Abstract
Bortezomib, lenalidomide, and dexamethasone (VRD), and bortezomib, doxorubicin, and dexamethasone (PAD), are commonly used in induction regimens for patients with newly diagnosed multiple myeloma (NDMM) in China. This real-world study enrolled 390 patients, 195 receiving VRD and 195 receiving PAD induction. The primary endpoint was progression-free survival (PFS) and stringent complete remission/complete remission. Across the entire cohort, VRD demonstrated significantly improved five-year overall survival (OS) (74% vs. 59%, p = 0.0024) and five-year PFS (67% vs. 37%, p = 0.0018) compared to PAD. Notably, the median OS and PFS were not reached for VRD-treated patients, while they were 77 months (60-not reached [NR]) and 46 months (36-NR), respectively, for PAD. In patients with standard-risk cytogenetics, VRD showed superior five-year OS (83% vs. 58%, p = 0.0038) and PFS (78% vs. 48%, p = 0.0091) compared to PAD. However, these differences were not statistically significant in high-risk patients. For transplanted patients, VRD was associated with superior five-year OS (91% vs. 67%, p = 0.014) and PFS (79% vs. 47%, p = 0.015) compared to PAD. In non-transplanted patients, VRD showed a trend towards improved five-year OS (p = 0.085) and PFS (p = 0.073) compared to the PAD group. In conclusion, VRD displayed superior OS and PFS outcomes in standard-risk patients and those who underwent transplantation. These findings suggest potential advantages of VRD over PAD in real-world clinical settings for NDMM treatment. However, due to the imbalance in transplantation rates between the VRD and PAD groups, limitations in testing for high-risk cytogenetic abnormalities (HRA), and the difference between the received cycles and salvage therapies, the conclusions of this study should be interpreted with caution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Significant advancements have transformed the treatment landscape for multiple myeloma (MM), the second most common hematologic malignancy. Though incurable, these developments have demonstrably impacted countless patients worldwide [1]. The rise of triplet regimens has fueled this progress, culminating in a remarkable rise in median overall survival (OS) to 126 months – a stark contrast to the 30-month median reported just two decades ago. Bortezomib, lenalidomide, and dexamethasone (VRD) induction therapy for newly diagnosed MM (NDMM) between 2006 and 2017 are credited with this dramatic improvement [2, 3].
Phase 1/2 clinical research paved the way for VRD as an induction therapy for NDMM [4]. Subsequent clinical trials solidified its efficacy and tolerability, demonstrating significant gains in both progression-free survival (PFS) and OS [5,6,7]. Notably, these studies also confirmed VRD’s favorable tolerability profile in the respective patient cohorts.
Bortezomib, doxorubicin, and dexamethasone (PAD) served as the initial therapeutic regimen for NDMM. Numerous clinical studies documented positive OS and PFS outcomes in PAD-treated patients [8,9,10]. While both VRD or PAD yielded positive clinical results, concerns surrounding uncommon but serious side effects, like bortezomib-induced peripheral neuropathy and chemotherapy-induced gastrointestinal (GI) distress, occasionally necessitated medication discontinuation [7, 11]. Furthermore, comparisons between VRD and PAD, two widely used NDMM induction regimens, remain limited.
Driven by this gap, we conducted a retrospective single-center study to analyze the efficacy and safety profiles of these two induction regimens. Our investigation prioritizes patients categorized by risk (high and standard), as well as transplant recipients and non-recipients.
Methods
Clinical data from Sun Yat-Sen University Cancer Center was retrospectively analyzed for NDMM patients treated with VRD or PAD between May 17, 2013, and August 23, 2023. October 20, 2023, served as the final follow-up date. Stringent procedures ensured data accuracy and completeness.
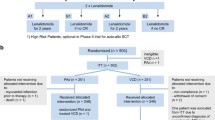
Inclusion criteria targeted Sun Yat-Sen University Cancer Center individuals receiving at least one cycle of VRD or PAD. Patients lost to follow-up were excluded, resulting in a final analysis of 390 patients. The median follow up was 27 months for patients in PAD cohort and 24 months for patients in VRD cohort.
The choice of the induction regimen was guided by medical recommendations, patient preferences, and financial considerations. Transplantation decisions were based on individual preferences and the medical team’s assessment. Bortezomib was administered weekly at a dose of 1.3 mg/m2, while doxorubicin was given at a dose of 30 mg/m2.
For transplant-eligible patients, the standard induction treatment consisted of 4 cycles followed by transplantation. After transplantation, it was recommended that these patients receive an additional 2 cycles of consolidation treatment. Non-transplant recipients underwent a standard induction treatment of 9 cycles. For some non-transplants who did not want to finish 9 cycles of induction treatment, the induction treatment cycles should be at least six cycles, even if they achieve complete remission (CR) during the initial phase of induction treatment. For non-transplants who do not wish to complete nine cycles of induction treatment due to intolerable adverse events, it is still recommended to complete 6 cycles of induction treatment. At our center, maintenance treatment is continued until disease progression, patient preference to discontinue maintenance, or the occurrence of intolerable adverse events. Salvage therapy for transplant recipients involves either advising them to participate in clinical trials or adding novel agent-based regimens such as daratumumab, carfilzomib, and pomalidomide-based induction treatment. Salvage treatment for non-transplants follows a similar approach. In our study, the median induction cycles before transplantation were 4 in the PAD group and 4 in the VRD group.
High-risk cytogenetics encompassed cytogenetic abnormalities like 1q+, t(4;14), t(14;16), and del(17p). High-risk patients were defined as those carrying at least one cytogenetic abnormality classified as high risk. Standard-risk patients were defined as those without high-risk cytogenetic abnormalities.
Response to induction treatment was assessed using International Myeloma Working Group (IMWG) criteria [12]. Minimal residual disease (MRD) evaluation, conducted throughout treatment via flow cytometry, established the lowest detectable MRD value at 10− 5 [13]. A P value less than 0.05 was considered as statistically significant.
Adverse events (AEs) data, sourced from patients’ medical records, included frequently reported events like diarrhea, vomiting, and peripheral neuropathy. AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE).
Statistical analysis
Baseline characteristics were compared between treatment groups using Fisher’s exact test and the Chi-squared test for categorical variables, and the Wilcoxon rank-sum test or Student’s t-test for continuous variables. OS was defined as the time between induction initiation and eventual death from any cause. PFS was the time from induction initiation to progression or death from any cause. Event-free survival (EFS) was the time from induction initiation to any of the following events: disease progression, modification of treatment post-VRD or PAD induction, or death from any cause. Kaplan-Meier curves were used to visualize OS, PFS, and EFS, and log-rank tests assessed differences between VRD and PAD groups. A multivariable Cox proportional hazards model was built to analyze the impact of induction therapy on OS and PFS, accounting for other relevant variables. At our institution, the number of patients undergoing transplantation was notably lower in the PAD group compared to the VRD group. There existed a statistically significant disparity in the rate of transplantation between the PAD and VRD groups (P = 0.002). The difference in the rate of transplantation between VRD and PAD group was considered as a confounding factor that may lead to superior survival outcomes in VRD group compared to PAD group. Consequently, Propensity Score Matching (PSM) analysis was additionally conducted to mitigate the impact of this baseline imbalance and affirm the primary findings of our study. Following PSM analysis, these was still a superior advantage in OS (P = 0.066) and PFS (P = 0.005) in VRD group, demonstrating the continued superiority of VRD induction over PAD induction (Table S1 and Figure S1). This approach aimed to validate the outcomes by addressing the potential bias introduced by the different rate of transplantation between two treatment groups.
Statistical significance was set at a p value less than 0.05. All analyses were performed using CRAN R Version 4.3.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
This study analyzed data from 390 patients with NDMM, treated with VRD and PAD induction therapy, at Sun Yat-sen University Cancer Center between May 17, 2013, and August 23, 2023. Within this cohort, 195 individuals received VRD, and 195 received PAD induction. Table 1 provides a detailed comparison of their baseline characteristics.
VRD-treated patients showed a greater tendency towards transplantation. While the median age for VRD recipients was 58 years (interquartile range [IQR] 53–67), for PAD it was 59 years (IQR 53–65). After induction, 37 patients (19%) in the PAD group, and 65 (33%) in the VRD group, underwent transplantation.
Various factors influenced the decision against transplantation. Some patients did not meet the IMWG criteria, others faced difficulties collecting hematopoietic stem cells, and some opted out for personal reasons [14]. Among VRD patients, 46% received maintenance treatment, either post-transplantation or after induction. In the PAD- group, this proportion was 48%. Lenalidomide was the most common maintenance drug.
Efficacy
The VRD group demonstrated a superior response to treatment compared to the PAD group. After induction, significantly more patients in the VRD group achieved complete remission (CR) or stringent CR (sCR) (30% vs. 20%, p = 0.02). After induction 26% of VRD patients and 15% of PAD patients achieving MRD negativity (p = 0.043) (Table 2).
Within transplanted patients, 35% of VRD recipients achieved sCR/CR and 40% achieved very good partial response (VGPR). Additionally, 30% of PAD recipients achieved sCR/CR and 45% achieved VGPR in the PAD group. Moreover, 30% of VRD patients and 25% of PAD patients achieved MRD-negative status after transplantation. Notably, there was not a statistically significant difference in the achievement of sCR/CR (P = 0.66) and MRD negativity (P = 0.78) between two treatment groups within transplant recipients (Table 3).
Survival analysis for all patients
OS, PFS, and EFS for all patients
OS: Patients treated with VRD had a five-year OS of 74% (95% confidence interval [CI]: 59–93%), significantly higher than the 59% (95% CI: 49–72%) observed in the PAD group (p = 0.0024, Fig. 1A). Notably, the median OS was not yet reached for VRD patients, while it was 77 months (95% CI: 60%-NR) for PAD. PSM confirmed the stability of these findings, with VRD maintaining an OS (P = 0.066) and PFS (P = 0.005) advantage even after balancing baseline characteristics (Figure S1A).
Overall survival (OS), progression-free survival (PFS), and event-free (EFS) survival of VRD versus PAD for the study participants. The Time was based on months. A: OS of VRD versus PAD for the study participants B: PFS of VRD versus PAD for the study participants C: EFS of VRD versus PAD for the study participants
PFS: VRD patients exhibited a significantly higher five-year PFS of 67% (95% CI: 57–80%) compared to the 37% (95% CI: 26–53%) in the PAD group (p = 0.0018, Fig. 1B). Similar to OS, the median PFS remained unreached for VRD patients, while it was 46 months (95% CI: 36-NR) for PAD. PSM again validated the initial results, with VRD retaining a statistically significant PFS advantage (p = 0.011) following baseline balancing (Figure S1B).
EFS: To minimize potential bias introduced by treatment deviations from IMWG guidelines at Sun Yat-sen University Cancer Center, an EFS analysis was conducted. This analysis estimated the likelihood of patients completing both induction regimens. The five-year EFS was significantly higher in the VRD group (60%, 95% CI: 49–72%) compared to the PAD group (28%, 95% CI: 18–42%) (p < 0.0001, Figure S1C).
Subgroup analysis
To comprehensively evaluate the effects of cytogenetic risk and transplantation on survival outcomes, we conducted a subgroup analysis. Patients were stratified by post-induction transplantation status, and further classified into high-risk and low-risk groups based on cytogenic abnormalities. Within the VRD group, 34% of patients exhibited high-risk cytogenetics, compared to 44% in the PAD group. Notably, 13% of VRD recipients and 12% in the PAD recipients had more than two high-risk cytogenic abnormalities. This stratification enabled examination of OS, PFS, and EFS within each risk group. Among VRD-induced patients, 65 (33%) underwent transplantation, compared to 37 (19%) in the PAD group.
OS and PFS outcomes in high-risk patients versus standard-risk patients
OS: For high-risk patients, the five-year OS was 58% (95% CI: 38–89%) in the VRD cohort, and 55% (95% CI: 43–71%) in the PAD cohort (p = 0.21, Fig. 2A). Median OS reached 77 months (95% CI: 44%-NR) for PAD patients, but the median OS was not reached for VRD patients. Both cohorts with standard-risk patients had an undetermined median OS, with five-year OS of 83% (95% CI: 65–100%) in the VRD group, and five-year OS of 61% (95% CI: 45–83%) in the PAD group, respectively (Fig. 2B).
PFS: With a p value of 0.31, the five-year PFS for VRD and PAD among high-risk patients was 48% (95%CI: 30–75%), and 21% (95% CI: 9.0–47%), respectively (Fig. 3A). Median PFS was 36 months (95%CI: 29-NR) for PAD patients, and 49 months (95%CI: 32-NR) for VRD patients. In the standard-risk category, with a p value of 0.0091, the five-year PFS was 79% (95%CI: 70–89%) for VRD, 52% (95%CI, 39–69%) for PAD. Median PFS reached 97 months (95%CI: 42-NR) for PAD patients but the median PFS was not reached for VRD patients (Fig. 3B).
OS and PFS for patients stratified by whether they receive transplantation
OS: Among transplant recipients, VRD demonstrated a significantly higher five-year OS (91%, 95% CI: 80–100%) compared to PAD (67%, 95% CI: 50–90%) (p = 0.014). Notably, the median OS remained unreached for both groups (Fig. 4A). For non-transplant patients, the five-year OS was 59% (95% CI: 36–97%) for VRD, and 57% (95% CI: 45–73%) for PAD (p = 0.085). While the median OS reached 77 months (60-NR) for PAD, it remained uncreased for VRD (Fig. 4B).
PFS: In transplant recipients, VRD showed significantly superior five-year PFS (79%, 95% CI: 67–94%) compared to PAD (47%, 95% CI: 31–71%) (p = 0.015) (Fig. 5A). The median PFS remained unreached for VRD, while it reached 39 months (95% CI, 32-NR) for PAD. Among non-transplant patients, VRD’s five-year PFS was higher at 58% (95% CI: 20–25%) compared to PAD’s 33% (95% CI: 20–55%) (p = 0.073). VRD again had an unreached median PFS, while PAD’s median PFS was 46 months (95% CI, 35-NR) (Fig. 5B).
High risk patients versus standard risk patients in VRD group and PAD group
OS: In VRD group, standard risk patients had a better OS than high risk patients (P = 0.00045). The median OS for high-risk patients was 49 months (95%CI: 44-NR). While the median OS was not reached for patients with standard risk cytogenetics (Fig. 6A). In PAD group, a trend toward improved OS was observed for patients in standard risk group compared to patients in high group (P = 0.076) (Fig. 6B). The median OS was 64 months (95%CI: 60-NR) for patients with standard risk cytogenetics and 77 months (95%CI: 44-NR) for patients with high-risk cytogenetics.
PFS: In VRD group, standard risk patients had a better PFS than high risk patients (P = 0.013). The median PFS for high-risk patients was 41 months (95%CI: 32-NR). While the median PFS was not reached for patients with standard risk cytogenetics (Fig. 7A). In PAD group, an improvement in PFS was observed for patients in standard risk group compared to patients in high-risk group (P = 0.049) (Fig. 7B). The median PFS was 55 months (95%CI: 36-NR) for patients with standard risk cytogenetics and 36 months (95%CI: 29-NR) for patients with high-risk cytogenetics.
COX multivariable analysis
Proportional hazards assumption test
Prior to conducting the Cox multivariable analysis, we assessed the validity of the proportional hazards assumption using a proportional hazards test. This test, performed using the COX.ZPH function in R, evaluates whether the hazards for different covariate levels remain proportional over time, a crucial requirement for the Cox model. The results supported the stability of the model, with all p values exceeding 0.05. This confirmation of the proportional hazards assumption allowed us to proceed confidently with the Cox multivariable analysis.
Multivariable analysis for all patients
The comprehensive multivariable analysis, incorporating all patients, revealed that PAD induction was associated with a significantly worse OS rate compared to VRD induction (HR 2.77, 95%CI: 1.47–5.23, p = 0.002). Age was also identified as a significant factor correlated with OS (HR 1.05, 95%CI: 1.02–1.09, p = 0.003). Furthermore, compared to the R-ISS Stage I, R-ISS Stage III was associated with a worse OS (HR 2.85, 95% CI: 1.08–7.55, p = 0.035). Similar to OS, PAD induction was significantly associated with worse PFS compared to VRD (HR 2.11, 95%CI: 1.37–3.27, p = 0.001). Additionally, R-ISS stage III compared to R-ISS stage I was associated with worse PFS (HR 2.75, 95%CI: 1.37–5.5, p = 0.004) (Table 4).
Safety profile
A comprehensive analysis of the entire patient cohort revealed that 36% of patients experienced peripheral neuropathy during or after induction, while 26% reported GI discomfort or other AEs (Table 5).
Peripheral neuropathy
Within the VRD-treated group, 66 patients (34%) developed symptoms, including sensory and motor nerve damage (≥ grade 2). In contrast, 74 PAD-treated patients (38%) experienced similar symptoms (≥ grade 2).
GI AEs
Regarding GI discomfort (vomiting, loss of appetite, nausea, etc.), 40 VRD patients (21%) encountered these issues. In contrast, 60 patients (31%) reported experiencing GI discomfort. Standard treatment involves medications to alleviate symptoms.
Cardiopulmonary AEs
Twelve VRD patients (6%) experienced cardiopulmonary AEs. In contrast, 23 PAD patients (12%) reported cardiopulmonary AEs.
Anaphylaxis
In the VRD group, 37 patients (19%) experienced anaphylaxis, compared to 49 PAD patients (25%).
Hematological AEs
Prevalent hematological AEs during treatment included neutropenia, thrombocytopenia, leukopenia, anemia, and lymphopenia. Notably, no statistically significant difference existed between VRD and PAD groups for these AEs.
Discontinuation due to AEs
Regarding discontinuation due to AEs, 20 VRD patients (10%) discontinued induction due to AEs, compared to 36 PAD patients (18%). Notably, bortezomib-induced peripheral neuropathy and GI disorders were the primary reasons for discontinuation.
Prophylactic therapy for adverse events
Long-acting G-CSFs were employed as prophylactic therapy for grade 2 granulocytopenia. Acyclovir served as prophylactic therapy for herpes infection. Patients in the PAD group received palonosetron hydrochloride injection as prophylactic therapy for vomiting. Aspirin was utilized as prophylactic therapy to prevent thromboembolism induced by lenalidomide for patients in VRD group.
Secondary primary malignancy in PAD group and VRD group
In the VRD group, no patients were diagnosed with secondary primary malignancies. In comparison, in the PAD group, one patient was diagnosed with secondary acute myeloid leukemia with maturation (AML-M2). The incidence of secondary primary malignancy in PAD group was 0.5% in our study.
Discussion
This real-world study, conducted at Sun Yat-sen University Cancer Center between 2013 and 2023, examined the effects of two induction regimens on survival outcomes in 390 patients with NDMM. VRD induction was compared to PAD induction, with a focus on PFS, OS, EFS, and depth of response. VRD exhibited a significantly higher five-year OS (74% vs. 59%, p = 0.0024) and PFS (67% vs. 37%, p = 0.0018) compared to PAD, despite an uneven distribution of transplant rates between groups. PSM further confirmed VRD’s superiority in survival analysis. VRD also demonstrated a higher rate of CR/sCR (30% vs. 20%, p = 0.02) and a more favorable five-year EFS (60% vs. 28%, p = 0.01). Notably, there was no statistically significant difference in OS and PFS between the VRD and PAD groups among patients who did not undergo transplantation and those classified as high risk.
A study published in the Journal of Clinical Oncology with a median follow-up time of 67 months revealed a median progression-free survival (PFS) of 65 months and a median overall survival (OS) of 126.6 months for patients treated with VRD [2]. Notably, median OS and PFS remained unreached for NDMM patients with a median follow-up of 77 months. Due to observed deviations in induction regimens during data collection, EFS was employed to mitigate statistical bias from regimen discontinuation, consistent with prior studies [15]. This approach aligned with our finding of superior EFS for VRD compared to PAD [7, 16, 17].
Subgroup analysis revealed significantly improved five-year OS (83% vs. 58%, p = 0.0038) and five-year PFS (78% vs. 48%, p = 0.0091) for VRD compared to PAD in standard-risk patients. However, in high-risk cytogenetics patients, differences in five-year OS (p = 0.34) and PFS (p = 0.31) were not statistically significant. Among transplant recipients, VRD was associated with enhanced five-year OS (91% vs. 67%, p = 0.014) and PFS (79% vs. 47%, p = 0.015) compared to PAD. Non-transplant recipients showed a trend towards better five-year OS (59% vs. 57%, p = 0.085) and PFS (58% vs. 33%, p = 0.073) with VRD. The findings from subgroup analysis indicate an improvement in survival time for patients with high-risk cytogenetics and RISS stage III subsequent to transplantation, advocating for transplantation as a robust recommendation for transplant eligible patients newly diagnosed with multiple myeloma [18,19,20].
VRD induction demonstrated improved tolerability compared to PAD, with lower rates of GI AEs, peripheral neuropathy, and cardiopulmonary AEs, as reported in previous studies [4, 21]. In our study, 21% of VRD patients experienced AEs compared to 41% in the PAD group. While 10% and 18% of patients in the VRD and PAD groups, respectively, discontinued induction due to AEs, with bortezomib-related peripheral neuropathy and GI disorders as the primary cause for discontinuation. Our results, which show a greater prevalence in the PAD group, reflect real-world situations of bortezomib-induced neuropathy. Cardiac incidents were reported by 12 patients (6%) and 23 patients (12%) in the VRD and PAD groups, respectively (p = 0.076), aligning with the expected likelihood of peripheral neuropathy for VRD and exceeding that reported in previous PAD trials for NDMM [5, 10].
In a phase III clinical trial comparing the VRD and daratumumab, bortezomib, lenalidomide and dexamethasone (DVRD) regimens for transplant-eligible patients, patients in the DVRD group had a 4-year PFS of 84.3% and transplant recipients who received VRD treatment in our study had a 4-year PFS of 84%. Furthermore, transplant eligible patients in the DVRD group of this trial achieved a CR rate of 87.9% and a MRD negative rate of 75.2%, contrasting with the CR rate of 35% and MRD negative rate of 30% for transplant recipients who received VRD treatment observed in our study [22]. Additionally, based on the results from the GRIFFIN trial, transplant eligible patients who received DVRD had a CR rate of 79.8% and a two-year PFS of 95.8% compared to a CR rate of 35% and a two-year PFS of 88.1% for transplant recipients who received VRD treatment in our study [23].
According to findings from the MAIA study, the clinical advantages of the daratumumab, lenalidomide and dexamethasone (DRD) regimen were evident in transplant ineligible patients with newly diagnosed multiple myeloma (NDMM). Based on the results from this study, the three-year PFS in the DRD group was 61.5% among transplant ineligible patients compared to a three-year PFS of 71% for non-transplants in the VRD group in our study. Additionally, transplant ineligible patients in the DRD group had a 43.6% CR rate and a 23.8% MRD negative rate compared to a 28.5% CR rate and a 12.8% MRD negative rate for non-transplants who received VRD treatment in our study [24]. The long term follow-up results showed that the five-year PFS was 52% and the five-year OS was 66.6%.
In the IMROZ trial, the results demonstrated a five-year PFS of 63.2% for transplant ineligible patients with NDMM who received isatuximab, bortezomib, lenalidomide, and dexamethasone. In our study, non-transplants who received VRD treatment had a three-year OS of 88% and a three-year PFS of 71% [25].
This real-world study, encompassing 390 patients, stands as the first exploration of treatment outcomes following VRD and PAD induction for MM. Leveraging a substantial sample size, comprehensive patient data, and adherence to MM guidelines, our study offers valuable insights into the actual survival outcomes of Chinese MM patients receiving VRD or PAD induction. While it firmly supports the use of VRD in standard-risk patients and emphasizes the benefits of post-VRD induction transplantation, the study acknowledges the lack of strict randomization between VRD and PAD groups, a characteristic of traditional randomized clinical trials. Despite employing multivariable Cox analysis to identify variables associated with OS and PFS, we recognize that potential heterogeneity in patient characteristics and physician decisions may limit the ability to definitively attribute results to the specific VRD or PAD regimens. Additionally, patients might alter their induction regimens due to financial constraints or other reasons. In our study, we aimed to mitigate the bias introduced by modifications to induction treatment through EFS analysis and address the bias related to differing transplantation rates between the two treatment groups using PSM analysis. Despite these additional analyses, the bias between the two induction groups could not be entirely eliminated due to the retrospective nature of our study. Therefore, our analysis results should be interpreted cautiously.
In conclusion, this real-world analysis provides valuable insights into the comparative effectiveness of VRD and PAD induction in MM patients, stratified by cytogenetic abnormalities and transplantation status. The findings strongly suggest a preference for VRD over PAD, empowering physicians to optimize treatment strategies for their patients. Future investigations should further explore the potential for incorporating Daratumumab into induction therapy.
Data availability
Data can be obtained from the corresponding author upon reasonable request.
References
Cowan AJ, Green DJ, Kwok M, et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327(5):464–77. https://doi.org/10.1001/jama.2022.0003.
Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38(17):1928–37. https://doi.org/10.1200/JCO.19.02515.
Bergsagel DE. Is aggressive chemotherapy more effective in the treatment of plasma cell myeloma? Eur J Cancer Clin Oncol. 1989;25(2):159–61. https://doi.org/10.1016/0277-5379(89)90001-1.
Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. J Am Soc Hematol Blood. 2010;116(5):679–86. https://doi.org/10.1182/blood-2010-02-268862.
Rosiñol L, Oriol A, Rios R, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. J Am Soc Hematol Blood. 2019;134(16):1337–45. https://doi.org/10.1182/blood.2019000241.
Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10(5):53. https://doi.org/10.1038/s41408-020-0311-8.
Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–27. https://doi.org/10.1016/S0140-6736(16)31594-X.
Sonneveld P, Schmidt-Wolf IGH, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946–55. https://doi.org/10.1200/JCO.2011.39.6820.
Mai EK, Bertsch U, Dürig J, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015;29(8):1721–9. https://doi.org/10.1038/leu.2015.80.
Popat R, Oakervee HE, Hallam S, et al. Bortezomib, doxorubicin and dexamethasone (PAD) frontline treatment of multiple myeloma: updated results after long-term follow‐up. Br J Haematol. 2008;141(4):512–6. https://doi.org/10.1111/j.1365-2141.2008.06997.x.
Huang BT, Tan Y, Zhao WH, et al. How to determine bortezomib-based regimen for elderly patients with multiple myeloma: PAD versus CBd, an observational study. J Cancer Res Clin Oncol. 2014;140(2):303–9. https://doi.org/10.1007/s00432-013-1570-6.
Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–46. https://doi.org/10.1016/S1470-2045(16)30206-6.
Costa LJ, Derman BA, Bal S, et al. International harmonization in performing and reporting minimal residual disease assessment in multiple myeloma trials. Leukemia. 2021;35(1):18–30. https://doi.org/10.1038/s41375-020-01012-4.
Palumbo A, Rajkumar SV, San Miguel JF, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32(6):587–600. https://doi.org/10.1200/JCO.2013.48.7934.
Landgren O, Siegel D, Kazandjian D, Costa L, Jakubowiak A. Treatments for newly diagnosed multiple myeloma: when endurance is interrupted. Lancet Oncol. 2020;21(12):e540. https://doi.org/10.1016/S1470-2045(20)30635-5.
Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311–20. https://doi.org/10.1056/NEJMoa1611750.
Sidiqi MH, Aljama MA, Bin Riaz I, et al. Bortezomib, lenalidomide, and dexamethasone (VRd) followed by autologous stem cell transplant for multiple myeloma. Blood Cancer J. 2018;8(11):106. https://doi.org/10.1038/s41408-018-0147-7.
Devarakonda S, Efebera Y, Sharma N. Role of stem cell transplantation in multiple myeloma. Cancers. 2021;13(4):863. https://doi.org/10.3390/cancers13040863.
Ntanasis-Stathopoulos I, Gavriatopoulou M, Kastritis E, Terpos E, Dimopoulos MA. Multiple myeloma: role of autologous transplantation. Cancer Treat Rev. 2020;82:101929. https://doi.org/10.1016/j.ctrv.2019.101929.
Martinez-Lopez J, Blade J, Mateos MV, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. J Am Soc Hematol Blood. 2011;118(3):529–34. https://doi.org/10.1182/blood-2011-01-332320.
O’Gorman P, Laubach JP, O’Dwyer ME, et al. Phase 2 studies of lenalidomide, subcutaneous bortezomib, and dexamethasone as induction therapy in patients with newly diagnosed multiple myeloma. Am J Hematol. 2022;97(5):562–73. https://doi.org/10.1002/ajh.26491.
Sonneveld P, Dimopoulos MA, Boccadoro M, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma[J]. N Engl J Med. 2024;390(4):301–13. https://doi.org/10.1056/NEJMoa2312054.
Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial[J]. Blood J Am Soc Hematol. 2020;136(8):936–45. https://doi.org/10.1182/blood.2020005288.
Facon T, Cook G, Usmani SZ, et al. Daratumumab plus Lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA[J]. Leukemia. 2022;36(4):1066–77. https://doi.org/10.1038/s41375-021-01488-8.
Facon T, Dimopoulos MA, Leleu XP, et al. Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone for multiple myeloma. NEW ENGL J MED. 2024. https://doi.org/10.1056/NEJMoa2400712.
Acknowledgements
Not applicable.
Funding
HW is supported by the National Natural Science Foundation of China (Grant No. 81700148) and Natural Science Foundation of Guangdong Province (Grant Nos. 2021A1515010093 and 2023A1515011862). YL is supported by Sun Yat-sen University Start-Up Funding grant 201603, the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2017ZT07S096) and National Natural Science Foundation of China grant 81873428.
Author information
Authors and Affiliations
Contributions
HW, YL and DL conceived the paper. DL, SRB, GJC, and DMF collected the data. DL wrote the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by Institutional Ethical Review Board of Sun Yat-Sen university cancer center. Informed consent requirements were also waived by Institutional Ethical Review Board of Sun Yat-Sen university cancer center. The International Conference on Harmonization’s Good Clinical Practice standards and the 1964 Helsinki Declaration and its later revisions served as the foundation for the study’s methodology.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, D., Bai, S., Feng, D. et al. Bortezomib, lenalidomide, and dexamethasone versus bortezomib, doxorubicin, and dexamethasone in newly diagnosed multiple myeloma. BMC Cancer 24, 1123 (2024). https://doi.org/10.1186/s12885-024-12880-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12880-9