Abstract
Background
In this report, we describe a case of proliferative diabetic retinopathy that developed into exudative changes confusing with central serous chorioretinopathy (CSCR) following extensive endolaser pan retinal photocoagulation.
Case description
A 49-year-old male patient with diabetic retinopathy in both eyes presented with vitreous hemorrhage and 6/60 visual acuity in his left eye. Optical coherence tomography (OCT) scans at presentation revealed serous PEDs in both eyes. On day 10 after vitreoretinal surgery and complete peripheral endolaser PRP for the left eye, there was serous retinal detachment (SRD) and an increase in PED heights, mimicking CSCR. No additional treatment was considered. At the three-week post-operative visit, OCT scans revealed that the SRD had resolved and the PED heights had decreased without rupture. At the final follow-up visit, 12 weeks after surgery, the SRD had not recurred, and the PEDs had stabilized. Despite no additional ocular therapy for the right eye, the serous PED height had decreased. The choroidal thickness (CT) at the fovea at various points during the follow-up visits revealed a reduction in both eyes.
Conclusion
This case demonstrated the course of SRD, PED, and CT following extensive PRP. These changes may be associated with intraocular VEGF changes. In the presence of SRD and serous PED, the PED morphology may help differentiate the condition from CSCR. Although caution should be exercised when performing PRP during surgery or as an outpatient procedure, the SRD usually resolves without problem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The main objective in the treatment of ischemic proliferative vascular retinopathy in diseases such as diabetic retinopathy is to decrease the production of vascular endothelial growth factor (VEGF). This can be achieved by either destroying the extramacular retinal pigment epithelium (RPE) through extensive pan retinal photocoagulation (PRP), which converts the hypoxic retina to anoxic conditions, or by immediately reducing VEGF levels using intravitreal antiVEGF injections [1, 2]. Retinal and choroidal complications have been observed due to laser burns that exceeded the energy-absorbing capacity of the RPE. The inflammation triggered by the thermal effect of photocoagulation on the retina and choroid, the reduction in choroidal blood flow in the peripheral photocoagulation lesion causing a compensatory increase in choroidal circulation in the untreated zones, a probable increase in vascular permeability due to an anti-VEGF rise, and the development of choroidal effusion caused by a disruption of the choriocapillaris may all alter choroidal thickness and cause complications such as choroidal detachment and serous retinal detachment. These changes typically subside with the healing process during the post-treatment period [3]. Additionally, there is a risk of developing new or worsening diabetic macular edema following PRP [4, 5]. It has emerged that the risk of these complications can be reduced by dividing the PRP into two or more sessions spaced at least two weeks apart [6]. The effect of diabetes and different stages of diabetic retinopathy on the choroid thickness (CT) remains poorly understood. Several studies have linked the changes in the CT to the elevated levels of VEGF observed in cases of proliferative diabetic retinopathy (PDR) and have observed that the choroid is thicker in these eyes [7, 8]. Furthermore, research has demonstrated a notable decrease in the CT following PRP treatment in eyes with PDR [9].
This case report illustrates the course of SRD, pigment epithelial detachment (PED), and CT changes in both eyes of a patient following vitrectomy and endolaser PRP in one eye for vitreous hemorrhage after PDR.
Case description
A man in his late 40’s diagnosed with uncontrolled type 2 diabetes mellitus reported at the retina clinic with sudden vision loss in the left eye over the last two days. He took injectable insulin and oral hypoglycaemics for uncontrolled blood sugars (HbA1C level = 9.7%). His right and left eyes’ vision was 6/6 and 6/60, respectively. The anterior segment examination showed a cataractous crystalline lens in both eyes and normal intraocular pressure. A dilated fundus exam in the right eye showed retinal neovascularisation elsewhere without diabetic macular edema, indicating a PDR and a serous RPE elevation. A dilated fundus exam of the left eye showed fresh vitreous hemorrhage from PDR, obscuring the macula. Both eyes showed serous PEDs on optical coherence tomography (OCT) scans, but no diabetic macular edema. Early vitrectomy surgery combined with cataract surgery, was recommended for the left eye and peripheral PRP for the right eye. The patient opted for the intraocular surgery on the left eye and decided to postpone treatment for the right eye.
Post-cataract surgery, a 25-gauge pars plana vitrectomy, with complete posterior cortical vitreous separation confirmed by using intravitreal triamcinolone acetonide, membrane removal, 360° endolaser application (Constellation®, Embedded Purepoint® Laser system, Alcon), and SF6 gas tamponade for an iatrogenic intraoperative retinal break were performed. The first post-operative day showed a well-attached retina through a gas-filled eye. Patient followed up 10 days after surgery. Left eye vision improved to 6/8. Left eye anterior segment examination was unremarkable. Left eye fundus showed partially absorbed residual gas bubble, well-attached retina, healing endolaser marks, and multiple PEDs. Left eye OCT scan showed increased PED heights and shallow SRD at the macula. The patient continued on prescribed post-operative medications without any modifications. After 10 days, the left eye’s SRD resolved and the PEDs reduced in height. At follow-up visits 3 and 9 weeks later, the SRD did not recur in the left eye, and PED heights were further reduced in both eyes. Subfoveal CT was measured which showed reduction in the thickness (Figs. 1 and 2and Table 1). At the last visit, the left eye’s visual acuity was restored to 6/6. Written informed consent was obtained from the patient to use clinical information and images for publication, with the understanding that his clinical identity would be kept anonymous.
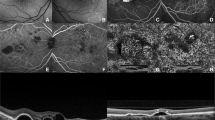
Clinical and optical coherence tomography (OCT) findings of the left eye at the various time points in the case study: Figures 1A, A show clinical and OCT findings of the left eye taken prior to surgery at presentation. Preretinal vitreous haemorrhage obscures the macula due to proliferative diabetic retinopathy. The OCT scan reveals no evidence of diabetic macular edema with two pockets of serous pigment epithelial detachments [PED] (one small pocket inferonasal and another large pocket superotemporal to the fovea). Figure 1C and D show a well-attached retina, a residual gas bubble, and appropriately spaced extensive endolaser burns at ten days after surgery. At this visit, an OCT scan revealed a serous retinal detachment at the macula with PED margins that were intact. Both PEDs’ heights appear to have increased during this visit. Figure 1E-J: Follow-up visits at weeks 3, 6, and 12 show resolution and no recurrence of the serous retinal detachment with a decline in the height of the PED at these visits
Clinical and optical coherence tomography (OCT) findings of the right eye at the various time points in the case study: Figures 2A, B show clinical and OCT findings of the right eye taken prior to surgery at presentation. The presence of a visible abnormal retinal neovascularisation temporal to the fovea within the retinal arcades, as well as serous retinal pigment epithelium elevation without coexisting diabetic macular edema, suggests the proliferative stage of diabetic retinopathy. The OCT scan obtained during this visit shows serous pigment epithelial detachment (PED) extending towards the foveal center but no serous retinal detachment. Figure 2C, D: This is the clinical photograph and OCT scan taken at the 12-week post-presentation visit. The patient did not seek treatment for his proliferative diabetic retinopathy. The clinical photograph depicts the expansion of the abnormal retinal neovascularization at the temporal macula. The OCT scan obtained during this visit shows a reduction in the serous PED with no serous retinal detachment
Discussion
Untreated PDR has been shown to cause increased CT [7,8,9]. In eyes with PDR, high VEGF levels could be responsible for breaking the blood-ocular barrier, leading to increased choroidal permeability and subsequently cause increased CT, and less often serous PED [10, 11]. Extensive and heavy-laser burns during PRP for PDR as part of intraoperative surgery or as an outpatient procedure leads to further breakdown in the outer blood-retinal barrier (RPE), allowing fluid to percolate into the neurosensory space from an already thick and leaky choroid, causing SRD [4].
It is important to distinguish SRD from other commonly seen choroidal vascular hyperpermeable diseases, mainly central serous chorioretinopathy (CSCR). A focal defect in the serous PED causes the tense PED to deflate immediately after leakage, causing SRD in CSCR [12]. In our case, the PED boundaries were intact and increased in height, indicating no RPE defect. Therefore, the PED and SRD in the current case cannot be attributed to an associated CSCR.
SRD caused by extensive PRP may resolve spontaneously or with oral steroids or intravitreal antiVEGF therapy [13, 14]. In this case, no additional treatment was required to reduce SRD, PED, or foveal CT. PRP stabilizes the blood-retinal barrier and lowers SRD, PED, and CT by decreasing RPE VEGF production with time [9]. In cases that do not respond spontaneously, treatment with oral steroids or intravitreal antiVEGF may be considered [13, 14].
Despite the lack of treatment for PDR in the contralateral eye, PED and CT height decreased spontaneously in this case. There is no obvious explanation for this observation. However, there could be a few reasons for this finding. (1) spontaneous VEGF waning; (2) PRP’s secondary collateral effect on the fellow eye, which reduces VEGF; and (3) stress relief following a successful visual outcome, all of which may eventually lead to the regression of pachychoroid manifestations. Also, we believe after PRP the intraocular VEGF levels are reduced due to the destruction of the RPE, which is responsible for VEGF production. The reduced ocular VEGF in the PRP-treated eye may have an effect on the contralateral eye via systemic VEGF reduction, potentially reversing the pachychoroid changes and leading to a reduction in choroidal and retinal fluid. Despite a thorough literature search, we found no studies that looked at the effects of PRP on the untreated fellow eye, such as clinical changes in diabetic retinopathy severity, diabetic macular edema reduction, or in-vivo VEGF levels. We do not have intraocular VEGF levels from both eyes before and after treatment to back up these theories.
In conclusion, learnings from this case report include a high risk of SRD and PED development or progression following extensive PRP, splitting the PRP into multiple sessions to avoid these problems, the PED morphology on OCT which aids in the differentiation of this condition from CSCR, and finally, PED and SRD resolution without causing significant vision loss. Also, more prospectively designed studies combined with intraocular VEGF assays are needed to understand the effects of PRP on the choroid.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- PDR:
-
Proliferative diabetic retinopathy
- PRP:
-
Pan retinal photocoagulation
- VEGF:
-
Vascular endothelial growth factor
- PED:
-
Pigment epithelial detachment
- RPE:
-
Retinal pigment epithelium
- SRD:
-
Serous retinal detachment
- CT:
-
Choroidal thickness
- OCT:
-
Optical coherence tomography
References
Nišić F, Pidro A, Lepara O, Fajkić A, Mioković AP, Suljić E, et al. Effect of pan-retinal laser photocoagulation on intravitreal vascular endothelial growth factor concentration in proliferative diabetic retinopathy. Rom J Ophthalmol. 2022;66:265–70.
Zhao Y, Singh RP. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context. 2018;7:212532.
Zhang Z, Meng X, Wu Z, Zou W, Zhang J, Zhu D, et al. Changes in Choroidal Thickness after Panretinal Photocoagulation for Diabetic Retinopathy: a 12-Week longitudinal study. Invest Ophthalmol Vis Sci. 2015;56:2631–8.
Reddy SV, Husain D. Panretinal Photocoagulation: a review of complications. Semin Ophthalmol. 2018;33:83–8.
Durmaz Engin C, Saatci AO. Macular oedema exacerbation and bacillary layer detachment following the scatter laser photocoagulation in a patient with proliferative diabetic retinopathy. Clin Experimental Optometry. 2024;107:465–8.
Liang JC, Huamonte FU. Reduction of Immediate Complications after Panretinal Photocoagulation: Retina. 1984;4:166–70.
Hamadneh T, Aftab S, Sherali N, Vetrivel Suresh R, Tsouklidis N, An M. Choroidal Changes in Diabetic patients with different stages of Diabetic Retinopathy. Cureus. 2020;12:e10871.
Rewbury R, Want A, Varughese R, Chong V. Subfoveal choroidal thickness in patients with diabetic retinopathy and diabetic macular oedema. Eye (Lond). 2016;30:1568–72.
Kim JT, Lee DH, Joe SG, Kim J-G, Yoon YH. Changes in Choroidal Thickness in Relation to the severity of Retinopathy and Macular Edema in type 2 Diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:3378.
Kinnunen K, Ylä-Herttuala S. Vascular endothelial growth factors in retinal and choroidal neovascular diseases. Ann Med. 2012;44:1–17.
Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in Ophthalmology. Mayo Clin Proc. 2012;87:77–88.
Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58:103–26.
Schatz P, Aldayel A, Taskintuna I, Abdelkader E, Mura M. Serous retinal detachment after panretinal photocoagulation for proliferative diabetic retinopathy: a case report. J Med Case Rep. 2017;11:265.
Nishikawa S, Kunikata H, Aizawa N, Nakazawa T. Bullous Exudative Retinal detachment after retinal pattern scan laser photocoagulation in Diabetic Retinopathy. Case Rep Ophthalmol. 2017;8:475–81.
Acknowledgements
None.
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
Authors’ contributionsR.V., J.C. – conceptualizing the study, data acquisition, analyzing the data, clinical management of the patient, interpreting the findings, writing & reviewing the manuscriptP.G., R.K., A.C. – Data acquisition and analyzing the data V.P., P.H. – critical review of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient for utilizing his clinical details for this manuscript. Permission for using the patient data for this report was obtained from Narayana Nethralaya institutional review board and ethics committee (C-2024-04-006).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gandhi, P., Prabhu, V., Hande, P. et al. Choroidal and retinal exudative changes following extensive endolaser pan retinal photocoagulation. BMC Ophthalmol 24, 357 (2024). https://doi.org/10.1186/s12886-024-03636-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03636-x