Abstract
Background
To assess the efficacy and safety of virtual reality-based visual training (VRVT) in myopia control among children.
Methods
The randomized, parallel-group, single-blind clinical trial conducted at the Department of Ophthalmology of Shanghai Tenth People’s Hospital enrolled 65 low-myopic children (aged 8 to 13 years) with cycloplegic spherical equivalent (SE) between − 0.50 and − 3.00 diopters (D), astigmatism less than − 1.00 D, anisometropia less than 1.50D, and best corrected visual acuity (BCVA) more than 0.0 logarithm (LogMAR) of the minimum angle of resolution. The participants were enrolled in December 2020, and the follow-up of this study concluded on August 2021. Children were assigned randomly to the intervention group (VRVT plus single-vision spectacle [SVS]) and the control group (only SVS without receiving VRVT). The intervention group was administered for 20 min per day with VRVT under parental supervision at home. The primary outcome was changes in axial length (AL) at 3 months. Macular choroidal thickness (mCT) was regarded as a key secondary outcome.
Results
Among 65 participants (mean age: 10.8 years, 52.3% male), 60 children (92.3%) who completed the 3-month intervention and 6-month follow-up were included in the analysis (30 in the intervention group and 30 in the control group). The changes of AL were 0.063 ± 0.060 mm (95% confidence interval [CI], 0.074 to 0.119 mm) in the intervention group and 0.129 ± 0.060 mm (95% CI, 0.107 to 0.152 mm) and in the control group at 3 months (t = − 2.135, P = 0.037), and the mean difference between the two groups was 0.066 mm. The change of mCT were 22.633 ± 36.171 μm (95% CI, 9.127 to 36.140 μm) in the intervention group and − 3.000 ± 31.056 μm (95% CI, − 14.597 to 8.597 μm) in the control group at 3 months (t = 2.945, P = 0.005). VR vertigo was the most common adverse event which was occurred in two children (2/30, 6.67%) in the intervention group.
Conclusions
VRVT is a promising method for myopia control in children with good user acceptability. Among children aged 8 to 13 years with low-myopia, nightly use of VRVT resulted in slowing myopia progression.
Trial registration
This protocol was registered with ClinicalTrials.gov (NCT06250920), retrospectively registered on 01 February 2024.
Similar content being viewed by others
Introduction
Myopia, one of the most common refractive errors, has raised significant international concern in recent decades, which may lead to blindness due to complications of high myopia [1,2,3]. Myopia is mainly manifested as the decline of distance vision, lengthening of the axial length, and thinning of the choroid. The choroid plays an important role in regulating eye growth and refractive development. Previous study reported that the thinner choroid was associated with higher amounts of myopia [4]. Choroidal thickness has also been suggested to be a biomarker for predicting future axial elongation (and thus myopia progression), with reduced axial elongation being tied to a thicker choroid, especially temporally [5].
Education and time outdoors have been regarded as the two major risk factors for school myopia, which have a strong and causal relationship with myopia [6]. First of all, nearwork (such as reading and writing) at school required more accommodation that would stimulate eye growth [6]. Secondly, spending time outdoors in bright light can protect against myopia development [7]. Previous studies have confirmed that visual training can improve the accommodation to slow the progression of myopia effectively [8]. Additionally, full-spectrum illumination has been shown to slow axial elongation in animal models [9], which has been confirmed in our previous study. Although the current mainstream methods including orthokeratology and low-concentration atropine have been proven to control myopia progression, they also have problems such as unknown long-term safety and infection risk [10]. It is necessary to explore non-invasive, effective, and convenient method to slow the progression of myopia, especially for children at high risk of developing myopia.
In medical research, virtual reality (VR) technology has been widely used in clinical practice [11]. The training treatment and diagnosis of amblyopia and strabismus with VR have been widely recognized [12,13,14]. Previous studies have shown that VR technology can simulate distance and near vision activities, effectively relieve asthenopia [15]. Also, VR can cause thickening of the choroidal thickness (possibly related to myopic defocus) [16].
Therefore, we intend to develop a visual training method based on VR technology, combining the convergence vision training method in the solar-like full-spectrum illumination environment, to compensate for the time of outdoor activities and exercise the function of ciliary muscle. The report presents the results of a randomized, parallel-group, single-blind clinical trial to assess the efficacy and safety of virtual reality-based visual training (VRVT) in myopia control among children.
Methods
Study design and setting
This study was a randomized, parallel-group, single-blind clinical trial that tried to assess the efficacy and safety of VRVT for myopia control which was conducted in Shanghai, eastern China for 3 months. It was registered at Clinicaltrials.gov (NCT06250920) and followed the tenets of the Declaration of Helsinki and CONSORT guidelines. Poster advertisements were used to inform and recruit participants at the study site. Participants were randomly assigned to either the intervention group (receiving 20 min of VRVT per day) or the control group (only SVS without receiving VRVT). The participants were enrolled in December 2020, and follow-up was completed in August 2021. All children submitted a written informed consent form signed by themselves and their parents or guardians. All examinations at baseline and follow-up visits were performed by the same examiners using the same protocol and equipment throughout. Investigators and key personnel involved in the present study were trained before the study commencement. This study was approved by the Ethics Committee of Shanghai Tenth People’s Hospital (identifier, SHSY-IEC-4.1/20–260/01). All the datasets used throughout the study were identified before being transferred to the study investigators.
Participants
Eligible participants were children aged 8 to 13 years with myopia of cycloplegic spherical equivalent (SE) between − 0.50 diopters (D) and − 3.00 D, astigmatism less than − 1.00 D, anisometropia less than 1.50D, and best corrected visual acuity (BCVA) more than 0.0 logarithm (LogMAR) of the minimum angle of resolution in either eye. Participants were willing to participate in the study and accept random allocation in grouping. They were asked to return to the hospital at recruitment, 1 month, 3 months, and 6 months (3 months after the end of all interventions) to cooperate with data collection.
Children were excluded if they had ocular diseases, such as amblyopia, strabismus, binocular vision abnormalities, and other ocular abnormalities in either eye. Children who were using orthokeratology or other optical methods for myopia control, and with systemic diseases (e.g., endocrine, cardiac, respiratory diseases) and developmental anomalies were also excluded.
Randomization and masking
Eligible children were allocated randomly to either the intervention or the control group with the random number table method, after verifying participant eligibility and obtaining written informed consent. Due to the nature of the intervention, participants and their guardians were aware of the study allocation. The intervention group of the participants was unknown to the outcome assessor (e.g., optometrists, and statisticians).
Intervention
All children were required to wear single-vision spectacles for participation throughout the study and updated their spectacles if needed. Children in the intervention group were instructed to receive VRVT, who were administered at home under the supervision of parents for 20 min per day with VRVT. Children in the control group lived without receiving VRVT. With the VR technology, twelve different scenarios were constructed in the solar-like full-spectrum illumination scenes, which have various training sessions and purposes. When the children’s binocular fixation point focuses on the set object like a butterfly, for a certain period of time, it will cause the object to move systematically (Supplement 1). Children were asked to follow the movement of the object with binocular fixation. The intervention adopted a virtual reality visual training system.
In the intervention group, children were asked to record a single English word that appeared randomly and feedback to investigators and their parents during the last training session every day for 3 months, and this method was used to ensure compliance with the intervention.
Study outcomes and adverse events
The outcome was the efficacy and safety of VRVT in myopia control. The primary outcome was changes in axial length (AL) at 3 months. Macular choroidal thickness (mCT) was regarded as a key secondary outcome. Children who underwent at least 1 session of VRVT intervention were analyzed for safety. A questionnaire on adverse events, including but not limited to VR vertigo and asthenopia, was collected from children at each follow-up and any unplanned visits if needed.
Measurements
Professional optometrists performed all examinations, including uncorrected visual acuity (UCVA) and BCVA at a standard testing distance of 4 m, near visual acuity (NVA) at 40 cm, non-cycloplegic and cycloplegic auto-refraction (KR-8900, Topcon, Japan), non-contact intraocular pressure (IOP) measurement (CT-IP, Topcon, Japan). After 1 drop of 0.5% Alcaine (Alcon, Fort Worth, USA), the cycloplegic agent used in this study was 1% cyclopentolate hydrochloride (Alcon, Fort Worth, USA); one drop was applied every 5 min for a total of three drops. Before observing pupil response and diameter, participants were asked to close their eyes and rest for 30 min. When the pupillary response disappears or the pupil diameter is greater than 6 mm, auto-refraction can be performed. The spherical equivalent was calculated as spherical power plus half of the cylinder power.
The axial length (AL) and anterior chamber depth (ACD) were measured using laser interferometry (IOL-Master 700, Carl Zeiss Meditec AG, Germany) which were measured three times and averaged. The subfoveal choroidal thickness (the distance between outer choroid episcleral margin and retinal pigment epithelium Bruch’s complex) was performed by enhanced depth imaging optical coherence tomography (CIRRUS HD-OCT 5000, Carl Zeiss Meditec AG, Germany). It was measured by the built-in measurement software. The measured data will be accepted when the image signal intensity index is more than 7. Stereoacuity was assessed by using the Titmus test (Stereo Optical Co., USA). All study data were collected at recruitment, at 1 month, at 3 months, and 6 months.
Sample size
Based on the preliminary studies, the change of AL was estimated to be 0.10 mm and 0.15 mm for the intervention and the control group respectively after 3 months. A sample size of 50 participants (25 per group) was selected to achieve 90% power at a significance level of 0.05. The final sample size of 60 participants (30 per group) was selected after factoring in a projected attrition rate of 20%.
Statistical analysis
Participants’ baseline demographic information and ocular parameters were summarized using descriptive statistics. Continuous variables were reported in terms of means, SDs, and 95% confidence interval (CI). Categorical variables were reported in frequencies and percentages. The independent-sample t-test was used to compare differences between the groups.
Intervention efficacy was calculated by dividing the between arm difference in values by the control arm value. Right eyes that met the enrollment criteria were used as the outcome data representing the participant. If the right eye did not meet inclusion criteria or if right eye data were missing, left eyes were used instead (n = 2). All adverse events were reported individually in detail.
All P-values were 2-sided and considered statistically significant when the values were 0.05. All statistical analyses were performed using SPSS Statistics software (version 25.0, IBM Corp., USA).
Results
Participant disposition and baseline characteristics
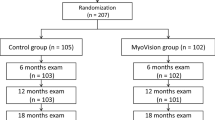
Of the 83 individuals assessed for eligibility, 65 low-myopic children were enrolled (Fig. 1). After verifying participant eligibility, 60 participants (92.3%, mean age: 10.8 years, 52.3% male) completed the 3-month study, consisting of 30 children (93.8%) in the VRVT group and 30 children (90.9%) in the control group. At baseline, the characteristics of the participants were similar in the two groups (Table 1). A total of two children in the VRVT group lost contact because of COVID-19, and three children in the SVS group switched to orthokeratology after their myopia progressed. The baseline characteristics of those included and excluded in the analysis were not statistically significantly different among the two groups.
Study outcome
The mean AL increases were 0.063 ± 0.060 mm (95% CI, 0.074 to 0.119 mm) in the VRVT group and 0.129 ± 0.060 mm (95% CI, 0.107 to 0.152 mm) in the control group at 3 months (t = − 2.135, P = 0.037), shown in Table 2; Fig. 2. The mean difference in axial elongation between the two groups was 0.066 mm. At the 6-month follow-up (3 months after the end of the VRVT intervention), the axial growth in the VRVT group was no different than the control group (t = − 1.382, P = 0.172).
At the 3-month follow-up visits, there were significant differences in mCT changes between the two groups. The change of mCT were 22.633 ± 36.171 μm (95% CI, 9.127 to 36.140 μm) in the VRVT group and − 3.000 ± 31.056 μm (95% CI, − 14.597 to 8.597 μm) in the control group (t = 2.945, P = 0.005). At the next follow-up, the VRVT group showed a continuous thickening, while the control group continued to thin (t = 1.476, P = 0.145).
In other ocular characteristics, UCVA and NVA of the VRVT group were improved − 0.067 ± 0.127 LogMAR (95% CI, − 0.114 to − 0.019 LogMAR, t = − 4.480, P = 0.000) and − 0.080 ± 0.096 LogMAR (95% CI, − 0.116 to − 0.044 LogMAR, t = − 2.168, P = 0.034 ) compared with that before training at 3 months.
Adverse events and intervention compliance
During the study, VR vertigo was the most common adverse event which was reported by two children (2/30, 6.67%) in the VRVT group. No children experienced asthenopia due to VR visual training. Under the supervision of parents, all children in the VRVT groups completed the training requirements well.
Discussion
The study explored and verified a new method of applying virtual reality technology with visual training to myopia control. Among low-myopic children (aged 8 to 13 years), the VRVT intervention represented the reduction in axial elongation compared with the control group (0.063 ± 0.060 vs. 0.129 ± 0.060) in this 3-month randomized controlled trial.
Possibility of VR for myopia control
Virtual reality, based on the principle of binocular disparity, is implemented by a head-mounted display with characteristics of immersion, interaction, and conception [17]. The possibility of VR for myopia prevention and control has attracted attention in past studies [16, 18]. In our study, the outdoor activities environment built with VR technology can make up for the reduction of outdoor activities time accompanied by study pressure. The standardized visual training link realized with VR technology can break through the limitations of traditional visual training conditions of sites and time. The solar-like full-spectrum illumination environment and the light brightness suitable for eye development can be simulated by using the advantages of spectral components and screen brightness. And full-spectrum illumination has been shown to slow axial elongation in our previous and other studies [9]. Children exposed to > 10,000 light intensity for 3 h per day have been proven to be effective in slowing down the occurrence of myopia [19, 20]. The reason may be that high-intensity light promotes the secretion of dopamine, thereby slowing down the progression of myopia. Some studies have also shown that it may be that high-intensity light can induce mydriasis, which in turn increases the depth of field, thereby improving the situation of visual blur and delaying the occurrence of myopia [21, 22].
Changes in ocular characteristics
Axial elongation is the primary factor driving myopic progression, which has been fully confirmed in clinical trials and myopic animal models [10]. The aim of myopic prevention and control is to delay the growth of axial length [10]. Studies have shown that there is a close relationship between myopia and choroidal thickness. With the increase of diopter and axial length, choroidal thickness showed a gradual thinning trend [23]. In this study, VRVT intervention showed a good control effect in delaying axial elongation and choroidal thinning, and UCVA and NVA were improved, which were well maintained at the end of the study follow-up. It may be related to the phenomenon that VR technology forms a myopic defocus state of the peripheral retina relative to the macular fovea, thereby preventing compensatory axial elongation [15]. At the same time, the light environment promotes the secretion of dopamine, which helps to increase the blood supply of the choroid in the eye and thickens the choroidal thickness. We also found the thickening of choroidal thickness in the VRVT group, so we speculated that its thickening could be a potential mechanism for myopia control, which has been identified as a potential predictor for treatment response to atropine eye drops and orthokeratology in myopic eyes in previous studies [4]. Due to the limitation of the study time and COVID-19, the changes in cycloplegic SE and the long-term intervention effects of axial length and choroidal thickness need to be further evaluated. In the future, targeted adjustment of light intensity and planning of training time may be an effective way to further improve the effect of VRVT intervention [18].
Efficacy in comparison with other interventions
Orthokeratology, specially designed spectacles, and low-concentration atropine eye drops are the most commonly used interventions for myopia [10]. Although they have shown varying degrees of myopia control efficacy in the evidence of randomized controlled trials, challenges still exist. Orthokeratology, through its special lens design, can cause myopic defocus in the peripheral retina, but there is a significant risk of infection [24]. Based on principles such as peripheral retinal myopic defocus, specially designed spectacles put forward certain requirements for wearing habit and duration, which are closely related to the effect of myopia control [25]. Low-concentration atropine has shown good potential in the control of myopia in children [26], but the accompanying photophobia, near blur, rebound effects, and unknown adverse reactions are the negative effects that have been widely discussed [27]. The repeated low-level red-light therapy has been reported with a certain effect on myopia control, but whether the “light” will cause changes in the structure of the fundus needs to be observed for a long time [28]. Due to the differences in study designs, it is difficult to directly compare the results of studies on these interventions. However, VRVT has the advantages of non-invasiveness, non-contact, and relatively few side effects. It may be a new and effective way to control myopia with a stronger competitiveness.
Safety
During the study, VR vertigo was the most common adverse event which was reported by two children (2/30, 6.67%) in the VRVT group. It is a common potential effect of VR, which is caused by the inconsistency between the effect seen visually and the condition perceived internally in the body, and the mechanism has not been elucidated [29]. However, no children withdrew from the intervention due to VR vertigo. During the follow-up period of 6 months, no adverse reactions such as decreased BCVA and ocular organic lesions occurred in children.
Limitations
This study has several limitations. First, the duration of the study was designed as 3 months which may not be long enough to observe myopic control effects. Second, the study was conducted in part during the COVID-19 pandemic, which made cycloplegic refraction difficult to collect. It was found to be associated with increasing myopic risk in children and may had an impact on the intervention effect of myopia [30]. Third, the effects of the myopia intervention were found only in the intervention protocol used in this study. Whether other intervention duration, intervention frequency, and other light levels have similar or even better intervention effects is worth exploring. Fourth, possible stop and rebound effects or carry-on effects need to be investigated. Fifth, the sample size was determined based on the primary outcomes, and caution is warranted in interpreting the power of the results for secondary outcomes. Sixth, this was a single-center study, and external validation at multiple centers was necessary. Finally, the participants were all Chinese, and the general applicability of the VRVT intervention to children of other ethnic groups other than Chinese needs to be further explored.
In conclusion, VRVT intervention is a novel, non-invasive, effective, and convenient method for myopia control and has good user acceptance, among Chinese children aged 8 to 13 years with low myopia. Further multi-center and large sample-size research is needed to replicate its long-term efficacy and potential underlying mechanisms and to assess safety. Whether the intervention can be used to prevent the occurrence of myopia will be the focus of future research.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Morgan IG, Ohno-Matsui K, Saw SM, Myopia. Lancet. 2012;379(9827):1739–48.
Xu L, Wang Y, Li Y et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006;113(7).
Ohno-Matsui K. Pathologic myopia. Asia Pac J Ophthalmol (Phila). 2016;5(6):415–23.
Ostrin LA, Harb E, Nickla DL, et al. IMI-The dynamic choroid: New insights, challenges, and potential significance for human myopia. Invest Ophthalmol Vis Sci. 2023;64(6):4.
Li Z, Wang W, Liu R, et al. Choroidal thickness predicts progression of myopic maculopathy in high myopes: a 2-year longitudinal study. Br J Ophthalmol. 2021;105:1744–50.
Morgan IG, Wu PC, Ostrin LA, et al. IMI Risk factors for myopia. Invest Ophthalmol Vis Sci. 2021;62(5):3.
Morgan IG, French AN, Ashby RS, et al. The epidemics of myopia: Aetiology and prevention. Prog Retin Eye Res. 2018;62:134–49.
Vasudevan B, Ciuffreda KJ, Ludlam DP. Accommodative training to reduce nearwork-induced transient myopia. Optom Vis Sci. 2009;86(11):1287–94.
Xu X, Shi J, Zhang C, et al. Effects of artificial light with different spectral composition on eye axial growth in juvenile guinea pigs. Eur J Histochem. 2023;67(1):3634.
Jonas JB, Ang M, Cho P, et al. IMI Prevention of Myopia and its progression. Invest Ophthalmol Vis Sci. 2021;62(5):6.
Yeung AWK, Tosevska A, Klager E, et al. Virtual and augmented reality applications in Medicine: analysis of the scientific literature. J Med Internet Res. 2021;23(2):e25499.
Ding J, Levi DM. Recovery of stereopsis through perceptual learning in human adults with abnormal binocular vision. Proc Natl Acad Sci U S A. 2011;108(37):E733–41.
Eastgate RM, Griffiths GD, Waddingham PE, et al. Modified virtual reality technology for treatment of amblyopia. Eye (Lond). 2006;20(3):370–4.
Miao Y, Jeon JY, Park G, Park SW, Heo H. Virtual reality-based measurement of ocular deviation in strabismus. Comput Methods Programs Biomed. 2020;185:105132.
Langhans K, Oltmann K, Reil S, et al. FELIX 3D display: humanmachine interface for interactive real threedimensional imaging. Berlin, Heidelberg: Springer; 2005: 22–31.
Turnbull P, Phillips JR. Ocular effects of virtual reality headset wear in young adults. Sci Rep. 2017;7(1):16172.
Sherman WR, Craig AB. Virtual reality. New York: Elsevier; 2003: 589–617.
Zhao F, Chen L, Ma H, Zhang W. Virtual reality: a possible approach to myopia prevention and control? Med Hypotheses. 2018;121:1–3.
Dolgin E. The myopia boom. Nature. 2015;519(7543):276–8.
Wu PC, Tsai CL, Wu HL, et al. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120(5):1080–5.
Xiong S, Sankaridurg P, Naduvilath T, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017;95(6):551–66.
Zhang J, Deng G. Protective effects of increased outdoor time against myopia: a review. J Int Med Res. 2020;48(3):300060519893866.
Flores-Moreno I, Lugo F, Duker JS, et al. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol. 2013;155(2):314–e3191.
Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53(11):7077–85.
Bao J, Huang Y, Li X, et al. Spectacle lenses with Aspherical Lenslets for Myopia Control vs single-vision spectacle lenses: a Randomized Clinical Trial. JAMA Ophthalmol. 2022;140(5):472–8.
Yam JC, Zhang XJ, Zhang Y, et al. Effect of low-concentration atropine eyedrops vs Placebo on Myopia incidence in children: the LAMP2 Randomized Clinical Trial. JAMA. 2023;329(6):472–81.
Gong Q, Janowski M, Luo M, et al. Efficacy and adverse effects of Atropine in Childhood Myopia: a Meta-analysis. JAMA Ophthalmol. 2017;135(6):624–30.
Jiang Y, Zhu Z, Tan X, et al. Effect of repeated low-level Red-Light Therapy for Myopia Control in children: a Multicenter Randomized Controlled Trial. Ophthalmology. 2022;129(5):509–19.
Kennedy RS, Drexler J, Kennedy RC. Research in visually induced motion sickness. Appl Ergon. 2010;41(4):494–503.
Choi KY, Chun RKM, Tang WC, To CH, Lam CS, Chan HH. Evaluation of an Optical Defocus Treatment for Myopia Progression among Schoolchildren during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2143781.
Acknowledgements
We thank all the study participants, along with their parents and guardians, for participating in this clinical trial, without whom the trial would not have been possible. We also thank our colleagues for all their work and dedication to this study.
Funding
The study is supported by the Project for Health Research of Anhui Province (AHWJ2023A20568). The funders had no role in the study design, collection, management, analysis, or interpretation of the data, writing of the manuscript, and the decision to publish the final manuscript.
Author information
Authors and Affiliations
Contributions
JY had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. ZX and AZ contributed to the data collection, data analysis, and manuscript edits. LL and JM collected the clinical data. YW and WC processed statistical data. All authors read, contributed to the research design, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted following the Declaration of Helsinki and approved by the Ethics Committee of Shanghai Tenth People’s Hospital (identifier, SHSY-IEC-4.1/20–260/01). All children submitted a written informed consent form signed by themselves and their parents or guardians.
Consent for publication
Not required.
Competing interests
JY and AZ : Patent - A virtual reality-based visual training method for myopia control (identifier, 202110298626.5 ).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Yu had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Z., Zou, A., Li, L. et al. Effect of virtual reality-based visual training for myopia control in children: a randomized controlled trial. BMC Ophthalmol 24, 358 (2024). https://doi.org/10.1186/s12886-024-03580-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03580-w