Abstract
Background
Endometrial cancer is the most common gynecological malignancy; however, there is no useful blood diagnostic biomarker. This study aimed to determine the utility of tissue factor pathway inhibitor 2 (TFPI2), a biomarker of ovarian cancer, as a diagnostic marker for endometrial cancer.
Methods
We examined serum TFPI2 levels in patients with endometrial cancer (n = 328) compared to those in healthy controls (n = 65) and evaluated the performance of serum TFPI2 levels as a diagnostic marker. We investigated the clinicopathological characteristics of patients with TFPI2-negative and TFPI2-positive endometrial cancer. Using immunohistochemistry (IHC), we examined TFPI2 expression in tumor tissues of 105 patients with type II endometrial carcinoma and evaluated the correlation between serum and tissue TFPI2 positivity.
Results
Patients with endometrial cancer had significantly higher serum TFPI2 levels than controls (196.7 pg/mL vs. 83.3 pg/mL; p < 0.001). The sensitivity and specificity were 54.3% and 95.4%, respectively (cutoff value, 191 pg/mL). Serum TFPI2 levels were significantly elevated along with the stage progression (stage I, 189.6 pg/mL; stage III, 230.9 pg/mL; stage IV, 312.5 pg/mL; p < 0.001). Patients with high-risk histology showed significantly elevated serum TFPI2 levels than those with low-risk histology (220.8 pg/mL vs. 187.7 pg/mL; p < 0.001). The positivity rate for TFPI2 was the highest among tumor markers, including CA125, CA19-9, and CEA. Serum TFPI2 and CA125 levels were almost independent (r = 0.203, p < 0.001), and the combined sensitivity increased to 58.8%. The 5-year survival rate was significantly worse in TFPI2-positive patients (≥ 191 pg/mL, n = 178) than in TFPI2-negative patients (< 191 pg/mL, n = 150) (hazard ratio, 8.22; 95% confidence interval, 2.49–27.1; p < 0.001). TFPI2 immunostaining revealed that 37.1% (39/105) of the samples were positive for TFPI2, with an IHC score of > 0. There was no significant difference in the immunostaining score according to histological type. Serum TFPI2 levels and immunostaining score showed poor agreement (kappa coefficient, -0.039).
Conclusions
The serum TFPI2 level is a promising marker for diagnosing and predicting the prognosis of endometrial cancer. No correlation exists between serum and tissue TFPI2 levels. Further multicenter clinical trials are needed to test the utility of TFPI2 as a diagnostic marker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Endometrial cancer is the fourth most common cancer in women, and its incidence is increasing in the United States [1]. In Japan, there were 18,338 new cases of endometrial cancer and 3,630 deaths from the disease reported in 2022 [2]. Cancer antigen 125 (CA125) is the most commonly used serum tumor marker in patients with endometrial cancer, but it has a low sensitivity of 23.5–34.9% [3, 4]. Although some new serum biomarkers for the diagnosis of endometrial cancer have been proposed, further investigation is needed for clinical use [5, 6]. Zhou et al. reported the efficacy of exosomal microRNA as a diagnostic biomarker, with an area under the curve value of 0.899 when combined with CA125 and carcinoembryonic antigen (CEA) [7]. However, microRNA testing is currently not commonly used for cancer diagnosis in clinical settings. Therefore, the identification of new serum biomarkers is essential to facilitate the diagnosis of endometrial cancer and determine the appropriate surgical approach.
Tissue factor pathway inhibitor 2 (TFPI2) is a secreted Kunitz-type inhibitor of serine proteases involved in various coagulation and fibrinolysis systems, including the TF/VIIa complex, plasmin, plasma kallikrein, trypsin, and chymotrypsin [8]. TFPI2 is highly expressed in the mature placenta [9, 10] and is physiologically produced in vascular endothelial cells [11], fibroblasts, platelets [12], and macrophages [13]. TFPI2 is a tumor suppressor gene that is silenced in many types of cancer [14, 15] and is involved in tumor apoptosis and in the inhibition of invasion, growth, and metastasis [16, 17]. We previously discovered that TFPI2 is specifically expressed in ovarian clear cell carcinoma [9, 16, 18,19,20]. Since 2021, the TFPI2 test has been included in the public health insurance coverage for ovarian cancer in Japan. For endometrial cancer, Kawaguchi et al. first demonstrated the efficacy of serum TFPI2 for the diagnosis of endometrial cancer in a retrospective single-center study of 207 patients [21]. Based on the receiver operating characteristic curve for predicting the prognosis of endometrial cancer, they established a cutoff value of 177 pg/mL. This group was also the first to demonstrate that TFPI2 is highly expressed in clear cell carcinoma of the endometrium and can differentiate this type from other histological types with 100% sensitivity and 73.8% specificity through immunohistochemistry [22]. To date, no additional studies exist, and no previous report has analyzed the correlation between TFPI2 expression in serum and tissue samples from the same patients.
In this study, we enrolled patients with endometrial cancer who had undergone surgical removal as their initial treatment. We first assessed the effectiveness of TFPI2 as a serum diagnostic marker by comparing patients with endometrial cancer with healthy controls. We also analyzed the clinicopathological characteristics and prognoses of TFPI2-positive and TFPI2-negative patients following initial treatment for endometrial cancer. Furthermore, we investigated the correlation between TFPI2 expression in serum and tissue samples in high-risk histological types of endometrial cancer. This study aimed to provide a more comprehensive understanding of the condition of patients with endometrial cancer based on serum TFPI2 levels. Overall, our findings demonstrate the potential utility of TFPI2 in the preoperative diagnosis of patients with endometrial cancer.
Methods
Study design and participants
This was a retrospective single-center study. The protocol was reviewed and approved by the Institutional Review Board of Kanagawa Cancer Center (approval no. Ethics-2021-60). We preoperatively collected serum samples from 401 patients with endometrial cancer who underwent primary treatment at the Kanagawa Cancer Center between 2010 and 2020. Among them, we included patients who were treated with primary surgery for endometrial cancer of the following histological types: endometrioid carcinoma with any grade, serous carcinoma, clear cell carcinoma, carcinosarcoma, and mixed-type adenocarcinoma, according to the World Health Organization classification 5th edition [23]. We excluded patients with a history of other malignancies, those who underwent neoadjuvant therapy, those who did not undergo primary surgery, and those with neuroendocrine carcinoma or mucinous carcinoma of the endometrium. We defined the type I histological type as low-risk histology (endometrioid carcinoma grades 1 and 2) and type II histological type as high-risk histology (endometrioid carcinoma grade 3, serous carcinoma, clear cell carcinoma, carcinosarcoma, and mixed adenocarcinoma) based on the World Health Organization 2020 classification [23,24,25]. We used serum from 65 women as healthy controls, which were purchased from Bio IVT [26] for 31 cases and Trina Bioreactives AG [27] for 34 international healthy volunteers. The immunohistochemical cohort comprised 105 patients with high-risk histological features of endometrial cancer who underwent primary surgery between 2010 and 2015. We analyzed TFPI2 expression levels in serum and tissue samples from 33 patients for whom samples were available.
Serum data
We employed an automated immunoassay analyzer (AIA) system (TOSOH, Japan) as described previously [16, 20]. This system uses a sandwich-type one-step immunofluorometric assay involving two different anti-TFPI2 monoclonal antibodies produced by TOSOH corporation, and immunoreaction reagents are provided as the E-test “TOSOH” II (TFPI2) (product number: 0025245; TOSOH, Japan). For our experiments, we utilized recombinant TFPI2 protein, which was obtained from SP 2/0 cells transfected with a TFPI2 expression vector and added it to the sample dilution buffer.
Tissue microarray
We used tissue microarrays (TMA) of high-risk histological endometrial carcinomas that were surgically removed at the Kanagawa Cancer Center. Five 2.0 mm-diameter tissue cores of formalin-fixed paraffin-embedded (FFPE) tissues for each case were prepared for TMA from three sites showing representative primary tumor histology, a site from the non-neoplastic endometrium, and a site from the non-neoplastic myometrium as normal controls.
Evaluation of TFPI2 immunohistochemistry for TMA
Immunohistochemistry (IHC) was performed using the same concentration of mouse monoclonal anti-TFPI2 antibody (sc-48380, diluted 1:200, Santa Cruz Biotechnology, Inc., CA, USA) as the primary antibody to ensure consistency of our experimental approach [20]. Immunohistological evaluations were conducted by two individuals (MU and YO) under the supervision of a board-certified surgical pathologist (SS). We selected and evaluated the cores that exhibited the highest staining intensity among the three cores. The IHC score was calculated by multiplying the intensity (0, 1+, 2+, 3+) and labeled tumor cell frequency (%) at the core of the most predominant intensity. For example, when the most predominant intensity was 2+, and the proportion of 2 + accounted for 30%, the IHC score was calculated as 2 × 30. An IHC score of 0 was considered negative. We analyzed the agreement between serum TFPI2 levels and IHC scores in high-risk histological endometrial cancer.
Clinical data analysis
The cutoff value of serum TFPI2 levels was set at 191 pg/mL, which is clinically established for the diagnosis of ovarian cancer, and a value less than the cutoff was defined as negative, while a higher value was defined as positive [18, 28]. We calculated sensitivity and specificity for endometrial cancer and analyzed clinical characteristics of patients, including serum TFPI2 level, age, parity, serum CA125 level, treatment period, histology, International Federation of Gynecology and Obstetrics (FIGO) stage (2009) [29], TNM classification (8th edition) [30], and ascites fluid cytology. The positivity rates of tumor markers (TFPI2, CA125, CA19-9, and CEA) were calculated, and the correlations between TFPI2 and other tumor markers were analyzed. Furthermore, we calculated the positivity of the combination of TFPI2 and CA125. The 5-year overall survival was compared between TFPI2-positive and TFPI2-negative groups (n = 328). Furthermore, the sensitivity and specificity were evaluated using the existing cutoff value of 177 pg/mL, which has been reported to be useful for predicting the prognosis of patients with endometrial cancer [21].
Statistics
Continuous variables were assessed for normality using the Shapiro–Wilk test. The Mann–Whitney U test was used for comparisons between two groups; the Kruskal–Wallis test was employed for comparisons among three or more groups. Categorical variables were analyzed using Fisher’s exact test. Pearson’s correlations were used for correlation analysis, and Cohen’s kappa test was used for agreement analysis. The 5-year overall survival rates were estimated using the Kaplan–Meier method and compared using the log-rank test. The Cox proportional hazards regression model was used for multivariate analysis of the 5-year overall survival. We used SPSS Statistics version 19 (IBM, Armonk, NY, USA) and EZR version 1.61 (64-bit) [31]. p < 0.05 was considered to indicate a statistically significant difference.
Results
Effectiveness of TFPI2 as a serum diagnostic marker
The clinicopathological characteristics of patients with endometrial cancer are shown in Table 1. In total, 328 patients were included and compared with 65 healthy controls (Additional file 1).
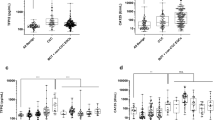
Patients with endometrial cancer had significantly higher serum TFPI2 levels than controls (median 196.7 pg/mL vs. 83.3 pg/mL; p < 0.001) (Fig. 1). Sensitivity and specificity were 54.3% and 95.4% for a cutoff value of 191 pg/mL and 66.2% and 93.8% for a cutoff value of 177 pg/mL (Additional file 2 A, 2B, Additional file 3) [21]. In the present study, we set the cutoff value at 191 pg/mL, which is clinically established for the diagnosis of ovarian cancer [18, 28].
Comparison of serum TFPI2 levels between healthy controls and patients with endometrial cancer. The Mann–Whitney U test showed that the serum TFPI2 levels of patients with endometrial cancer were significantly higher than those of healthy controls (median 196.7 pg/mL vs. 83.3 pg/mL). *** indicates p < 0.001. The dotted line indicates 191 pg/mL. TFPI2, tissue factor pathway inhibitor 2
Serum TFPI2 levels of patients with endometrial cancer by histological types and FIGO stages. (A) TFPI2 levels were significantly higher in the high-risk group than in the low-risk group (220.8 pg/mL vs. 187.7 pg/mL, p < 0.001). (B) TFPI2 levels were significantly higher in stage III and IV than in stage I (stage I, 189.6 pg/mL; stage III, 230.9 pg/mL; stage IV, 312.5 pg/mL). *** indicates p < 0.001 in the Mann–Whitney U test. The dotted line indicates 191 pg/mL. CS, carcinosarcoma; EMG, endometrioid carcinoma grade; FIGO, International Federation of Gynecology and Obstetrics; TFPI2, tissue factor pathway inhibitor 2
Clinicopathological characteristics of the TFPI2-positive and TFPI2-negative groups
Patients in the TFPI2-positive group were older than those in the TFPI2-negative group (median 57.0 years vs. 59.5 years, p = 0.005) and had higher proportions of CA125-positive cases (10.0% vs. 28.7%, p < 0.001), high-risk histological cases (14.7% vs. 35.4%, p < 0.001), and cases with stage II or higher according to the FIGO classification (13.3% vs. 32.0%, p < 0.001) (Table 1). There were significant differences in surgical procedures and whether patients underwent adjuvant chemotherapy between TFPI2-negative group and TFPI2-positive group (Additional file 4).
Next, we evaluated serum TFPI2 levels according to the histological type and FIGO stage. TFPI2 levels were significantly higher in the high-risk histological group than in the low-risk histological group (220.8 pg/mL vs. 187.7 pg/mL, p < 0.001) (Fig. 2A). The positivity rate was higher in the high-risk histological group than in the low-risk histological group (74.1% vs. 47.3%, p < 0.001) (Additional file 5 A). Compared to stage I, stages III and IV had significantly higher TFPI2 levels (stage I, 189.6 pg/mL; stage III, 230.9 pg/mL; stage IV, 312.5 pg/mL; p < 0.001, Fig. 2B). The TFPI2 positivity rate was also higher in stages III and IV (stage I, 48.2%; stage II, 63.2%; stage III, 75.6%; stage IV, 84.6%; p < 0.001) (Additional file 5B, Additional file 6).
Correlation between TFPI2 and other tumor markers
The positivity rates for TFPI2, CA125, CA19-9, and CEA were 54.3%, 20.1%, 24.7%, and 20.1%, respectively (Additional file 7). Combining TFPI2 and CA125 increased the positivity to 58.8% (Additional file 7). The correlation between serum TFPI2 and other tumor markers was low or absent; for example, TFPI2 and CA125 only had a low correlation (r = 0.203; p < 0.001, kappa coefficient, 0.176; 95% confidence interval [CI], 0.074–0.278) (Fig. 3, Additional file 8, Additional file 9.
Efficacy of serum TFPI2 for prognostic prediction
We analyzed the association between preoperative serum tumor marker levels and the 5-year survival rates. The 5-year survival rate was significantly worse in the TFPI2-positive group than in the TFPI2-negative group (hazard ratio [HR], 8.22; 95% CI, 2.49–27.1; log-rank test, p < 0.001) (Fig. 4). Serum TFPI2, CA125, CA19-9, and CEA levels, parity, treatment period, and age were used as covariates in the Cox proportional hazards regression model. Multivariate analysis showed that TFPI2 positivity was associated with poor prognosis (HR, 6.00; 95% CI, 1.79–20.1); CA125 positivity (HR, 3.05; 95% CI, 1.34–6.95) and age over 65 years (HR, 1.05; 95% CI, 1.01–1.09) were also associated with poor prognosis (Table 2).
Kaplan–Meier curve of preoperative serum TFPI2 levels and 5-year survival. Serum TFPI2 positivity is associated with poor prognosis in patients with endometrial cancer. P-value was calculated using the log-rank test (HR, 8.22; 95% CI, 2.49–27.1; p < 0.001). CI, confidence interval; HR, hazard ratio; TFPI2, tissue factor pathway inhibitor 2
Immunohistological analysis of TFPI2
IHC for TFPI2 was performed on 105 patients with endometrial cancer of high-risk histological types, and immunolabeled TFPI2 was detected in the cytoplasm of the tumor cells (Fig. 5). An IHC score greater than 0 was considered positive, and the positivity rate was 37.1% (Additional file 10). There were no significant differences in age, parity, CA125 levels, treatment period, histological type, FIGO stage, or TNM stage between the positive and negative groups. None of the non-neoplastic control tissues from the endometrium or the myometrium exhibited TFPI2 expression (Additional file 11). There were no significant differences in the IHC scores according to histological type (p = 0.427) (Fig. 6). In addition, the agreement rate of TFPI2 positivity between the serum and tissue was indicated by a kappa coefficient of -0.039, demonstrating poor agreement (95% CI, -0.344–0.265); there were no significant differences between serum TFPI2 levels of the IHC-positive and IHC-negative groups (p = 0.427) (Additional file 12, Additional file 13).
Microscopic immunohistochemistry images of TFPI2 expression in endometrial cancer tissues. Microscopic images show immunohistochemistry for TFPI2 in (A) clear cell carcinoma with intensity 0, (B) endometrioid carcinoma grade 3 with intensity 1+, (C) clear cell carcinoma with intensity 2+, and (D) serous carcinoma with intensity 3+. There were both IHC-positive and IHC-negative cases for all histological types. HE, hematoxylin eosin; IHC, immunohistochemistry; TFPI2, tissue factor pathway inhibitor 2
Discussion
This study demonstrates the potential of TFPI2 as a serum diagnostic marker for endometrial cancer, using the cutoff value of 191 pg/mL clinically applied for ovarian cancer, with elevated performance in combination with serum CA125. The evaluation of serum TFPI2 levels before surgery alone also raises the possibility of predicting patient prognosis. The serum level of TFPI2 was not simply correlated with its expression in tumor cells.
Kawaguchi et al. reported that TFPI2 with a cutoff value of 177 pg/mL may serve as an independent prognostic factor in such patients (69.4% sensitivity; 69.4% specificity) [21]. In the present study, we validated the results of this previous study with an increased number of patients from another institution. Using a cutoff value of 191 pg/mL, the established value for ovarian cancer diagnosis, decreased the sensitivity from 66.2 to 54.3% but slightly elevated the specificity from 93.8 to 95.4%, compared to a cutoff value of 177 pg/mL. This decrease in sensitivity partly improved to 58.8% when combined with serum CA125 testing. TFPI2 is independent of CA125; therefore, a combination of the two biomarkers may benefit more patients with endometrial cancer in terms of diagnosis and disease monitoring. However, we should use these markers in combination with other examinations, such as sonography and endometrial cytology, because the individual sensitivity values of these markers are not sufficiently high for accurate diagnosis.
Generally, TFPI2 acts as a suppressor of cancer progression by inhibiting the proteolytic degradation of proteins, leading to the suppression of tumor proliferation, invasion, and angiogenesis, as well as the promotion of apoptosis [11, 32]. Epigenetic silencing by the aberrant methylation of CpG islands in the TFPI-2 promoter has been reported in many types of cancer [33,34,35]. In breast and pancreatic cancers, low TFPI2 expression in tumor cells is associated with poor patient survival [36, 37]. One study reported the pro-tumorigenic effect of TFPI2 on the hepatocyte growth factor-induced invasion of hepatocellular carcinoma cells [32]. However, the effect of serum TFPI2 levels on cancer progression remains unclear. The present study revealed that serum TFPI2 levels were elevated in patients with high-risk histological types and advanced stages of endometrial cancer. Although the pro-tumorigenic aspects of TFPI2 remain to be clarified biologically in endometrioid cancer, this finding indicates that even with low-risk histology on pathologic examination or in early-stage disease by imaging, elevated serum TFPI2 levels should lead to consideration of the possibility of high-risk histology or advanced disease when planning preoperative treatment strategies.
We demonstrated that TFPI2 was detected via IHC in all high-risk histological types. In a previous study involving 55 patients with endometrial cancer, TFPI2 IHC was able to differentiate clear cell carcinoma from other histological types with a sensitivity of 100% and specificity of 73.8% [22]. However, in the present study, no differences in the IHC scores were observed across the various histological types of endometrial carcinomas. Nonetheless, several factors differed between the two studies, i.e., the sample sizes were different, and the target populations were notably different. The previous study included grade 1 and 2 endometrioid carcinoma (11 out of 55) but excluded carcinosarcoma and mixed-type endometrial carcinomas. Furthermore, a prior study reported a localized positive staining pattern for TFPI2, which might have been overlooked in our TMA examination. We used the same antibody as that used by Kawaguchi et al. [22] but adopted the same concentration as that used in our previous research on ovarian cancers [20], which differed from that used by Kawaguchi et al. [22]. The sensitivity and specificity of TFPI2 immunostaining for clear cell endometrial carcinoma require further characterization.
We conducted the first analysis comparing TFPI2 expression levels in serum and tissues derived from the same patients, demonstrating a low agreement rate. This may be partly consistent with our findings in ovarian cancer that TFPI2 was exclusively found in clear cell carcinoma [20], whereas elevated serum TFPI2 was observed in 29.4% of serous adenocarcinoma cases [16, 18]. It is conceivable that the secretory capacity of TFPI2 varies among cells; however, several other factors can be postulated for this apparent discrepancy. First, in some cases, TFPI2 expression may be localized and prone to be missed by immunostaining of representative sections or TMAs [22]. Second, TFPI2 secreted from tumor cells can be trapped on the cell surface or in the extracellular matrix (ECM), preventing its migration into the bloodstream. Both TFPI and TFPI2 carry strong positive charges at their C-terminal regions, allowing them to adhere to negatively charged ECM or cell surface heparan sulfate proteoglycans, such as glypican-3, via glycosylphosphatidylinositol anchors [38]. Third, TFPI2 is released or secreted into the serum by non-cancer cells physiologically producing TFPI2, such as vascular endothelial cells [11], platelets [12], or macrophages [13], under conditions that are currently undefined. In endometrial cancer, discordance between serum levels and tissue IHC staining for CA125 has been reported, and CA125 production from sources other than endometrial cancer tissues has been suggested [39]. The mechanism of elevated serum TFPI2 remains largely unknown; thus, understanding this mechanism may promote the clinical use of this biomarker.
Limitations
This study has several limitations. First, it was a retrospective single-center study, and the results may not be generalizable to other settings. Second, we compared the serum TFPI2 levels of patients with endometrial cancer with those from commercially available serum samples of international healthy volunteers used as controls. Consequently, we were unable to [1] accurately assess the incidence with precise sensitivity and specificity, [2] determine an accurate cutoff value for TFPI2 from the ROC curve, and [3] thoroughly evaluate the efficacy of combining of TFPI2 with CA125 versus using CA125 alone in terms of specificity, positive predictive value, and negative predictive value. To address these limitations, a future prospective study is necessary. Third, we did not use the FIGO 2023 classification but instead applied the FIGO 2009 classification for endometrial cancer owing to the lack of molecular and biological data [29, 40]. Fourth, when evaluating protein expression levels by IHC using FFPE specimens, the heterogeneity of formalin fixation intensity within and between specimens is known to affect stainability, which is another limitation of this study. Paired TFPI2 analyses of IHC using FFPE specimens and transcriptomics with frozen tissues in at least some cases are needed in future research. Fifth, we attempted to incorporate other factors such as family histories of endometriosis, endometrial cancer, and other types of cancer in multivariate analysis for prognosis of patients with endometrial cancer. However, our medical records did not provide complete data on these variables, thus we were unable to include these factors in our analysis. To address this limitation, we recommend conducting prospective, multicenter collaborative research to ensure the clinical application of TFPI2 as a serum biomarker for endometrial cancer.
Conclusions
Serum TFPI2 has been suggested as a useful diagnostic and prognostic predictor of endometrial cancer.
Data availability
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CA:
-
Cancer antigen
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- ECM:
-
Extracellular matrix
- FFPE:
-
Formalin-fixed paraffin-embedded
- FIGO:
-
International Federation of Gynecology and Obstetrics
- HR:
-
Hazard ratio
- IHC:
-
Immunohistochemistry
- TFPI2:
-
Tissue factor pathway inhibitor 2
- TMA:
-
Tissue microarray
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
World Cancer Research Fund International. Endometrial cancer statistics 2024. https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/. Accessed 15 July 2024.
Chao A, Tang YH, Lai CH, Chang CJ, Chang SC, Wu TI, et al. Potential of an age-stratified CA125 cut-off value to improve the prognostic classification of patients with endometrial cancer. Gynecol Oncol. 2013;129:500–4.
Jiang T, Huang L, Zhang S. Preoperative serum CA125: a useful marker for surgical management of endometrial cancer. BMC Cancer. 2015;15:396.
Wang C, Li J, Liu W, Li S, Zhang Y, Jin Y, et al. Comprehensive analysis and experimental validation reveal elevated CLCN4 is a promising biomarker in endometrial cancer. Aging. 2023;15:8744–69.
Meijuan C, Meng X, Fang L, Qian W. Synaptotagmin-like protein 1 is a potential diagnostic and prognostic biomarker in endometrial cancer based on bioinformatics and experiments. J Ovarian Res. 2023;16:16.
Zhou L, Wang W, Wang F, Yang S, Hu J, Lu B, et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol Cancer. 2021;20:57.
Sierko E, Wojtukiewicz MZ, Kisiel W. The role of tissue factor pathway inhibitor-2 in cancer biology. Semin Thromb Hemost. 2007;33:653–9.
Miyagi Y, Koshikawa N, Yasumitsu H, Miyagi E, Hirahara F, Aoki I, et al. cDNA cloning and mRNA expression of a serine proteinase inhibitor secreted by cancer cells: identification as placental protein 5 and tissue factor pathway inhibitor-2. J Biochem. 1994;116:939–42.
Rao CN, Reddy P, Liu Y, O’Toole E, Reeder D, Foster DC, et al. Extracellular matrix-associated serine protease inhibitors (Mr 33,000, 31,000, and 27,000) are single-gene products with differential glycosylation: cDNA cloning of the 33-kDa inhibitor reveals its identity to tissue factor pathway inhibitor-2. Arch Biochem Biophys. 1996;335:82–92.
Kempaiah P, Chand HS, Kisiel W. Human tissue factor pathway inhibitor-2 is internalized by cells and translocated to the nucleus by the importin system. Arch Biochem Biophys. 2009;482:58–65.
Vadivel K, Ponnuraj SM, Kumar Y, Zaiss AK, Bunce MW, Camire RM, et al. Platelets contain tissue factor pathway inhibitor-2 derived from megakaryocytes and inhibits fibrinolysis. J Biol Chem. 2014;289:31647–61.
Pou J, Rebollo A, Piera L, Merlos M, Roglans N, Laguna JC, et al. Tissue factor pathway inhibitor 2 is induced by thrombin in human macrophages. Biochim Biophys Acta. 2011;1813:1254–60.
Yang Y, Zhang C, Li S, Liu J, Qin Y, Ge A. Tissue factor pathway inhibitor 2 suppresses the growth of thyroid cancer cells through by induction of apoptosis. Asia Pac J Clin Oncol. 2021;17:e48–56.
Kobayashi H, Imanaka S. Toward an understanding of tissue factor pathway inhibitor-2 as a novel serodiagnostic marker for clear cell carcinoma of the ovary. J Obstet Gynaecol Res. 2021;47:2978–89.
Arakawa N, Kobayashi H, Yonemoto N, Masuishi Y, Ino Y, Shigetomi H, et al. Clinical significance of tissue factor pathway inhibitor 2, a serum biomarker candidate for ovarian clear cell carcinoma. PLoS ONE. 2016;11:e0165609.
Xu Y, Qin X, Zhou J, Tu Z, Bi X, Li W, et al. Tissue factor pathway inhibitor-2 inhibits the growth and invasion of hepatocellular carcinoma cells and is inactivated in human hepatocellular carcinoma. Oncol Lett. 2011;2:779–83.
Miyagi E, Arakawa N, Sakamaki K, Yokota NR, Yamanaka T, Yamada Y, et al. Validation of tissue factor pathway inhibitor 2 as a specific biomarker for preoperative prediction of clear cell carcinoma of the ovary. Int J Clin Oncol. 2021;26:1336–44.
Arakawa N, Miyagi E, Nomura A, Morita E, Ino Y, Ohtake N, et al. Secretome-based identification of TFPI2, a novel serum biomarker for detection of ovarian clear cell adenocarcinoma. J Proteome Res. 2013;12:4340–50.
Ota Y, Koizume S, Nakamura Y, Yoshihara M, Takahashi T, Sato S, et al. Tissue factor pathway inhibitor–2 is specifically expressed in ovarian clear cell carcinoma tissues in the nucleus, cytoplasm and extracellular matrix. Oncol Rep. 2021;45:1023–32.
Kawaguchi R, Maehana T, Yamanaka S, Miyake R, Kawahara N, Iwai K, et al. Preoperative serum tissue factor pathway inhibitor–2 level as a prognostic marker for endometrial cancer. Oncol Lett. 2023;26:463.
Kawaguchi R, Maehana T, Sugimoto S, Kawahara N, Iwai K, Yamada Y, et al. Immunohistochemical analysis of the tissue factor pathway inhibitor-2 in endometrial clear cell carcinoma: a single-center retrospective study. Int J Gynecol Pathol. 2024;43:25–32.
Board WCoTE. WHO Classification of Tumours, 5th edition: WORLD HEALTH ORGANIZATION; 2020.
Dueholm M, Hjorth IM, Dahl K, Marinovskij E, Ørtoft G. Preoperative prediction of high-risk endometrial cancer by expert and non-expert transvaginal ultrasonography, magnetic resonance imaging, and endometrial histology. Eur J Obstet Gynecol Reprod Biol. 2021;263:181–91.
Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505.
Li L, Li D, Heyward S, Wang H. Transcriptional regulation of CYP2B6 expression by hepatocyte nuclear factor 3β in human liver cells. PLoS ONE. 2016;11:e0150587.
Anckaert E, Jank A, Petzold J, Rohsmann F, Paris R, Renggli M, et al. Extensive monitoring of the natural menstrual cycle using the serum biomarkers estradiol, luteinizing hormone and progesterone. Pract Lab Med. 2021;25:e00211.
Maehana T, Kawaguchi R, Nishikawa K, Kawahara N, Yamada Y, Kimura F. Investigating the efficacy of tissue factor pathway inhibitor–2 as a promising prognostic marker for ovarian cancer. Oncol Lett. 2024;28:302.
Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109.
Brierley JD, Gospodarowicz MK, Wittekind C. Gynaecological tumours. In: O’Sullivan B, Mason M, Asamura H, Lee A, Van Eycken E, Denny L, Amin MB, Gupta S, editors. TNM classification of malignant tumours. 8th ed. Hoboken: John Wiley & Sons, Ltd.; 2017. pp. 171–4.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Neaud V, Hisaka T, Monvoisin A, Bedin C, Balabaud C, Foster DC, et al. Paradoxical pro-invasive effect of the serine proteinase inhibitor tissue factor pathway inhibitor-2 on human hepatocellular carcinoma cells. J Biol Chem. 2000;275:35565–9.
Jee CD, Kim MA, Jung EJ, Kim J, Kim WH. Identification of genes epigenetically silenced by CpG methylation in human gastric carcinoma. Eur J Cancer. 2009;45:1282–93.
Dong Y, Tan Q, Tao L, Pan X, Pang L, Liang W, et al. Hypermethylation of TFPI2 correlates with cervical cancer incidence in the Uygur and Han populations of Xinjiang, China. Int J Clin Exp Pathol. 2015;8:1844–54.
Lo Nigro C, Wang H, McHugh A, Lattanzio L, Matin R, Harwood C, et al. Methylated tissue factor pathway inhibitor 2 (TFPI2) DNA in serum is a biomarker of metastatic melanoma. J Invest Dermatol. 2013;133:1278–85.
Xu C, Wang H, He H, Zheng F, Chen Y, Zhang J, et al. Low expression of TFPI-2 associated with poor survival outcome in patients with breast cancer. BMC Cancer. 2013;13:118.
Zhai LL, Cai CY, Wu Y, Tang ZG. Correlation and prognostic significance of MMP-2 and TFPI-2 differential expression in pancreatic carcinoma. Int J Clin Exp Pathol. 2015;8:682–91.
Mast AE, Higuchi DA, Huang ZF, Warshawsky I, Schwartz AL, Broze GJ. Glypican-3 is a binding protein on the HepG2 cell surface for tissue factor pathway inhibitor. Biochem J. 1997;327(Pt 2):577–83.
Yamazawa K, Hirashiki K, Usui H, Mitsuhashi A, Matsui H, Sekiya S. Discordance between serum level and tissue immunohistochemical staining of CA125 in endometrioid adenocarcinoma of the uterine corpus. Int J Gynecol Pathol. 2005;24:254–9.
Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162:383–94.
Acknowledgements
Our sincere thanks go to the teams in the Gynecology and Pathology Department at Kanagawa Cancer Center Hospital for their invaluable support.
Funding
This study was funded by clinical trial expenses of Kanagawa Cancer Center.
Author information
Authors and Affiliations
Contributions
MU was a major contributor to the drafting of the manuscript and data analysis. YO contributed to the manuscript drafting, data analysis, and project administration. YS provided formal analytical instructions and drafted the manuscript. AY contributed to data collection and investigation. HN contributed to the formal analysis instruction. SK contributed to supervision, review, and editing. SS performed histological examination and visualization. YN created TMAs. SM produced the figures and collected data. NO performed serum measurements and contributed to data curation. HS curated and investigated the data. EM contributed to conceptualization and funding acquisition. YM supervised the pathological experiments and the project and contributed to funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental protocol of the present study was reviewed and approved by the Institutional Review Board of the Kanagawa Cancer Center Hospital (Ethics-2021-60). Written informed consent was obtained from the patients for publication of the study and accompanying images.
Consent for publication
Not applicable.
Competing interests
SM and NO are employees of Tosoh Corporation, which provides E-test Tosoh II, an assay for measuring Tissue Factor Pathway Inhibitor 2 (TFPI2), a marker used to assist in the diagnosis of ovarian cancer. EM obtained a grant from the Tosoh Corporation outside the submitted work. YM obtained grants from the Tosoh Corporation, both for this work and outside of the submitted work. The other authors declare no conflicts of interest directly relevant to the content of this article.The analysis of serum TFPI2, CA125, CA19-9, and CEA levels was outsourced to the Tosoh Corporation by the Kanagawa Cancer Center.
Footnotes
Springer Nature remains neutral regarding its jurisdictional claims in its published maps and institutional affiliations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
: Baseline characteristics of patients with endometrial cancer and healthy controls. The Mann–Whitney U test showed significant age differences between healthy controls and patients with endometrial cancer
Supplementary Material 2
: Sensitivity and specificity of serum TFPI2 levels. (A) Sensitivity and specificity were 54.3% and 95.4%, respectively, when the cutoff was 191 pg/mL, which is used for ovarian cancer in clinical practice. (B) Sensitivity and specificity were 66.2% and 93.8%, respectively, when the cutoff was 177 pg/mL, as reported in a previous study. TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 3
: ROC curve of serum TFPI2 levels in patients with endometrial cancer. The ROC curve was obtained using serum TFPI2 levels of healthy controls and preoperative patients with endometrial cancer. The AUC was 0.948, and the cutoff with the least false negative and false positive results was determined to be 130.748 pg/mL, with a sensitivity of 86.15% and specificity of 96.65% (95% CI, 0.913–0.982). AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic curve; TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 4
: Surgical and chemotherapy procedures in patients with endometrial cancer. We included patients who underwent at least 3 cycles of chemotherapy including platinum-containing drug postoperatively and classified them as “Yes”. P-values were calculated using Fisher’s exact test. ARH, abdominal radical hysterectomy; BSO, bilateral salpingo-oophorectomy; mRH, modified radical hysterectomy; PALA, para-aortic lymphadenectomy; PLA, pelvic lymphadenectomy; TAH, total abdominal hysterectomy; TFPI2, tissue factor pathway inhibitor 2; TLmRH, total laparoscopic modified radical hysterectomy
Supplementary Material 5
: Serum TFPI2 status by histological groups and FIGO stages. Data are presented as numbers (%). FIGO, International Federation of Gynecology and Obstetrics; TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 6
: Serum TFPI2 concentrations by stage and histological type. Kruskal-Wallis test followed by Dunn’s test showed that (A) serum TFPI2 levels were significantly elevated in patients with advanced FIGO stages in both low-risk and high-risk histological types (low-risk type, p = 0.037; high-risk type, p = 0.013) and that (B) no correlation between serum TFPI2 levels of stages and serum TFPI2 levels of any histological types due to small population. I, II, III, and IV correspond to FIGO stages I, II, III, and IV, respectively. ns, not significant. * indicates p < 0.05. CS, carcinosarcoma; EMG, endometrioid carcinoma grade; FIGO, International Federation of Gynecology and Obstetrics; TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 7
: Comparison of TFPI2 with other tumor markers. Positive and negative rates of the serum tumor markers TFPI2, CA125, CA19-9, and CEA and the combination of TFPI2 and CA125 among 328 patients with endometrial cancer. TFPI2 has the highest positive rate among these individual four markers. Positivity increases to 58.8% when TFPI2 and CA125 are combined. The sensitivity of “Combination of TFPI2 and CA125” indicates CA125-positive or TFPI2-positive cases. Data are presented as numbers (%). CA, cancer antigen; CEA, carcinoembryonic antigen; TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 8
: Pearson’s correlations between serum TFPI2 and CA19-9/CEA levels. Pearson’s correlation analysis revealed (A) a significant correlation between TFPI2 and CA19-9 levels (r = 0.169, p = 0.002) and (B) no significant correlation between TFPI2 and CEA levels (r = 0.000, p = 0.463). CA, cancer antigen; CEA, carcinoembryonic antigen; TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 9
: Correlation of serum TFPI2 and CA125 levels. Kappa statistics (kappa coefficient, 0.176) showed that the positivity and negativity of TFPI2 levels and those of CA125 levels had low agreement. CA, cancer antigen; TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 10
: Clinical characteristics of the TFPI2 IHC-positive and IHC-negative groups. Data are presented as numbers (%). P-values were calculated using Fisher’s exact test. CA, cancer antigen; EMG1, endometrioid carcinoma grade 1; FIGO, International Federation of Gynecology and Obstetrics; IHC, immunohistochemistry; TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 11
: IHC images of TFPI2 in non-cancerous tissues. Both the endometrial epithelium and myometrium are negative for TFPI2 in patients with endometrial cancer. HE, hematoxylin eosin; IHC, immunohistochemistry; TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 12
: Correlation between serum TFPI2 levels and TFPI2 IHC. Kappa statistics (kappa coefficient, -0.039) showed poor agreement between the serum TFPI2 levels and TFPI2 expression according to IHC. IHC, immunohistochemistry; TFPI2, tissue factor pathway inhibitor 2
Supplementary Material 13
: Boxplot of serum TFPI2 levels in the TFPI2 IHC-negative and IHC-positive groups. The Mann–Whitney U test was used to compare the serum TFPI2 levels of the IHC-negative and IHC-positive groups, but no significant differences between the two groups were found (p = 0.427). IHC, immunohistochemistry; TFPI2, tissue factor pathway inhibitor 2
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Uomoto, M., Ota, Y., Suzuki, Y. et al. Tissue factor pathway inhibitor 2 as a serum biomarker for endometrial cancer: a single-center retrospective study. BMC Cancer 24, 1058 (2024). https://doi.org/10.1186/s12885-024-12827-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12827-0