Abstract
Background
Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation is a rare autosomal dominant disease caused by mutations in KIF11 which disrupt EG5 protein function, impacting the development and maintenance of retinal and lymphatic structures due to its expression in the retinal photoreceptor cilia. The primary ocular finding in MCLMR is chorioretinopathy. Additional features can include microphthalmia, angle-closure glaucoma, persistent hyperplastic primary vitreous, cataract, pseudo-coloboma, persistent hyaloid artery, and myopic or hypermetropic astigmatism. The appearance of the chorioretinal lesions as white to pinkish, round, non-elevated atrophic areas devoid of blood vessels resembles the lacunae in Aicardy syndrome. Due to the lack of systematic description of the lesions and significant phenotypical variability, there is an impending need for a detailed report of each case.
Case presentation
A child with microcephaly detected in the third trimester of gestation began her following in the ophthalmology department due to a non-visually significant cataract. Shortly after, she developed nystagmus and large-angle alternating esotropia with cross-fixation. Her fundus initially showed a pallid optic disc and pigmentary changes, developing thereafter retinal lacunae and a retinal fold. Her differential diagnosis accompanied the dynamic changes in her fundus, which included congenital infections, Leber´s Congenital Amaurosis and Aicardy syndrome. At 19 months old, genetic testing identified a heterozygous mutation (c.1159 C > T, p.Arg387*) in the KIF11 gene. The patient underwent bilateral medial rectus muscle recession surgery at 2 years old for persistent esotropia, with significant improvement. Refraction revealed a hyperopic astigmatism in both eyes (+ 0.25 -2.50 × 180 OD and + 0.75 -2.00 × 170 OS). She continues to require right eye patching for 2 hours daily.
Conclusions
This case report expands the phenotypic spectrum of MCLMR by demonstrating a unique combination of retinal features which sheds new light on differential diagnosis from Aicardy syndrome. Our findings emphasize the significant phenotypic variability associated with MCLMR, particularly regarding ocular involvement. This underscores the importance of detailed clinical evaluation and comprehensive reporting of cases to improve our understanding of the disease spectrum and genotype-phenotype correlations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation (MCLMR) is a rare autosomal dominant disease (OMIM: 152950) caused by mutations in the KIF11 gene on chromosome 10q23. This gene encodes EG5, a homotetrameric kinesin motor protein crucial for spindle formation during cell division. Mutations in KIF11 disrupt EG5 function, impacting the development and maintenance of retinal and lymphatic structures due to its expression in the retinal photoreceptor cilia [1].
MCLMR exhibits significant phenotypic variability. Only 25 families have been described worldwide [2], highlighting the disease’s rarity. The severity of microcephaly can range from mild to severe, and intellectual disability may not always be present. A characteristic facial gestalt is observed, including upslanting palpebral fissures, a broad nose with a rounded tip, a long philtrum with a thin upper lip, and prominent chin and ears. Lymphedema, when present, typically affects the dorsal aspects of the feet, manifesting as lymphedema nails and coarse hair follicles ([3]; [1]).
The primary ocular finding in MCLMR is chorioretinopathy. Additional features can include microphthalmia, angle-closure glaucoma, persistent hyperplastic primary vitreous, cataract, pseudo-coloboma, persistent hyaloid artery, and myopic or hypermetropic astigmatism. In rare cases, microcornea and a pallid optic disc have also been reported (Feingold, 1992 [3]; Birtel, 2017 [1]).
MCLMR demonstrates remarkable phenotypic variability within and between families. This includes variable expressivity, where the degree of symptom manifestation varies, and reduced penetrance, where not all individuals with the KIF11 mutation develop the disease (Birtel, 2017 [1]). Notably, the retinal phenotype exhibits both intrafamilial and intraindividual variability.
Retinal lacunae, a characteristic finding in MCLMR, are white to pinkish, rounded, non-elevated atrophic areas devoid of blood vessels. These lesions result from the excavation of the retinal pigment epithelium exposing the underlying sclera and degeneration of the photoreceptor cells (rods and cones). Interestingly, retinal lacunae are also a hallmark feature of Aicardi Syndrome, a distinct genetic disorder characterized by a triad of infantile spasms, corpus callosum agenesis, and retinal lacunae (Fruhman, 2012 [4]).
Materials and methods
Description of the clinical presentation of a single patient, a 4-year-old girl with a confirmed diagnosis of MCLMR through genetic testing. A comprehensive ophthalmological examination was performed, including pattern-onset visual evoked potentials (VEP), full-field electroretinography (ERG), and retinography. Due to limitations in patient cooperation, optical coherence tomography (OCT) could not be reliably obtained and will not be included in the analysis.
Case presentation
Microcephaly was identified on prenatal ultrasound in the third trimester of gestation. This finding was confirmed after birth with a head circumference of 29.5 cm (at 39 weeks + 5 days), which remained below the expected range for gestational age. Height and weight were within normal limits at birth (47 cm and 2510 g, respectively). Amniocentesis revealed normal cytomegalovirus (CMV) DNA screening. Aneuploid screening, and comparative genomic hybridization (CGH) array were also tested with normal results. Gestational ultrasounds did not detect any major fetal malformations. Magnetic resonance imaging (MRI) of the brain showed a reduction in the skull-to-face ratio consistent with microcephaly and simplified gyral patterns.
The patient presented to ophthalmology at 3 months of age with a superotemporal lens opacity outside the visual axis, suggesting a non-visually significant cataract. Shortly thereafter, nystagmus and large-angle alternating esotropia with cross-fixation were identified. The ocular movements were normal. The Fundus examination revealed a pale optic disc and retinal pigmentary changes. Differential diagnoses at this stage included congenital infections and Leber’s Congenital Amaurosis (LCA). However, normal serological studies for infections in the first trimester and the absence of sluggish pupillary light reflex and characteristic bone spicules in the retina argued against these diagnoses, respectively. Subsequent fundus examinations showed a “salt and pepper” appearance with well-demarcated areas resembling retinal lacunae. Aicardi syndrome was briefly considered due to the presence of retinal lacunae, but other characteristic features were lacking. Treatment for esotropia with botulinum toxin injection at 1 year of age yielded minimal improvement.
At 19 months old, genetic testing identified a heterozygous mutation (c.1159 C > T, p.Arg387*) in the KIF11 gene, confirming the diagnosis of MCLMR. The patient underwent bilateral medial rectus muscle recession surgery at 2 years old for persistent esotropia, with significant improvement. Refraction revealed a hyperopic astigmatism in both eyes (+ 0.25 -2.50 × 180 OD and + 0.75 -2.00 × 170 OS). She continues to require right eye patching for 2 h daily.
Retinography revealed extensive pigmentary changes, retinal lacunae, retinal fold, and a pale optic disc (Fig. 1). Electroretinography (ERG) demonstrated cone-rod dysfunction (Fig. 2).
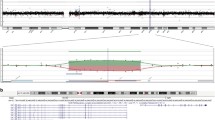
Eletrophyology exams were obtained showing significant cone-rod dysfunction and a better visual function of the right eye (Figs. 3 and 4).
Full-field ERG performed using skin electrodes. The first row reflects the findings in our patient, the second row reflects the usual responses observed in a normal patient using corneal electrodes. Dark-adapted (DA) responses are present, although reduced. No response was identified in the light-adapted (LA) phase. These findings suggest cone-rod dysfunction
Discussion
Mirzaa et al. (2014) [5] also reported simplified gyral patterns on brain MRI in an MCLMR case, similar to our patient. This finding suggests potential central nervous system involvement beyond microcephaly.
Birtel et al. (2017) [1] proposed a classification system for MCLMR-associated retinal changes. Type 1 includes subclinical findings with only retinal thinning and abnormal full-field ERG. Type 2 involves retinal dystrophy with specific alterations on OCT and fundus autofluorescence (FAF) imaging, including marked outer retinal atrophy and characteristic FAF patterns. Type 3 presents with chorioretinal atrophy in well-defined areas, primarily outside the retinal vascular arcades. Finally, type 4 manifests with features resembling familial exudative vitreoretinopathy (FEVR), such as retinal folds, detachment, and peripheral avascularity.
FEVR [6] includes a group of genetic diseases in which abnormal retinal angiogenesis is in the center of the pathological process leading to incomplete retinal vascularization, ischemia and neovascularization. The neovascularization subsequently leads to fibrosis, traction to the posterior pole, retinal detachment and retinal folds. Expressivity is highly variable and may be asymmetric. Of the main important signs of the disease highlights the avascularity of the peripheral retina that its often in V-shaped and located in the temporal region. The lack of signs of avascularity and the relative slow worsening of the patient´s visual function contradicts this diagnosis on this case as it was expected that the retinal folds would be rapidly followed by retinal detachment with drastic decay of her visual acuity [7]. On the other hand, an overlap of both diseases as already been described with mutations on the KIF11 gene (p.A218Gfs*15, p.E470X, p.R221G, c.790-1G > T, p.R47X), leading to stipulations that microcephaly could serve as a marker to look for KIF11 mutations in patients with FEVR and that avascular regions do not exclude MCLMR diagnosis in patients with FEVR. In conclusion, overlapping FEVR diagnosis can never be fully excluded.
Our case demonstrates the significant phenotypic variability documented in MCLMR, particularly regarding ocular involvement. It presents a unique overlap of features from different Birtel classifications, including extensive pigmentary changes (type 2), retinal folds (type 4), and lacunar areas(type 3).
While these lacunae share some resemblance to those seen in Aicardy syndrome, crucial distinctions exist. Unlike Aicardy syndrome, our case exhibits involvement of both central and peripheral retinal regions, less rounded lacunae, and the presence of blood vessels traversing these areas. Additionally, the combination of pigmentary changes and FEVR-like features further supports the differentiation between these two disorders.
The specific c.1159 C > T (p.Arg387*) mutation in our patient has not been previously described in association with detailed retinographic findings or strabismus. Ostergaard et al. (2012) [8] reported four patients with this mutation, only two of whom had ocular involvement characterized as non-specific chorioretinopathy. Our case expands the phenotypic spectrum associated with this specific mutation by providing a detailed description of the retinal findings and the presence of strabismus.
Conclusion
This case report expands the phenotypic spectrum of MCLMR by demonstrating a unique combination of retinal features. The patient exhibited extensive pigmentary changes, retinal folds, and lacunar areas, overlapping characteristics from previously described classifications (Birtel et al., 2017 [1]). Importantly, the morphology of the lacunar areas differed from those observed in Aicardy syndrome, highlighting the need for careful clinical distinction between these disorders.
Our findings emphasize the significant phenotypic variability associated with MCLMR, particularly regarding ocular involvement. This underscores the importance of detailed clinical evaluation and comprehensive reporting of cases to improve our understanding of the disease spectrum and genotype-phenotype correlations. By sharing detailed case reports, we can contribute to the development of more accurate diagnostic criteria and potentially guide future treatment strategies for this rare and complex condition.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- MCLMR:
-
Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation
References
Birtel J, Gliem M, Mangold E, Tebbe L, Spier I, Müller PL, et al. Novel insights into the Phenotypical Spectrum of KIF11 -Associated Retinopathy, including a New Form of Retinal Ciliopathy. Invest Opthalmology Visual Sci. 2017;58(10):3950.
Orphanet. (n.d.). Retrieved from https://www.orpha.net/en/disease/detail/2526
Feingold M, Bartoshesky L. Microcephaly, lymphedema, and chorioretinal dysplasia: a distinct syndrome? Am J Med Genet. 1992;43(6):1030–1.
Fruhman G, Eble TN, Gambhir N, Sutton VR, Van den Veyver IB, Lewis RA. Ophthalmologic findings in Aicardi syndrome. J Am Association Pediatr Ophthalmol Strabismus. 2012;16(3):238–41.
Mirzaa GM, Enyedi L, Parsons G, Collins S, Medne L, Adams C, et al. Congenital microcephaly and chorioretinopathy due to de novo heterozygous KIF11 mutations: five novel mutations and review of the literature. Am J Med Genet A. 2014;164(11):2879–86.
Gilmour DF. Familial exudative vitreoretinopathy and related retinopathies. Eye. 2015;29(1):1–14.
Robitaille JM, Gillett RM, LeBlanc MA, Gaston D, Nightingale M, Mackley MP, et al. Phenotypic overlap between familial exudative vitreoretinopathy and Microcephaly, Lymphedema, and Chorioretinal Dysplasia caused by KIF11 mutations. JAMA Ophthalmol. 2014;132(12):1393.
Ostergaard P, Simpson MA, Mendola A, Vasudevan P, Connell FC, van Impel A, et al. Mutations in KIF11 cause autosomal-Dominant Microcephaly variably Associated with congenital Lymphedema and Chorioretinopathy. Am J Hum Genet. 2012;90(2):356–62.
Acknowledgements
Are extended to all the health staff involved in the caring of the patient for their professionalism that contributed to the completion of this study. Their assistance is greatly appreciated.
Funding
We state that none of the authors has received funding for the divulgation of this case report. This research was conducted without external financial support or funding from any organization.
Author information
Authors and Affiliations
Contributions
N.S gathered all clinical and scientifical information and wrote the manuscript. R.S and A.M reviewed the manuscript and were the attending physicians of the child. R.S interpreted the eletrophysiological findings. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We affirm that we have obtained ethics approval and consent to participate in the research study as required by ethical guidelines. The parents of the patient involved in the study provided informed consent before participation, ensuring they were fully aware of the study’s purpose, procedures, and potential risks. We grant permission for the publication of study findings in academic or public domains. We are committed to upholding ethical standards and respecting participants’ rights to privacy and confidentiality throughout the publication process.
Consent for publication
We declare that an informed consent was obtain beside the child´s parents to publish all exams and all relevant clinical anonymized information. A copy of the consent is provided.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Silva, N.Á., Silva, R.E.S. & Magalhães, A.A. Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation (MCLMR)- the new lacunae: a case report. BMC Ophthalmol 24, 372 (2024). https://doi.org/10.1186/s12886-024-03627-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03627-y