Abstract
Background
To compare clinical outcomes of trifocal intraocular lens in patients with and without prior history of laser in situ keratomileusis (LASIK).
Methods
A retrospective study included patients who underwent bilateral cataract surgery and PanOptix trifocal intraocular lens (IOLs) implantation. Patients were grouped: Group A for patients with history of LASIK and Group B for patients without history of LASIK. Postoperative outcome measures comprised distance, intermediate, and near visual acuity, manifest refraction, defocus curve, contrast sensitivity, visual quality, patient satisfaction, and the rate of spectacle independence.
Results
A total of 288 eyes (144 patients) were included: 132 eyes in Group A and 156 eyes in Group B. At 6 months post-surgery, patients of both groups achieved a continuous satisfying visual acuity from 33 cm to distance. 73% of eyes in Group A and 75% of eyes in Group B were within ± 0.50 D of emmetropia (P > 0.05). The percentages of eyes within ± 1.00 D of emmetropia were 98% for Group A and 96% for Group B (P > 0.05). The total scores of satisfaction were 52.58 ± 3.46 for Group A and 53.23 ± 3.46 for Group B (P > 0.05). Most of patients (98% for Group A, 99% for Group B) were able to be spectacle independence for daily living. 53% of patients in Group A and 51% in Group B experiencd mild to moderate negative visual symptoms, which made it a little or moderate difficult to drive at night.
Conclusions
Cataract patients with and without history of LASIK could safely undergo implantation of the PanOptix IOLs, which results in precise refractive outcomes and satisfactory visual acuity. Although contrast sensitivity decreased and some negative visual symptoms were observed, patients’ satisfaction was generally high due to the high rate of spectacles independence. There were no statistically significant differences between the study groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Given the high prevalence of myopia in China, millions of patients have undergone refractive corneal laser surgery, with laser in situ keratomileusis (LASIK) being the predominant method used over the past three decades. LASIK is widely regarded as a safe and effective treatment for myopia [1, 2]. However, over time, many post-LASIK patients have developed presbyopia or cataract [3, 4]. Having become accustomed to spectacle independence following corneal refractive surgery, these patients have high expectations for being spectacle-free after cataract surgery as well [5, 6]. To enhance visual acuity for daily activities and maintain spectacle independence, the implantation of a trifocal intraocular lens (IOL) presents a viable solution [7, 8].
Trifocal IOLs aim to enable patients a spectacles independent life after cataract surgery [4]. However, the use of trifocal intraocular lenses (MIOLs) for presbyopia and cataracts after previous corneal refractive surgery is considered controversial [9, 10]. Eyes previously treated with Excimer laser in situ keratomileusis (LASIK) have traditionally been considered poor candidates for implantation of MIOLs [1]. One reason is that corneal refractive surgery complicates the accurate calculation of the intraocular lens (IOL) power [11,12,13], which may affect the visual outcome after cataract surgery. Another concern of implanting trifocal IOL in the post-LASIK eye is the potential side effects on contrast sensitivity due to corneal ablations and the design of the lens [9].
Although, a few studies have reported on the visual performance of trifocal IOLs after corneal refractive surgery [1, 14, 15], experience in this area is still limited. More in-depth research is still needed to confirm this. Moreover, there is no relevant studies or reports in the literature focusing on comparing clinical outcomes of trifocal intraocular lens in patients with and without previous myopic corneal refractive surgery. The purpose of this study is to evaluate medium-term (6 months) visual outcome and safety in post-myopia LASIK eyes after PanOptix trifocal IOLs implantation, compared to the virgin eyes. The results of this study could help cataract surgeons make informed decisions regarding trifocal IOL implantation after previous myopic laser surgery.
Methods
Study design and participants
This retrospective study analyzed patients who underwent bilateral PanOptix IOL implantation for cataract between July 2020 and September 2023 at Hangzhou MSK Eye Hospital, Hangzhou, China. The conduct of the study was in strict accordance with the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Hangzhou MSK Eye Hospital.
The inclusion criteria required patients to meet the following conditions: (1) presence of cataract with decreased vision; (2) have undergone femtosecond laser-assisted phacoemulsification cataract surgery with PanOptix IOL implantation; (3) preoperative corneal astigmatism of less than 1.00 D; (4) have a pupil diameter greater than 2.5 mm under photopic conditions and less than 6 mm under mesopic conditions; (5) exhibit total corneal HOA ≤ 0.5 μm (with undilated pupil, 4 mm diameter) before cataract surgery; (6) have corneal spherical aberration (SA) ranging from − 0.04 to 0.12 μm (with undilated pupil, 4 mm diameter); (7) potential acuity meter (PAM) score of ≥ 0.8.
The exclusion criteria included: (1) corneal decentered ablation with decentration exceeding 0.5 mm; (2) presence of corneal scar, retinoschisis, haze, myopic retinopathy, retinoschisis, or retinal detachment following myopia excimer laser correction; (3) other ocular or neurological conditions affecting trifocal IOL implantation, such as corneal disease, lens dislocation, uveitis, glaucoma, or retinopathy; (4) absence of follow-up data for 6 months.
Patients were categorized into two groups based on their history of prior myopic corneal refractive surgery using laser in situ keratomileusis (LASIK). Group A consisted of patients with a history of myopic LASIK prior to cataract surgery, whereas Group B included patients with no prior corneal refractive surgery, representing eyes without previous surgical intervention.
Preoperative examination
Patients underwent a thorough ophthalmologic assessment, which included the following evaluations: uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest and cycloplegic refractions, keratometry, slit-lamp microscopy, intraocular pressure (IOP) measurement, endothelial cell density (ECD) assessment (NSPC; KONAN, Japan), ultrasound A and B scans (Aviso, Quantel Medical, France), dilated indirect fundoscopy, Potential acuity meter (PAM-1; GASUSH, China), anterior segment tomography (Sirius; CSO, Florence, Italy), biometry (IOL Master 700; Carl Zeiss, Jena, Germany), and optical coherence tomography (OCT) (Cirrus HD-OCT 5000; Carl Zeiss, Jena, Germany).
Intraocular lenses
A trifocal IOL (AcrySof IQ PanOptix, TNFT00, Alcon, Fort Worth, TX, USA) was implanted in all cases. The spherical aberration (SA) value for PanOptix intraocular lenses was − 0.1 μm. PanOptix IOL has been expected to provide acceptable visual acuity at distance, intermediate, and near by the design of splitting incoming light into 3 different focal points, with 50% for far vision, 25% for intermediate vision, and 25% distributed for near vision [16]. The IOL calculation was performed using Zhang & Zheng (ZZ) formula (published on BMC Ophthalmology 2021 [3]). ZZ IOL calculation for virgin eyes can be accessed at https://www.zzcal.com/calc/en/iol and for post-LASIK eyes can be accessed at https://www.zzcal.com/calc/en/iol_old. The IOL power was selected to target emmetropia.
Surgical technique
Femtosecond laser-assisted phacoemulsification cataract surgery with PanOptix IOL implantation were performed by experienced surgeons. The surgical procedure was conducted as detailed in our previous article [3]. No complications were noted in any of the cases.
Outcome assessment
Follow-up evaluations were conducted at 1 week, 1 month, 3 months, and 6 months postoperatively. The study assessed the following outcome measures: (1) uncorrected distance visual acuity (UDVA), uncorrected intermediate visual acuity (UIVA) at 60 centimeters, and uncorrected near visual acuity (UNVA) at 40 centimeters, at 1 week, 1 month, 3 months, and 6 months; (2) corrected distance visual acuity (CDVA), corrected intermediate visual acuity (CIVA) at 60 centimeters, and corrected near visual acuity (CNVA) at 40 centimeters, at 1 week, 1 month, 3 months, and 6 months; (3) manifest refraction spherical equivalent (MRSE) and refractive cylinder at 6 months; (4) the binocular defocus curve, which was collected in the same room and was performed from + 1.00 D to -4.00 D with 0.50 D step at 6 months; (5) contrast sensitivity, measured using a binoptometer (Binoptometer 4P; Oculus Optikgeräte GmbH), at 6 months; (6) patient satisfaction, evaluated using the VF-14 questionnaire, at 6 months; (7) A questionnaire assessing negative visual symptoms such as glare, halos, starburst, or ghosting at 6 months; (8) rate of spectacle independence at 6 month.
Statistical analysis
Statistical analysis was performed using SPSS software (version 22.0, SPSS, Inc., USA). The Kolmogorov-Smirnov test was applied to assess the normality of the data. Data with a normal distribution were reported as means ± standard deviations (SD). Visual acuity data were converted to logMAR values. Categorical variables were expressed as numbers and percentages. The independent-sample t test was used to compare data between Group A and Group B when the data followed a normal distribution; otherwise, the Mann-Whitney test was applied. Statistical significance was set at a P value of less than 0.05. Sample size calculation was performed using PASS 15.0 software (NCSS, LLC).
Results
A total of 288 eyes (144 patients) were included in this study. 132 eyes (66 patients) in Group A had a history of LASIK, and 156 eyes (78 patients) in Group B had no history of LASIK. Table 1 showed the patients’ baseline demographic data of both groups. All the patients had uneventful surgical procedures and completed the 6-month follow-up period.
Visual acuity
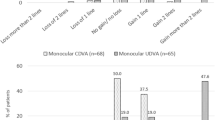
Table 2 and Fig. 1 showed visual acuity for different distances of both groups at postoperative 6 months. Patients of both groups achieved a full range of satisfying visual acuity. For Group A, the mean postoperative UDVA, UIVA and UNVA at 6-month were 0.00 ± 0.09 logMAR, 0.01 ± 0.06 logMAR and 0.03 ± 0.09 logMAR respectively. For Group B, the mean postoperative UDVA, UIVA and UNVA at 6-month were − 0.01 ± 0.07 logMAR, -0.01 ± 0.04 logMAR and 0.03 ± 0.05 logMAR respectively. All eyes of both groups had monocular visual acuities ≥ 0.3 logMAR at distance, intermediate and near (Fig. 1). The percentages for UDVA ≥ 0.1 logMAR were 94% for Group A and 99% for Group B (P < 0.05) (Fig. 1-A). The percentage for UIVA ≥ 0 logMAR of Group A was lower than that of Group B (86% vs. 96%, P < 0.05) (Fig. 1C). UNVA was 0.1 logMAR or better in 89% of the patients of Group A, lower than 97% of Group B (P < 0.05). (Fig. 1E).
Visual acuity of both groups at postoperative 6 months. A UDVA. B CDVA. C UIVA. D DCIVA. E UNVA. F DCNVA. * Significant difference between Groups (P< 0.05). Abbreviations: UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; DCIVA, distance-corrected intermediate visual acuity; UNVA, uncorrected near visual acuity; DCNVA, distance-corrected near visual acuity
Refraction
The mean postoperative MRSE was − 0.02 ± 0.46D for Group A and 0.09 ± 0.45D for Group B at 6 months after surgery. 73% of eyes in Group A and 75% of eyes in Group B were within ± 0.50 D of emmetropia. The percentages of eyes within ± 1.00 D of emmetropia were 98% for Group A and 96% for Group B. There was no significant difference between the two groups (P > 0.05) (Fig. 2A).
The mean postoperative refractive cylinder was − 0.55 ± 0.32D for Group A and − 0.53 ± 0.31D for Group B at 6 months after surgery. For Group A, 61% of eyes were within ± 0.50 D and 97% of eyes were within ± 1.00 D. For Group B, the percentage of eyes within ± 0.50 D and within ± 1.00 D was 70%, and 94%, respectively. There was no significant difference between the two groups (P > 0.05) (Fig. 2B).
Defocus curve
Figure 3 showed binocular distance-corrected defocus curve of both groups at 6 months postoperatively. The best visual acuity of the two groups both appeared with defocus of 0.00 D and – 2.50 D, equivalent to distance and 40 cm, respectively. Within the range between the peaks, the curve dropped to its lowest point at 0.12 logMAR with defocus of – 1.00D in Group A, and 0.08 logMAR with defocus of – 1.50D in Group B. Additionally, the defocus of -3.00D (corresponding to a distance of 33 cm) resulted in a visual acuity of 0.09 logMAR for Group A and 0.06 logMAR for Group B. Thus, patients maintained a continuous satisfying visual acuity from 33 cm to distance, indicating a functional range of visual acuity. There was no significant difference between the two groups (P > 0.05).
Contrast sensitivity
Figure 4 showed uncorrected visual acuity changes for distance, intermediate and near between different contrast threshold levels at postoperative 6 months. When the contrast thresholds decreased from 100 to 80%, the percentage of eyes with no change or gaining 1 snellen line in UDVA was 44% for Group A and 52% for Group B; in UIVA was 38% for Group A and 44% for GroupB; in UNVA was 28% for Group A and 40% for Group B. The percentage of eyes losing 1 snellen line in UDVA was 41% for Group A and 38% for Group B; in UIVA was 45% for Group A and 40% for GroupB; in UNVA was 51% for Group A and 46% for Group B.
Visual acuity changes under different contrast threshold levels for both groups at postoperative 6 months. A Changes in Snellen Lines of UDVA under contrast threshold levels 80% vs 100%. B Changes in Snellen Lines of UDVA under contrast threshold levels 40% vs 100%. C Changes in Snellen Lines of UIVA under contrast threshold levels 80% vs 100%. D Changes in Snellen Lines of UIVA under contrast threshold levels 40% vs 100%. E Changes in Snellen Lines of UNVA under contrast threshold levels 80% vs 100%. F Changes in Snellen Lines of UNVA under contrast threshold levels 40% vs 100%. Abbreviations: UDVA, uncorrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; UNVA, uncorrected near visual acuity
When the contrast thresholds decreased from 100 to 40%, the percentage of eyes losing 1 snellen line or more worse in UDVA was 99% for Group A and 97% for Group B; in UIVA was 98% for Group A and 95% for GroupB; in UNVA was 96% for Group A and 89% for Group B. Most of the eyes had 2 to 3 snellen lines of vision loss.
Group A seemed to be a little more sensitive to contrast threshold than Group B, but there was no significant difference between the two groups (P > 0.05).
Patient satisfaction
Table 3 summarized the results of the visual function questionnaire (VF-14). The total scores of satisfaction were 52.58 ± 3.46 for Group A and 53.23 ± 3.46 for Group B. There was no significant difference between the two groups (P > 0.05). Distributions of vision satisfaction scores of different visual activities for both groups were shown in Fig. 5. Among all the activities evaluated, patients’ satisfaction for driving at night was the lowest. 55% of patients in Group A and 56% of patients in Group B had a little or moderate difficulty for driving at night.
Patients were also asked about negative visual symptoms, such as halos, glare, starburst and ghosting. The percentage of patients experiencing such symptoms was 53% for Group A, and 51% for Group B (Table 4). Most of these symptoms were mild. There was no significant difference between the two groups (P > 0.05).
Spectacle independence
The majority of patients (98% in Group A and 99% in Group B) achieved spectacle independence for daily activities. Only one patient in each group required spectacles to improve near visual acuity. All patients in both groups demonstrated spectacle independence for distance and intermediate visual acuity.
Discussion
In this study, we systematically investigated visual outcomes, refractive accuracy, quality of vision and patient satisfaction after the implantation of PanOptix IOLs in post-LASIK eyes and the virgin eyes. Patients of both groups achieved a continuous satisfying visual acuity from 33 cm to distance, indicating a functional range of visual acuity. Refractive accuracy in post-LASIK eyes was as good as that in the virgin eyes. 73% of eyes in Group A and 75% of eyes in Group B were within ± 0.50 D of emmetropia (P > 0.05). The percentages of eyes within ± 1.00 D of emmetropia were 98% for Group A and 96% for Group B (P > 0.05). Patients’ satisfaction was generally high, and most of them (98% for Group A, 99% for Group B) were able to be spectacle independence for daily living. 53% of patients in Group A and 51% in Group B experiencd mild to moderate negative visual symptoms, such as halos, glare, starburst and ghosting, which made it a little or moderate difficult for them to drive at night. However, the visual symptoms after the implantation of trifocal IOLs were not exacerbated by previous myopic LASIK procedures by comparing their performances of both groups.
As we know, monofocal intraocular lenses (IOLs) provide a fixed focus for distance vision [6]. Bifocal IOLs, which have two focal points, offer good distance and near visual acuity [14], but intermediate vision is not as clear. With advancements in IOL technology, trifocal IOLs have been designed with three useful focal points to provide a wider range of vision for distance, intermediate and near [17]. Therefore, theoretically, the intermediate vision with trifocal IOLs should be improved [18]. Some studies have reported good visual outcomes with AT LISA tri 839MP (Carl Zeiss Meditec) [7, 14], FineVision Micro-F (PhysIOL) [1], and FineVision Pod-F (PhysIOL) [1] trifocal IOLs. In our study, the intraocular IOLs used were PanOptix trifocal IOLs. To accurately evaluate the visual outcomes after implantation of PanOptix trifocal IOLs, we recorded monocular UDVA, UIVA and UNVA at follow-up visit. Our results showed that the postoperative visual acuities were significantly better than preoperative at all distances. The percentage of eyes with prior history of LASIK achieving 20/20 or better for UDVA, UIVA and UNVA was 72%, 86%, and 65%, respectively. Our results were similar to John’s report [4], in which the percentage of eyes achieving 20/20 or better for UCVA, best-corrected distance visual acuity (BCVA), UIVA, and UNVA was 28.6% (10/35 eyes), 77.1% (27/35 eyes), 77.8% (21/27 eyes), and 65.6% (21/32 eyes), respectively. These findings indicated that cataract patients with previous myopic corneal refractive surgery could restore good distance, intermediate and near visual acuity by cataract surgery and PanOptix trifocal IOLs implantation.
To comprehensively evaluate the visual capabilities over the entire range, we also recorded defocus curve at 6 months post-surgery. The best visual acuity of the two groups both appeared with defocus of 0.00 D and – 2.50 D, equivalent to distance and 40 cm, respectively. Additionally, the defocus of -3.00D (corresponding to a distance of 33 cm) resulted in a visual acuity of 0.09 logMAR for Group A and 0.06 logMAR for Group B. Thus, patients maintained a continuous satisfying visual acuity from 33 cm to distance, indicating a functional range of visual acuity. There was no significant difference between the two groups (P > 0.05). With regard to spectacle independence, only one patient in each group required glasses to improve their near-distance visual acuity. Being glasses free for daily living greatly improved patient satisfaction. In Jonker’s study [19], the defocus curves of the trifocal IOL group demonstrated a more continuous performance than the bifocal IOL group at the intermediate range. All these results further support the notion that trifocal IOLs could provide good and wide range visual acuity after previous corneal refractive laser surgery for myopia.
Since the IOL power calculation in eyes with previous corneal refractive laser surgery is difficult, prediction accuracy remains a challenge with cataract surgery and IOL implantation [4, 13, 19]. The difficulty is caused by two major factors: (1) inaccurate determination of corneal refractive power [20]; (2) incorrect effective lens position estimation [21, 22]. To address these issues, we used the ZZ IOL power formula for IOL calculation, targeting emmetropia. In our previous study [3], we have compared the clinical accuracy of this formula with other reported IOL formulas. The ZZ IOL formula may offer several advantages. Firstly, it is applicable to a broad range of patients and is not constrained by axial length (AL). Sencondly, it does not rely on clinical history information, which can often be lost over the long interval between corneal refractive surgery and cataract surgery. In this study, 73% of eyes with prior history of LASIK and 75% of the virgen eyes were within ± 0.50 D of emmetropia. The percentages of eyes within ± 1.00 D of emmetropia were 98% for the post-LASIK eyes and 96% for the virgin eyes. There was no significant difference between the two groups (P > 0.05) These results suggested that the prediction accuracy could be achieved by appropriate IOL calculation formula for post-corneal refractive sugery eyes.
Patients who underwent trifocal IOLs implantation have high requirements for quality of life after cataract surgery. So visual quality evaluation of trifocal IOLs has always been important [7]. In contrast sensitivity test, patients’ visual acuity decreased noticeably as contrast decreased. This result might explain some patients’ complaint about blurred vision in a low-light environment. In addition, some patients (53% for Group A and 51% for Group B) reported experiencing mild negative visual symptoms, such as halos, glare, starburst and ghosting after IOL implantation. As a result, they felt a little difficult for driving at night, and the mean satisfaction score was lower than other activities. Similarly, halos, night glare, and starbursts were common in Chang’s study and about 50% of patients perceived them as moderate to very severe [23].
Furthermore, we found there was no statistically significant difference in visual quality between post-myopic LASIK eyes and virgin eyes in our study. This indicates that the visual symptoms after the implantation of trifocal IOLs were not exacerbated by previous myopic LASIK procedures [7]. This results might be related to our strict surgical indication before implantation of PanOptix IOLs. Patients with corneal decentered ablation (decentration > 0.5 mm) were not recommended. Inclusion criteria such as pupil diameter and total corneal HOA were also important for visual quality [24].
The current study has several limitations. First, follow-up duration of 6 months was still limited, long-term evaluations was required. Second, trifocal IOLs used in this study was not compared with other lenses, such as bifocal IOLs, enhanced depth of focus (EDOF) or monofocal IOLs. More comparisons of different IOLs will be made in future study. Third, the changes of corneal higher order aberrations (HOAs) were not analysed. The effects of HOA on postoperative visual quality require more in-depth study.
Conclusions
In conclusion, the findings of this study indicate that cataract patients with and without history of myopic LASIK could safely undergo implantation of the PanOptix trifocal IOL, which results in precise refractive outcomes and satisfactory visual acuity. Although contrast sensitivity decreased and some negative visual symptoms were observed, patients’ satisfaction was generally high due to the high rate of spectacles independence.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on reasonable request.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- LASIK:
-
Laser in situ keratomileusis
- IOLs:
-
Intraocular lens
- UDVA:
-
Uncorrected distance visual acuity
- CDVA:
-
Corrected distance visual acuity
- UIVA:
-
Uncorrected intermediate visual acuity
- DCIVA:
-
Distance-corrected intermediate visual acuity
- UNVA:
-
Uncorrected near visual acuity
- DCNVA:
-
Distance-corrected near visual acuity
References
Ortega-Usobiaga RC-SJ, Rodríguez-Gutiérrez B, Víctor, Tejerina F, Llovet B, Casco J. Baviera. Trifocal intraocular lens implantation in eyes with previous corneal refractive surgery for myopia and hyperopia. J Cataract Refract Surg. 2021;47(10):1265–1272.
Kim TI, Del Alió JL, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. 2019;393:2085–98.
Zhang J, Shao J, Zheng L, Shen Y, Zhao X. Comparative clinical accuracy analysis of the newly developed ZZ IOL and four existing IOL formulas for post-corneal refractive surgery eyes. BMC Ophthalmol. 2021;21(1):231.
John F, Blaylock, Brad J. Hall. Refractive outcomes following trifocal intraocular lens implantation in post-myopic LASIK and PRK eyes. Clin Ophthalmol 2022; 16:2129;36.
Khor WB, Afshari NA. The role of presbyopia-correcting intraocular lenses after laser in situ keratomileusis. Curr Opin Ophthalmol. 2013;24:35–40.
Yang S, Rong YHX. Presbyopia-correcting intraocular lenses implantation in eyes after corneal refractive laser surgery: a meta-analysis and systematic review. Front Med (Lausanne). 2022;9:834805.
Chow SSW, Chan TCY, Ng ALK, Kwok AKH. Outcomes of presbyopia-correcting intraocular lenses after laser in situ keratomileusis. Int Ophthalmol. 2019;39(5):1199–204.
Javier L, Fernández-García A, Llovet-Rausell J, Ortega-Usobiaga. Rafael Bilbao-Calabuig, Fernando Llovet-Osuna, Vasyl Druchkiv, Alfonso Arias-Puente. Unilateral versus bilateral refractive lens exchange with a trifocal intraocular lens in emmetropic presbyopic patients. Am J Ophthalmol. 2021;223:53–9.
Vrijman V, van der Linden JW, van der Meulen IJE, Mourits MP, Lapid-Gortzak R. Multifocal intraocular lens implantation after previous corneal refractive laser surgery for myopia. J Cataract Refract Surg. 2017;43(7):909–14.
Chan TC, Liu D, Yu M, Jhanji V. Longitudinal evaluation of posterior corneal elevation after laser refractive surgery using swept-source optical coherence tomography. Ophthalmology. 2015;122(4):687–92.
Gimbel HV, Sun R. Accuracy and predictability of intraocular lens power calculation after laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:571–6.
Ferdinando Cione M, De Bernardo M, Gioia, et al. A No-History Multi-formula approach to improve the IOL power calculation after laser refractive surgery: preliminary results. J Clin Med. 2023;12(8):2890.
Kohnen T, Mahmoud K, Bühren J. Comparison of corneal higher- order aberrations induced by myopic and hyperopic LASIK. Ophthalmology. 2005;112(10):1692.
Li QM, Wang F, Wu ZM, et al. Trifocal diffractive intraocular lens implantation in patients after previous corneal refractive laser surgery for myopia. BMC Ophthalmol. 2020;20:293.
Ortega-Usobiaga RB-CJ, Mayordomo-Cerdá F. Jaime Beltrán-Sanz, Javier Fernández-García, Rosario Cobo-Soriano. Trifocal versus monofocal intraocular lens implantation in eyes previously treated with laser in situ keratomileusis (LASIK) for myopia. Indian J Ophthalmol. 2024;72(Suppl 2):S254–9.
Thomas Kohnen M, Herzog E, Hemkeppler S, Schönbrunn N, De Lorenzo. Kerstin Petermann, Myriam Böhm. Visual performance of a quadrifocal (Trifocal) intraocular lens following removal of the crystalline lens. Am J Ophthalmol. 2017;184:52–62.
Plaza-Puche AB, Alio JL. Analysis of defocus curves of different modern multifocal intraocular lenses. Eur J Ophthalmol. 2016;26(5):412–7.
Ken Hayashi M, Yoshida C, Igarashi, Akira Hirata. Effect of refractive astigmatism on all-distance visual acuity in eyes with a trifocal intraocular lens. Am J Ophthalmol. 2021;221:279–86.
Soraya MR, Jonker, Noël JC, Bauer, Natalia Y, Makhotkina, et al. Comparison of a trifocal intraocular lens with a + 3.0 D bifocal IOL: results of a prospective randomized clinical trial. J Cataract Refract Surg. 2015;41(8):1631–40.
Seitz B, Langenbucher A, Nguyen NX, Kus MM, Kuchle M. Underestimation of intraocular lens power for cataract surgery after myopic photorefractive keratectomy. Ophthalmology. 1999;106(4):693–702.
Martinez-Enriquez E, Perez-Merino P, Duran-Poveda S, Jimenez-Alfaro I, Marcos S. Estimation of intraocular lens position from full crystalline lens geometry: towards a new generation of intraocular lens power calculation formulas. Sci Rep. 2018;8:9829.
Pinero DP, Camps VJ, Ramon ML, Mateo V, Perez-Cambrodi RJ. Error induced by the estimation of the corneal power and the effective lens position with a rotationally asymmetric refractive multifocal intraocular lens. Int J Ophthalmol. 2015;8:501–7.
Chang JS, Ng JC, Chan VK, Law AK. Visual outcomes, quality of vision, and quality of life of diffractive multifocal intraocular lens implantation after myopic laser in situ keratomileusis: a prospective, observational case series. J Ophthalmol 2017:2017:6459504. doi: 10.1155/2017/6459504. Epub 2017 Jan 4.
Zhang J, Zheng L, Zhang Y, Wang K. Analysis of Asphericity and corneal longitudinal spherical aberration of 915 Chinese myopic adult eyes. Clin Ophthalmol. 2023;17:591–600.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
HW devised idea, designed and supervised the study. ZJ and SJ collected, analyzed the data. CXF wrote the main manuscript text. ZJ revised the manuscript. All authors reviewed and approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study adhered to the tenets of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Hangzhou MSK Eye Hospital. Due to the retrospective design of the study, the Medical Ethics Committee of Hangzhou MSK Eye Hospital waived the requirement for informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, X., Zhang, J., Shao, J. et al. Comparing clinical outcomes of trifocal intraocular lens in patients with and without prior history of laser in situ keratomileusis for myopia. BMC Ophthalmol 24, 406 (2024). https://doi.org/10.1186/s12886-024-03671-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03671-8