private-pay option [2,3,4].
The results of multiple marker screening generally yield (i) a screen-positive result for trisomy 21 or for trisomy 18, in which case follow-up investigations are offered to further define the risk of aneuploidy (through cfDNA screening) or confirm/refute the positive result through diagnostic cytogenetic testing; or (ii) a screen-negative result, for which no follow-up investigations are indicated. However in some instances, the result is unexpectedly reported as positive for both trisomies 21 and 18 at once, referred to as a ‘double-positive‘ result; in some of these cases, diagnostic testing confirms neither of these trisomies [5]. The patterns of biochemical marker levels that confer a positive result are different for the two trisomies, and at times in opposition [6], thus a double-positive result is both rare and difficult to interpret.
In a report of 32 pregnancies with double-positive results in 2002, 20 had no reported chromosomal abnormality, yet only 5 resulted in a live birth without obstetric or perinatal complications [7]. A subsequent study compared pregnancy outcomes between 33 patients with a double-positive result for which a prenatal diagnosis of trisomies 21 and 18 had been excluded and 66 age-matched pregnancies with screen-negative results, finding an increased risk of adverse outcomes including spontaneous abortion and lower gestational age at delivery for the double-positive group [5]. This study was limited by its small sample size and potential for selection bias resulting from restriction of the study to pregnancies in which prenatal cytogenetic investigations had been conducted. Nevertheless, the finding that a double-positive result may indicate a higher risk of preterm birth is particularly concerning given the clinical importance of preterm birth for perinatal and child health outcomes [8].
Although the occurrence of double-positive results appears to be rare (prevalence 0.3 − 0.4%), the paucity of evidence on this type of result hinders patient counseling [5, 9]. The present study aimed to investigate the relationship between a double-positive multiple marker screening result and preterm birth, as well as other adverse obstetrical and perinatal outcomes, on a population level.
Methods
Study design & data sources
We conducted a population-based retrospective cohort study using data from the Better Outcomes Registry & Network, the prescribed perinatal registry in Ontario, Canada. Data from all multiple marker screening, cfDNA screening, and cytogenetic tests performed in Ontario are collected in the registry, which also captures pregnancy, obstetrical, and perinatal outcomes. All pregnancy outcomes beyond 20 weeks’ gestation are captured in the registry, while outcomes for pregnancies lasting less than 20 weeks’ gestation are captured less systematically. Data from the Canadian Institute for Health Information Hospital Discharge Abstract Database were used to supplement the capture of pregnancy outcome, preeclampsia, and neonatal intensive care admission (see Supplemental Table 1) [10].
Setting and study population
In Ontario, multiple marker screening is offered as the first-tier screen; modalities have varied over time and are described in Supplemental Table 2. The same screening modalities are used to generate a risk estimate for trisomies 21 and 18, although some biomarkers, namely first trimester alpha fetoprotein and placental growth factor and second trimester inhibin A, are excluded from the calculation of the risk of trisomy 18, while included in the calculation of trisomy 21 (Supplementary Table 2). cfDNA screening is universally covered under the province’s publicly-funded health care for those meeting specific eligibility criteria, including a positive multiple marker screen. Individuals may also choose to self-pay for cfDNA screening if the eligibility criteria are not met [11, 12].
All singleton pregnancies with an estimated date of delivery from September 1, 2016, to March 31, 2021, and a multiple marker screening result were included in the study (Fig. 1).
Study exposure
The double-positive group was defined as pregnancies with a multiple marker screening result that was positive for both trisomies 21 and 18 and where we considered the result to be false positive due to: (i) a cytogenetic testing result, prenatally or postnatally, that excluded a diagnosis of trisomies 21 and 18; or (ii) a cfDNA screening result that indicated low risk of these trisomies. The low-risk cfDNA screening result was considered sufficient evidence to rule out trisomies 21 and 18 given its negative predictive value of > 99.99%, (95%CI 99.97–100.00) [13]. Pregnancies with double-positive results with a diagnosis of mosaic or partial trisomies 21 or 18 were excluded, as were pregnancies for which no follow-up investigations were conducted as such lack of follow-up precluded the exclusion of a diagnosis of trisomies 21 and 18 (Fig. 1).
Pregnancies with double-positive results as defined here were compared to pregnancies with a negative multiple marker screening result; pregnancies with a diagnosis of full, partial or mosaic trisomy 21 or 18 were excluded from this reference group (Fig. 1).
Study outcome
The primary outcome for this study was preterm birth, defined as a live birth before 37 weeks’ gestation [14]. Among pregnancies resulting in a live birth, secondary outcomes included small for gestational age (< 10th percentile), cesarean section, diagnosis of preeclampsia, and admission to the neonatal intensive care unit for more than 12 h [15].
Additional secondary outcomes were included for all pregnancies: chromosomal abnormalities other than trisomies 21 and 18, and a composite of pregnancy loss, stillbirth, or termination.
Chromosomal abnormalities were identified through cytogenetic testing including rapid aneuploidy detection techniques, karyotype, and microarray tests. During the study period, among pregnancies in which cytogenetic testing is performed, 86.7% included a microarray analysis. Because not all pregnancies receive cytogenetic investigations, additional sources of information were used to ascertain whether pregnancies without such testing could reasonably be defined as not having a chromosomal abnormality. This was achieved based on cfDNA screening results and the findings of the clinical examination at birth, as recorded in the registry, in which live births with no phenotypic features or congenital anomalies were recorded were assumed not to have any chromosomal abnormality.
Statistical analysis
The study population was described using means and standard deviations for continuous variables and frequencies and proportions for categorical variables.
We computed the cumulative incidence of all study outcomes for both groups and used modified Poisson regression models with robust variance estimation to generate risk ratios and risk differences. All models were adjusted for potential confounders identified a priori: characteristics of the pregnant individual (age, pre-pregnancy weight, racial origin, insulin dependent diabetes mellitus, smoking) and conception through assisted reproductive technologies, all factors for which screening laboratories make adjustments and for which an association with adverse perinatal outcomes has been reported [16, 17].
To address missing covariate data (11.6% of the study population were missing data on at least one covariate), we used multiple imputations by chained equations with 20 imputed data sets (Supplemental Tables 3 and 4) [18].
Sensitivity analyses
Given the potential association between double-positive results and chromosomal abnormalities other than trisomies 21 and 18, we conducted a subgroup analysis excluding all pregnancies with identified chromosomal abnormalities to evaluate the impact on our main results.
In the main analysis, only pregnancies with double-positive results in which a diagnosis of trisomies 21 and 18 had been ruled out by follow-up investigations were included. However, 20 of the pregnancies that were excluded from the analysis due to having a double-positive result but no follow-up investigations (i.e., no cytogenetic or cfDNA screening were performed) resulted in a live birth. To determine the impact of the exclusion of these pregnancies, we performed a sensitivity analysis in which these pregnancies were included as part of the double-positive group.
Complete case analyses including only pregnancies for which data on all covariates were available were also conducted as a further sensitivity analysis.
Some individuals in the study population may have had more than one pregnancy over the course of the study period. Due to the complexity of the model given the multiple imputations, we were unable to account for non-independence of such pregnancies in the main model. We performed a sensitivity analysis using one of the imputed data sets to determine the impact of not accounting for this clustering in our main analysis (Supplemental Table 5).
We performed the analyses using SAS, version 9.4 (SAS Institute Inc, Cary, NC) and R, version 4.3.1 for the multiple imputations and regression models.
This study received approval by the research ethics board of the Children’s Hospital of Eastern Ontario (protocol # 22/05PE) and the University of Ottawa (protocol # H-06-22-8269). As this study involved secondary use of data from a prescribed registry, individual patient consent was not required.
This study is reported using the RECORD guidelines [19].
Results
From 634 146 singleton pregnancies recorded in the registry during the study period, 458 240 (72.3%) had multiple marker screening. After excluding 25 651 with a positive screen for trisomy 21, 917 for trisomy 18, and 2 132 where no screening report could be issued, 429 540 pregnant individuals remained, of whom 428 677 had a screen negative result and 863 (0.2%) had a double-positive result (Fig. 1). Over two-thirds (67.1%) of pregnancies with double-positive results had cytogenetic testing; 49.4% had prenatal cytogenetic testing specifically (Fig. 2). For pregnancies with screen negative results, 2.8% had cytogenetic testing at any time and 0.6% had prenatal cytogenetic testing specifically.
Among the 863 pregnancies with a double-positive results, a diagnosis of trisomy 21 or 18 was confirmed by cytogenetic testing in 326 (37.8%, Fig. 1). An additional 46 (5.3%) had a high-risk cfDNA screening result for trisomies 21 or 18, presuming a likely diagnosis of aneuploidy. An additional 117 pregnancies were excluded from the double-positive group as a diagnosis of trisomy 21 or 18 could not be excluded (uninformative cytogenetic testing, failed cfDNA screening, or no follow-up investigations performed).
After these exclusions, 374 pregnancies with double-positive results were included in the analytic sample, 203 of which resulted in a live birth (Fig. 1). These pregnancies were compared with 428 466 pregnancies with a screen negative result, after excluding 211 screen-negative pregnancies in which a full, partial or mosaic trisomy 21 or 18 was diagnosed; 411 937 of the included screen-negative pregnancies resulted in a live birth (Fig. 1).
In the analytic sample, pregnant individuals in the group of 374 pregnancies with double-positive results were older than those in the group of 428 466 individuals with screen-negative results (mean age at estimated date of delivery of 35.5 years versus 31.2 years, respectively) and they were also less likely to be nulliparous (22.9% versus 46.4%; Table 1). In pregnancies with double-positive results, 44.1% (152/345) had a multiple marker screening result for which the fetal nuchal translucency measurement was below the threshold of 3.5 mm, compared to 99.96% (396 017/396 186) for pregnancies in the group with screen-negative results.
The biomarker patterns varied in the two groups and are described in the Supplemental Table 6: in pregnancies with double-positive results, the nuchal translucency measurement, and second trimester total human chorionic gonadotropin and inhibin A were increased, while all other biomarkers were decreased. A low PAPP-A, defined as < 0.4 MoM, was observed in 237 (69.7%) of pregnancies with double-positive results compared to 20 341 (5.1%) of pregnancies with screen-negative results.
Analyses of primary and secondary outcomes
Outcomes among pregnancies resulting in a live birth
Among pregnancies resulting in a live birth, the incidence of preterm birth was higher among pregnancies with double-positive results (17.0%) compared to the screen-negative group (6.1%; Table 2). The adjusted risk ratio (aRR) for preterm birth in those with double-positive results versus screen negative results was 2.6 (95%CI 2.0-3.6) and the adjusted risk difference (aRD) was 10.5% (95%CI 5.4–15.7) (Table 2).
The incidence of all secondary outcomes for pregnancies resulting in a live birth was also higher in the double-positive group compared to the group with screen-negative results, with some variation in the magnitude of the increased aRRs and aRDs (cesarean delivery, admission to the neonatal intensive care unit, and small for gestational age, preeclampsia) (Table 2).
Outcomes among all pregnancies
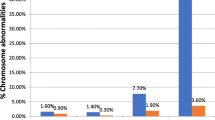
In the analyses including all pregnancies, those in the group with double-positive results had an increased risk of chromosomal abnormalities other than trisomies 21 and 18 (34.4%) compared to the screen-negative group (0.4%), with an aRR of 81.1 (95%CI 69.4–94.8) and aRD of 34.0% (95%CI 29.2–38.8) (Table 2; detailed numbers for specific chromosomal abnormalities, Supplemental Table 7). The proportion of pregnancies ending in a composite of pregnancy loss, stillbirth or termination versus live birth was also higher in the group with double-positive results (20.7%) compared to screen-negative results (0.7%), with an aRR of 26.9 (95%CI 20.8–34.7; Table 2) and aRD of 19.9% (95%CI 15.0-24.9; Table 2). Further, a higher proportion of pregnancies with double-positive results had no pregnancy outcome recorded (31.6%) compared to the screen-negative group (3.2%) (Supplemental Table 8).
Sensitivity analyses
Excluding all identified chromosomal abnormalities
In a sensitivity analysis where all identified chromosomal abnormalities other than trisomies 21 and 18 were additionally excluded (Table 3), the risk of preterm birth was still increased in pregnancies with double-positive results, compared to pregnancies with screen negative results (aRR 2.6 (95%CI 1.9–3.7), aRD 10.0% (95%CI 4.8–15.3)). The results for the secondary outcomes also remained similar in magnitude.
Other sensitivity analyses
The complete case analysis for preterm birth excluded 47 838 pregnancies (11.6%) due to missing data for one or more covariates; the estimates were similar in magnitude and direction, but less precise (Supplemental Tables 3 and 9).
In our primary analysis, we excluded from the analytic sample 102 pregnancies with double-positive results but no follow-up investigations (Fig. 2), as we relied on these confirmatory results to rule out trisomies 21 and 18. In 20 such pregnancies, there was a live birth recorded. When these pregnancies were included in the double-positive group, the results for preterm birth were very similar to the main analysis (aRR of 2.5, 95%CI 1.9–3.4 and aRD of 9.9%, 95%CI 5.0-14.8; Supplemental Table 10).
Additionally, in the sensitivity analysis of a single imputed data set in which we accounted for clustering among individuals with more than one pregnancy in the study cohort, the results were virtually identical to the main analyses (Supplemental Table 5).
Finally, because some methods of determining the estimated date of delivery of a pregnancy are more accurate (first trimester ultrasound) than others (second trimester ultrasound, last menstrual period), we reviewed this information for pregnancies based on multiple marker screening results. The methods of estimated date of delivery determination were similar in the two groups, with 94.1% and 95.6% of pregnancies with double-positive results and screen-negative results using first trimester ultrasound, respectively. We reviewed the cases of preterm birth among pregnancies with double-positive results where the estimated date of delivery was not determined by first trimester ultrasound and calculated the association if all these pregnancies had been misclassified as having a preterm birth; the crude risk ratio would decrease from 2.8 to 2.5 (Supplemental Table 11).
Among the 114 pregnancies with double-positive results and a nuchal translucency measurement < 3.5 mm with a recorded live birth, 21 (18.4%) were recorded to result in a preterm birth, and among the 52 live births with double-positive results and a PAPP-A ≥ 0.4 MoM, 8 (15.4%) were delivered prematurely (Supplemental Table 12). In pregnancies with nuchal translucency measurements < 3.5 mm, the risk of chromosomal abnormalities other than trisomies 21 and 18 was increased in pregnancies with double-positive results (15.8%) compared to pregnancies with screen-negative results (0.4%) (Supplemental Table 13).
Discussion
Main findings
In this large population-based study, we found that double-positive multiple marker screening results are relatively rare, occurring in 0.2% of pregnancies, often confirming a diagnosis of trisomy 21 or 18 (37.8%) or indicating a high suspicion of aneuploidy in a further 5.3% of pregnancies with a high-risk cfDNA screening result. Among pregnancies with double-positive results in which cytogenetic testing or cfDNA screening ruled out a diagnosis of trisomy 21 or 18, we observed a substantial increase in the risk of preterm birth, compared to pregnancies with a screen-negative result. These pregnancies resulting in a live birth were also at increased risk of admission to the neonatal intensive care unit, small for gestational age, and delivery by cesarean section. These elevated risks remained similar in magnitude when pregnancies with any identified chromosomal abnormality were excluded from the analysis.
There was also an increased risk of chromosomal abnormalities other than trisomies 21 and 18 among pregnancies with a double-positive result, and an elevated risk of a composite of pregnancy loss, stillbirth or termination.
As previous studies have reported that lower PAPP-A levels are associated with preterm birth [20], we investigated whether the risk of preterm birth may be explained by low PAPP-A levels or by high nuchal translucency levels; we found that the risk of preterm birth remained elevated among those with nuchal translucency measurements under 3.5 mm and those with PAPP-A levels greater or equal to 0.4 MoM.
Strengths and limitations
An important strength of this study is its population-based design, avoiding the potential for selection bias that may occur when only high-risk individuals who elected to have prenatal diagnosis are included. Our study also included a large sample size, enabling relatively precise estimates of risk for outcomes associated with this rare finding. For assessment of chromosomal abnormalities other than trisomies 21 and 18, we were able to investigate copy number variants, as cytogenetic testing results included microarray analyses. Finally, our detailed description of the study population (Fig. 2) will serve as a useful reference to inform clinicians and programs regarding the type of follow-up investigations received by patients with this type of screening result.
An important limitation of this study was that pregnancy outcomes were not available for all pregnancies that ended before reaching 20 weeks’ gestation, as these are captured less systematically in the birth registry. Such pregnancies could represent early losses or terminations, which would most likely result in an underestimation of the association between double-positive multiple marker screening results and pregnancy outcomes given the higher proportion of pregnancies without an outcome recorded in the group with double-positive results. This would also apply to chromosomal abnormalities, as their incidence would be expected to be higher among pregnancy losses and terminations.
An additional limitation is possible misclassification of chromosomal abnormalities since not all pregnancies receive cytogenetic testing. We included a follow-up period of a minimum of three months after birth to identify the results of postnatal cytogenetic testing, but for some conditions with more subtle clinical features that may not prompt cytogenetic investigations prior to three months of age, a cytogenetic diagnosis may not have yet been established. The misclassification could potentially be differential, due to surveillance bias in pregnancies with double-positive screening results, resulting in a potential overestimation of the risk of chromosomal abnormalities in pregnancies in this group.
Clinical and research implications
To our knowledge, only a descriptive case series and small comparative study exist on the topic of double-positive screening results [5, 7]. Similar to our study, the comparative study found an increased risk of pregnancy loss and termination of pregnancy among 33 pregnancies with double-positive results, along with a lower gestational age at birth and lower birth weight [5]. The study was underpowered to identify associations with the other outcomes investigated, including preterm birth, low birth weight, and preeclampsia, whereas our study had a much larger sample. Further, the population in that prior study included only pregnancies with normal prenatal diagnosis investigations on karyotype, which could represent a higher risk population if additional findings other than the screening result led them to have prenatal diagnosis. Our study therefore brings important evidence to confirm that pregnancies with double-positive multiple marker screening results are at increased risk of adverse perinatal outcomes, including preterm birth. Additionally, our study produced findings that are more applicable to current practice than previous studies: chromosomal microarray is now routinely offered in the context of prenatal diagnosis and incorporating microarray results enabled us to identify additional chromosomal abnormalities beyond those that could be detected by karyotype investigations.
Given that many jurisdictions offer similar multiple marker screening modalities, the results of our study may be applicable to other settings. Indeed, although uptake of cfDNA screening has had a major impact on the prenatal screening landscape, most publicly funded screening programs still offer multiple marker screening as the main screening modality due to the higher cost of cfDNA screening [2, 4, 20].
Our conclusions are broadly important to inform clinicians and screening programs about the potential increased risk of adverse perinatal outcomes for this rare but clinically important finding, which could inform follow-up testing and monitoring of the pregnancy. Our findings indicate that a double-positive result may be indicative of chromosomal abnormalities other than trisomies 21 and 18, including some abnormalities that are not routinely identified by cfDNA screening; this may inform the type of follow-up investigations offered and clinical management following a double-positive result. Indeed, the findings of this study will provide useful information about elevated risk to counsel patients when a double-positive result is identified, allowing them to determine if they wish to have prenatal diagnosis as a first intention rather than cfDNA screening given the high probability of identifying a chromosomal abnormality. For example, cfDNA screening could result in (1) a high-risk result that would need to be confirmed through prenatal diagnosis; (2) a low-risk result, in which case there would remain a residual risk of chromosomal abnormalities not identifiable on cfDNA screening; or (3) a failed screen that could delay the investigations.
Although a consistent signal of increased risk among pregnancies with double-positive results was observed across different adverse perinatal outcomes, more information about the underlying mechanism is needed to inform how best to care for these patients. Given the observed biomarker patterns and their relation to placental function [21, 22], we postulate that placenta-mediated factors could play a key role in the mechanism by which double-positive results are associated with adverse perinatal outcomes. Investigating the role of structural defects in mediating the association between double-positive results and chromosomal abnormalities, pregnancy outcome, and adverse neonatal outcomes would also be important future research directions.
Conclusions
This population-based cohort study provides robust evidence to support previous notions that, although rare, double-positive multiple marker screening results are associated with an increased risk of adverse perinatal outcomes compared to pregnancies with screen negative results. Pregnancies with double-positive results are at increased risk of preterm birth, which can contribute to adverse perinatal and child health outcomes. They are also at an increased risk of chromosomal abnormalities beyond trisomies 21 and 18 and are less likely to result in a live birth. These findings should be taken into consideration in patient counselling and program decisions regarding follow-up investigations and clinical management.
Data availability
This study was conducted using secondary data from Ontario’s prescribed perinatal registry, Better Outcomes Registry & Network. To comply with privacy requirements of the registry, all analyses were conducted within the secure environment of the registry, and only aggregate results were released for the purposes of this publication. Individuals wishing to request data from BORN Ontario can do so by contacting Science@BORNOntario.ca.
Abbreviations
- cfDNA:
-
cell-free DNA
- RD:
-
risk difference
- RR:
-
risk ratio
- aRD:
-
adjusted risk difference
- aRR:
-
adjusted risk ratio
- CI:
-
confidence interval
- EDD:
-
estimated date of delivery
- NICU:
-
neonatal intensive care unit
References
Cuckle H, Maymon R. Development of prenatal screening—A historical overview. Semin Perinatol. 2016;40(1):12–22.
Wolfberg A. The evolution of prenatal testing: how NIPT is changing the landscape in fetal aneuploidy screening. Med Lab Obs. 2016;48(1):18.
Cuckle H, Benn P, Pergament E. Cell-free DNA screening for fetal aneuploidy as a clinical service. Clin Biochem. 2015;48(15):932–41.
Gadsbøll K, Petersen OB, Gatinois V, Strange H, Jacobsson B, Wapner R, et al. Current use of noninvasive prenatal testing in Europe, Australia and the USA: a graphical presentation. Acta Obstet Gynecol Scand. 2020;99(6):722–7309.
Yee LM, Valderramos SG, Pena S, Cheng YW, Bianco K. Perinatal outcomes in euploid pregnancies with ‘double-positive’ first trimester prenatal screening for trisomy 18 and 21. J Perinatol. 2013;33(11):836–40.
Russo ML, Blakemore KJ. A historical and practical review of first trimester aneuploidy screening. Semin Fetal Neonatal Med. 2014;19(3):183–7.
Summers AM, Huang T, Wyatt PR. Pregnancy outcomes of women with positive serum screening results for Down syndrome and trisomy 18. Prenat Diagn. 2002;22(3):269–71.
Pinto F, Fernandes E, Virella D, Abrantes A, Neto MT. Born Preterm: a Public Health Issue. Port J Public Health. 2019;37(1):38–49.
Baer RJ, Currier RJ, Norton ME, Flessel MC, Goldman S, Towner D, et al. Obstetric, Perinatal, and fetal outcomes in pregnancies with false-positive Integrated Screening results. Obstet Gynecol. 2014;123(3):603–9.
Murphy MSQ, Fell DB, Sprague AE, Corsi DJ, Dougan S, Dunn SI, et al. Data Resource Profile: Better Outcomes Registry & Network (BORN) Ontario. Int J Epidemiol. 2021;50(5):1416–h1417.
Bellai-Dussault K, Meng L, Huang T, Reszel J, Walker M, Lanes A, et al. A 2‐year review of publicly funded cell‐free DNA screening in Ontario: utilization and adherence to funding criteria. Prenat Diagn. 2020;40(2):164–72.
Prenatal Screening Ontario [Internet]. [cited 2022 Jan 6]. https://prenatalscreeningontario.ca/en/pso/index.aspx
Dougan SD, Okun N, Bellai-Dussault K, Meng L, Howley HE, Huang T, et al. Performance of a universal prenatal screening program incorporating cell-free fetal DNA analysis in Ontario, Canada. Can Med Assoc J. 2021;193(30):E1156–63.
Preterm birth [Internet]. World Health Organization. [cited 2023 Oct 31]. https://www.who.int/news-room/fact-sheets/detail/preterm-birth#:~:text=Preterm is defined as babies,to less than 32 weeks).
Low birth weight [Internet]. World Health Organization; [cited 2023 Oct 31]. https://www.who.int/data/nutrition/nlis/info/low-birth-weight
Zou G. A modified Poisson Regression Approach to prospective studies with Binary Data. Am J Epidemiol. 2004;159(7):702–6.
Holmberg MJ, Andersen LW. Estimating risk ratios and risk differences: Alternatives to odds Ratios. JAMA. 2020;324(11):1098.
mice. Multivariate Imputation by Chained Equations [Internet]. R Documentation; [cited 2023 Oct 31]. https://search.r-project.org/CRAN/refmans/mice/html/mice.html
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies conducted using Observational routinely-collected health data (RECORD) Statement. PLOS Med. 2015;12(10):e1001885.
Xie X, Wang M, Goh ESY, Ungar WJ, Little J, Carroll JC, et al. Noninvasive Prenatal Testing for Trisomies 21, 18, and 13, sex chromosome aneuploidies, and microdeletions in Average-Risk pregnancies: a cost-effectiveness analysis. J Obstet Gynaecol Can. 2020;42(6):740–e74912.
Poon LC, Nicolaides KH. First-trimester maternal factors and biomarker screening for preeclampsia. Prenat Diagn. 2014;34(7):618–27.
Huang T, Hoffman B, Meschino W, Kingdom J, Okun N. Prediction of adverse pregnancy outcomes by combinations of first and second trimester biochemistry markers used in the routine prenatal screening of Down syndrome. Prenat Diagn. 2010;30(5):471–7.
Acknowledgements
Not applicable.
Funding
Doctoral Award Frederick Banting and Charles Best Canada Graduate Scholarships, Canadian Insitutes of Health Research (Funding Reference Number FBD-170866).
There was no involvement from the funding source in the design, collection, analysis, interpretation of the study findings or manuscript preparation.
Author information
Authors and Affiliations
Contributions
KBD led the design of this study, performed the statistical analyses, interpreted the study results, and drafted the manuscript. The co-authors BP, SDD, DBF, SH, CLV, JL, LM, MW, TH, NO, and CA contributed to the conceptualization of the study, the interpretation of the study findings, and reviewed the manuscript. All authors made significant contributions to this work, critically reviewed the manuscript, and have approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received approval by the research ethics board of the Children’s Hospital of Eastern Ontario (protocol # 22/05PE) and the University of Ottawa (protocol # H-06-22-8269). As this study involved secondary use of data from a prescribed registry, individual patient consent was not required.
Consent for publication
Not applicable.
Conflict of interest
The other authors report no conflict of interest.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bellai-Dussault, K., Dougan, S.D., Fell, D.B. et al. Pregnancies with ‘double-positive’ multiple marker screening results: a population-based study in Ontario, Canada. BMC Pregnancy Childbirth 24, 584 (2024). https://doi.org/10.1186/s12884-024-06774-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06774-8