Abstract
Purpose
To evaluate retinal nerve fiber layer thickness (RNFLT) and radial peripapillary capillaries (RPC) density in adults with different degrees of myopia using optical coherence tomography angiography (OCTA) and explore their relationship with ocular factors, such as axial length (AL) and disc area.
Methods
A total of 188 subjects were included in this cross-sectional study. The eyes were divided into four groups according to AL. OCTA was used for the assessment of RNFLT, RPC density, and other optic disc measurements, such as disc area. One-way analysis of variance was performed to compare differences between four groups, and P value < 0.01 was considered significant.
Results
The RNFLT was significantly thinner in high myopia (HM) group at inferior nasal (IN) quadrant (P = 0.004) than low myopia (LM) group, but thicker at temporal inferior (TI) quadrant (P = 0.006). The RPC density of nasal superior (NS) quadrant, nasal inferior (NI) quadrant, and inferior nasal (IN) quadrant significantly decreased as AL increasing. By simple linear regression analysis, the inside disc RPC (iRPC) density tended to be correlated significantly with AL (0.3997%/mm, P < 0.0001). Peripapillary RPC (pRPC) density was in significant correlation with AL (-0.2791%/mm, P = 0.0045), and peripapillary RNFLT (pRNFLT) was in significant correlation with disc area (0.2774%/mm2, P = 0.0001).
Conclusion
RNFLT and RPC density were closely associated with AL and disc area. They might be new indexes in assessing and detecting myopia development via OCTA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Myopia, the most common type of refractive error all over the world, has exhibited a rising incidence in recent years [1]. It is noteworthy that several pathological complications including glaucoma, myopic maculopathy, choroidal neovascularization (CNV) and retinal detachment occur more frequently in highly myopic patients, particularly those exhibiting excess axial elongation [2]. Koh et al. found that patients with high myopia (HM) were more likely to have optic neuropathy such as peripapillary atrophy (PPA) than healthy subjects, suggesting that myopia might be a risk factor for optic nerve impairment [3]. The changes in radial peripapillary capillaries (RPC), as the direct source of oxygen and nutrition for neuroretinal layers, have attracted much attention for its possibility of leading to unbalanced metabolic supply and optic nerve function abnormalities.
In recent years, advancements of optical coherence tomography angiography (OCTA), a novel non-invasive imaging technique that employs motion contrast imaging to obtain high-resolution volumetric blood flow information, have enabled the quantitative assessment of retinal blood flow in multiple layers [4]. Previous studies have presented evidence of various alterations in choriod-retinal microcirculation in HM, and indicated thinner retinal nerve fiber layer thickness (RNFLT) that in myopic eyes than in non-myopic eyes [5]. Notably, there have been different RNFL distribution patterns in various studies, which may lead to inaccurate diagnosis [6]. However, few studies have assessed optic nerve blood perfusion in HM, in which conflicting results were reported. For example, Mo J et al. found that in HM group, RPC density decreased significantly in nasal quadrant and inferior nasal quadrant [7]. On the contrary, A. C. Yaprak’s study showed that RPC density decreased significantly in all quadrants except inferior quadrant [8]. Consequently, the purpose of this study is to compare the differences in RNFLT and RPC density from different quadrants between four refraction groups and investigate the correlation between them with axial length (AL) and disc area.
Methods
This cross-sectional study was approved by the ethics committee of The First Affiliated Hospital of Soochow University, and followed all recruitment and procedures strictly adhered to the principles of the Declaration of Helsinki. All participants comprehensively understood the study protocol, and the informed consent was obtained from each participant prior to their inclusion in the study.
Subjects
This project included 188 subjects who visited the Department of Ophthalmology of the First Affiliated Hospital of Soochow University between May 2022 and August 2023. Participants were categorized into the following four groups based on the AL: emmetropia group (EM group, 34 eyes, 23.5 mm ≤ AL < 24.5 mm), low myopia group (LM group, 53 eyes, 24.5 mm ≤ AL ≤ 25.5 mm), moderate myopia group (MM group, 56 eyes, 25.5 mm ≤ AL < 26.49 mm), and high myopia group (HM group, 45 eyes, 26.5 mm ≤ AL < 29.00 mm). The exclusion criteria were as follows: intraocular pressure (IOP) ≥ 21mmHg; astigmatism < − 2 D or > + 2 D; best-corrected visual acuity (BCVA) < 6/20; evidence of ocular disease other than myopia; history of ocular trauma and ocular surgery; systemic disease that might affect the blood flow, such as hypertension and diabetes mellitus; systemic drug use that might affect the blood flow; age < 18 years or > 45 years; quality of OCTA scan images < 6/10.
Ophthalmic examinations
All participants underwent a complete ophthalmic examination, including refraction (model KR-8900; Topcon, Tokyo, Japan), BCVA, intraocular pressure (IOP, Full Auto Tonometer TX-F; Topcon), and slit-lamp. Central corneal thickness (CCT), lens thickness (LT), anterior chamber depth (ACD), and axial length (AL) were measured using IOL Master (version 3.02, Carl Zeiss Meditec, Germany).
OCTA
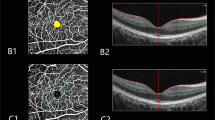
All patients were examined using spectral-domain OCTA (RTVue-XR, Optovue, Fremont, CA, USA) by a single experienced technician with an undilated pupil. The device frequency was set to 70 kHz, the wavelength is controlled at 840 mm, and the frequency width is set to 35 mm. To evaluate optic disc vascular structures, 4.5 × 4.5 mm scanning range was used. Each scan was automatically segmented to visualize the RNFLT, RPC density, and disc area using AngioVue. The obtained RNFL and RPC images were averaged to eight quadrants, that is nasal superior (NS) quadrant, nasal inferior (NI) quadrant, inferior nasal (IN) quadrant, inferior temporal (IT) quadrant, temporal inferior (TI) quadrant, temporal superior (TS) quadrant, superior temporal (ST) quadrant, and superior nasal (SN) quadrant thickness (Fig. 1d). Moreover, peripapillary retinal nerve fiber layer thickness (pRNFLT), peripapillary radial peripapillary capillary (pRPC) density, inside disc RPC (iRPC) density, and whole RPC (wRPC) density were also measured.
Pictorial representation of the structure and capillary plexus of optic disc. a optic disc, b retinal nerve fiber layer, c radial peripapillary capillaries, d superior nasal (SN), nasal superior (NS), nasal inferior (NI), inferior nasal (IN), inferior temporal (IT), temporal inferior (TI), temporal superior (TS), superior temporal (ST) quadrants
Statistical analysis
All data were analyzed using SPSS software (version 26.0; SPSS, Inc. an IBM company, Chicago, IL, USA). Qualitative variables were presented as numbers. Continuous values were presented as means and standard deviations for normally distributed data, and median and interquartile range for non-normally distributed data. The normality was tested using Shapiro-Wilk normality test. One-way analysis of variance was conducted to compare the differences among four groups. Linear regression analysis was employed to assess the relationship between RNFLT or RPC density with AL or disc area. P value < 0.05 was considered statistically significant.
Results
Demographic characteristics were summarized in Table 1. A total of 154 eyes from 154 myopia patients and 34 eyes from 34 healthy subjects were included in this study. The right eyes were selected for examination. The mean subject age was 27.25 ± 6.04 years (range: 18–45 years), and the mean AL was 25.60 ± 1.25 mm (range: 23.50–29.00 mm). There was no significant difference in age, sex, IOP, ACD, and LT among four groups (all P > 0.05).
The RNFLT and RPC density of eight quadrants in EM, LM, MM, and HM groups were presented in Table 2. The RNFLT of IN quadrant was significantly thinner in HM group than LM group (P = 0.004). By contrast, HM group showed significantly thicker RNFLT than LM group at TI quadrant (P = 0.006). The RPC density of NS, NI, IN quadrants and pRPC density decreased as AL increased, and differences between HM group and EM group were all statistically significant (all P < 0.05, Table 2). Instead, iRPC density was significantly greater in HM group than EM group (P = 0.006). The linear regression analysis revealed that iRPC density to be correlated significantly with AL (0.3997%/mm, P < 0.0001, Fig. 2C) and disc area (0.3124%/mm2, P < 0.001, Fig. 2G). Additionally, RPC density tended to be in significant correlation with AL (-0.2791%/mm, P=0.0045, Fig. 2D), and pRNFLT tended to be in significant correlation with disc area (0.2774%/mm2, P = 0.0001, Fig. 2E). There was, however, no significant correlation between wRPC density and AL as well as disc area.
Scatter plot of simple linear regression between (A) peripapillary retinal nerve fiber layer (RNFL) thickness and axial length (AL) (P = 0.066), (B) whole radial peripapillary capillaries (RPC) density and AL (P = 0.2223), (C) inside disc RPC density and AL (P < 0.0001), (D) peripapillary RPC density and AL (P = 0.0029), (E) peripapillary RNFL thickness and disc area (P = 0.0001), (F) whole RPC density and disc area (P = 0.8313), (G) inside disc RPC density and disc area (P < 0.001), (H) peripapillary RPC density and disc area (P = 0.6064)
Associations among pRNFLT and wRPC density were also evaluated by linear regression analysis, with wRPC density as the dependent variable and pRNFLT as the independent variable. The following equation represented the effect of pRNFLT on wRPC density:
From the equation, pRNFLT showed significant effects on wRPC density, and larger wRPC density was associated with increasing pRNFLT. Multivariable linear regression analysis showed that IOP, ACD, and CCT had no significant effects on wRPC density.
Discussion
In this study, we found RPC density decreased with increasing AL, which was in accordance with findings of previous studies [9, 10]. It was speculated that the excessive elongation of eyeball could cause thinning of retina, which might lead to decrease in blood circulation [11, 12]. Nevertheless, the mechanism underlying the reduced blood flow in eyes with HM still required further investigation. In most previous studies, only wRPC density was evaluated, so we examined RPC in more detail in this study. Notably, our results showed all RPC density decreased when emmetropia progressed towards low-to-moderate myopia, except iRPC density increased. We speculated the tension on the posterior pole of eyeball increased as its axial elongation, which would affect more significantly in peripapillary area than in center of optic disc [13]. Besides, there might be potential compensatory mechanism to improve blood supply inside optic disc to maintain normal function, which required further research to study specific mechanism underlying the increasing iRPC density in HM eyes. However, Yaprak et al. did not detect decrease of iRPC density in HM group, while they did not observe any correlation between AL and iRPC. They thought the stabilization of iRPC density might be related to only non-pathological HM included in their study [8]. We speculated differences of their results from ours might be attributed mainly to insufficient sample size, for 188 subjects in our research and 75 subjects in Yaprak’s research. Few studies have reported OCTA iRPC density results, so further studies with larger sample size were necessary. iRPC density, the only vessel density indicator changed in LM or MM compared to EM in our study, maintained significantly increasing trend in whole progression of myopia degree. Therefore, we speculated increase of iRPC density might indicate patients more likely to develop into HM in the future, and its increase could be used as key monitoring indicator of myopia progression. Additionally, our results found RPC density of NS, NI, IN quadrants remarkably reduced when LM or MM developed further to HM. Previous studies also showed the vessel loss in nasal quadrant was most serious in HM than LM group [14]. We suggested that RPC density in nasal quadrant might be more susceptible to myopic-related structural changes than in other quadrants, so its decline could be used as important monitoring indicator of myopia progression. In current study, we adopted AL rather than dioptric values to measure the degree of myopia, because dioptric values could be affected by crystalline lens status and it was known that axial elongation is related to the mechanism of myopic optic disc change [15]. Liu et al. also showed that AL was more strongly associated with myopic retinal change than refractive error [16].
We found a negative correlation between AL and RNFLT in most quadrants, which accorded well with some previous studies [17,18,19]. This could be due to the elongation of globe leading to mechanical stretching and thinning of scleral and retina, although it was yet to be ascertained whether the RNFLT was decreased at the histologic level. Additionally, we found RNFLT decreased in temporal quadrant, but increased in temporal quadrant as AL increasing, which suggested redistribution of RNFL might occur in the development of myopia. Sung Ganekal et al. also reported RNFLT thinning was seen in all quadrants except in temporal quadrants [5]. Kim et al. demonstrated that retina was dragged toward the temporal horizon as AL increasing. In this progress, RNFL layers are compressed against the bundles originating from the opposite hemisphere at the horizontal raphe, and this would result in thickening of RNFLT in the temporal quadrant. In contrast, the nasal retina would become thinner as it is stretched [17]. However, there were some quadrantal differences of RNFLT in different studies. Leung et al. found RNFLT significantly thicker in HM group, especially in the 12, 1, and 7 o’ clock sectors [18]. Instead, in the study of Seo et al., the RNFLT of the 1, 2, 5, 6, and 12 o’ clock sectors were significantly thinner in HM group than in LM group. At the same time, they found HM group showed significantly thicker RNFLT than LM group at the 8, 9, and 10 o’clock sectors [14]. We speculated that these differences might be attributed mainly to the OCT imaging equipment used. Seo used a Cirrus HD-OCT device with software version 6.0, whereas Leung used Stratus OCT version 3 that obtained 12 RNFLT values from each scan. Besides, regional ethnic differences or insufficient sample size should be considered as well. Additionally, the default was that each quadrant is a whole, so statistic data may also cancel each other if the results of the internal parts were contradictory.
Of note, when emmetropia progressed towards low-to-moderate myopia and high myopia, RPC density changed earlier, and its range of changing quadrants was larger than RNFLT. Therefore, we speculated that the susceptibility of RPC density was higher than RNFLT, which might be new observational indexes in the diagnosis of myopia. Nevertheless, the confusion whether this retinal response preceded or followed the vascular compromise was still controversial. Our study shares the limitations associated with cross-sectional studies, which cannot confirm causation, so further longitudinal studies and specific mechanisms research are required for additional investigation. In addition, we found RPC density positively correlated with RNFLT, which was in accordance with the findings of previous studies [20,21,22]. Sung et al. speculated that RNFL thinning might affect regional oxygen demand or the need of vascular supply in peripapillary region, and thereby triggered the retinal vascular adjustment via autoregulatory mechanisms [15]. Similarly, it was possible that an increase of RNFLT could lead to increase in oxygen and nutrient demands, thereby increasing retinal perfusion [23]. However, we thought this conclusion might be doubtful, for the changed quadrants of RNFLT and RPC density not synchronizing. Sung et al. speculated that morphologic changes of optic disc caused additional mechanical strain to microvasculature at the peripapillary region and resulted in deep peripapillary microvascular remodeling [15]. Additionally, Jonas et al. reported that the excessive elongation of eyeball caused stretching and thinning especially in the posterior pole, which might result in both enlargement of optic disc and thinning of RNFL [24]. Therefore, we should recognize that in addition to considering the effect of AL on the OCT RPC measurements in myopic adults, disc area should also be considered for analysis [14]. Besides, we found pRNFLT positively correlated with disc area. It has been postulated that eyes with larger optic disc area may retain more retinal nerve fiber axons [15, 25]. Alternatively, the larger the optic disc area, the shorter the distance between machine scanning circle and disc margin, which might result in the overestimation of RNFLT [26].
Moreover, as the integrity of RNFL is a recognized surrogate for glaucomatous change [18], we think impact of both HM and glaucoma on RNFLT should be comprehensively considered when using RNFLT to evaluate the progression of optic neuropathy in HM patients with glaucoma. On the one hand, the increased risk of development of glaucomatous change may be related to the already reduced RNFLT in myopic eyes. On the other hand, the reduced RNFLT in myopia may itself represent a risk factor for development of glaucoma.
Strengths and limitations
The main goal of this study was to observe the RNFLT and RPC density in myopic adults using OCTA technology and evaluate the influence of AL and disc area on them. It has some notable strengths compared with previous studies. Firstly, we analyzed eight quadrants values of optic disc from the healthy and myopic eyes. To our knowledge, study results will be different for different methods of dividing regions by OCT machines, so changes of RPC density with development of myopia can be obtained as much as possible by dividing the quadrants in detail. Moreover, only young subjects were enrolled in this study, thus minimizing the impact of aging on OCT parameters. The thickness of RNFL is systematically thinner with age, which means that the association between myopia and OCT measurements may vary depending on the age of the subject [25, 27, 28], so reducing the effects of aging can provide more clear insights into effects of myopia on OCT parameters.
Of course, this study also has some limitations. First, ocular magnification can partially affect RNFL measurements. Several studies have produced conflicting results on the relationship between OCT RNFL parameters and myopia after magnification correction [19], so further research are needed to investigate this relationship. Moreover, we only recruited young subjects of uniform age and ethnicity to eliminate potential confounding factors, so further investigation should focus on subjects of other age groups or ethnicities, and further studies with larger sample size are needed to provide identical guidance for clinical diagnosis.
Conclusions
We evaluated RNFLT and RPC density of different myopia degrees from quadrant perspectives using OCTA. The information revealed alterations of RNFLT and RPC density might be related to myopia pathogenesis, which demonstrated myopia could independently cause optic nerve changes. Therefore, ophthalmologists should take advantage of OCTA to improve the early diagnosis of myopia, as well as evaluate the subsequent progression of myopia, especially changes of iRPC density in early myopia and changes of RPC density at nasal quadrants alterations in high myopia. Additionally, RPC density had greater susceptibility to AL than RNFLT. We should emphasize the importance of RPC density as new observational indexes in the diagnosis and monitoring of myopia.
Availability of data and materials
The data uesd and analyzed in this study are available from the corresponding author.
Abbreviations
- RNFL:
-
Retinal nerve fiber layer
- RPC:
-
Radial peripapillary capillaries
- RNFLT:
-
Retinal nerve fiber layer thickness
- pRNFLT:
-
Peripapillary retinal nerve fiber layer thickness
- pRPC:
-
Peripapillary radial peripapillary capillaries
- iRPC:
-
Inside disc radial peripapillary capillaries
- wRPC:
-
Whole radial peripapillary capillaries
- CNV:
-
Choroidal neovascularization
- PPA:
-
Parapapillary atrophy
- OCTA:
-
Optical coherence tomography angiography
- SE:
-
Spherical equivalent
- BCVA:
-
Best corrected visual acuity
- IOP:
-
Intraocular pressure
- AL:
-
Axial length
- LT:
-
Lens thickness
- CCT:
-
Central corneal thickness
- ACD:
-
Amber chamber depth
- EM:
-
Emmetropia
- LM:
-
Low myopia
- MM:
-
Moderate myopia
- HM:
-
High myopia
- SN:
-
Superior nasal
- NS:
-
Nasal superior
- NI:
-
Nasal inferior
- IN:
-
Inferior nasal
- IT:
-
Inferior temporal
- TI:
-
Temporal inferior
- TS:
-
Temporal superior
- ST:
-
Superior temporal
References
Yekta A, et al. The prevalence of anisometropia, amblyopia and strabismus in schoolchildren of Shiraz. Iran Strabismus. 2010;18(3):104–10.
Moriyama M, et al. Topographic analyses of shape of eyes with pathologic myopia by high-resolution three-dimensional magnetic resonance imaging. Ophthalmology. 2011;118(8):1626–37.
Koh V, et al. Myopic maculopathy and optic disc changes in highly myopic young Asian eyes and impact on visual acuity. Am J Ophthalmol. 2016;164:69–79.
Read SA, et al. Choroidal changes in human myopia: insights from optical coherence tomography imaging. Clin Exp Optom. 2019;102(3):270–85.
Ganekal S, Sadhwini MH, Kagathur S. Effect of myopia and optic disc area on ganglion cell-inner plexiform layer and retinal nerve fiber layer thickness. Indian J Ophthalmol. 2021;69(7):1820–4.
Hong SW, et al. Analysis of peripapillary retinal nerve fiber distribution in normal young adults. Invest Ophthalmol Vis Sci. 2010;51(7):3515–23.
Mo J, et al. Vascular flow density in pathological myopia: an optical coherence tomography angiography study. BMJ Open. 2017;7(2):e013571.
Yaprak AC, Yaprak L. Retinal microvasculature and optic disc alterations in non-pathological high myopia with optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2021;259(11):3221–7.
Benavente-Pérez A, et al. Ocular blood flow measurements in healthy human myopic eyes. Graefes Arch Clin Exp Ophthalmol. 2010;248(11):1587–94.
Wang X, et al. Is the peripapillary retinal perfusion related to myopia in healthy eyes? A prospective comparative study. BMJ Open. 2016;6(3):e010791.
Shin YJ, et al. Aqueous humor concentrations of vascular endothelial growth factor and pigment epithelium-derived factor in high myopic patients. Mol Vis. 2012;18:2265–70.
Sawada O, et al. Negative correlation between aqueous vascular endothelial growth factor levels and axial length. Jpn J Ophthalmol. 2011;55(4):401–4.
Rosenfeld PJ, et al. ZEISS Angioplex™ spectral domain optical coherence tomography angiography: technical aspects. Dev Ophthalmol. 2016;56:18–29.
Seo S, et al. Ganglion cell-inner plexiform layer and retinal nerve fiber layer thickness according to myopia and optic disc area: a quantitative and three-dimensional analysis. BMC Ophthalmol. 2017;17(1):22.
Sung MS, et al. Clinical features of superficial and deep peripapillary microvascular density in healthy myopic eyes. PLoS ONE. 2017;12(10):e0187160.
Liu W, et al. Peripapillary atrophy in high myopia. Curr Eye Res. 2017;42(9):1308–12.
Kim MJ, Lee EJ, Kim TW. Peripapillary retinal nerve fibre layer thickness profile in subjects with myopia measured using the Stratus optical coherence tomography. Br J Ophthalmol. 2010;94(1):115–20.
Leung CK, et al. Retinal nerve fiber layer measurements in myopia: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2006;47(12):5171–6.
Savini G, et al. The influence of axial length on retinal nerve fibre layer thickness and optic-disc size measurements by spectral-domain OCT. Br J Ophthalmol. 2012;96(1):57–61.
Chen Q, et al. Exploration of peripapillary vessel density in highly myopic eyes with peripapillary intrachoroidal cavitation and its relationship with ocular parameters using optical coherence tomography angiography. Clin Exp Ophthalmol. 2017;45(9):884–93.
Li M, et al. Retinal Microvascular Network and Microcirculation assessments in high myopia. Am J Ophthalmol. 2017;174:56–67.
Xu H, et al. Microcirculatory responses to hyperoxia in macular and peripapillary regions. Invest Ophthalmol Vis Sci. 2016;57(10):4464–8.
Yu J, et al. Relationship between retinal perfusion and retinal thickness in healthy subjects: an Optical Coherence Tomography Angiography Study. Invest Ophthalmol Vis Sci. 2016;57(9):Oct204–10.
Jonas JB, et al. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992;33(6):2012–8.
Mwanza JC, et al. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(11):7872–9.
Yamashita T, et al. Relationship between position of peak retinal nerve fiber layer thickness and retinal arteries on sectoral retinal nerve fiber layer thickness. Invest Ophthalmol Vis Sci. 2013;54(8):5481–8.
Kanamori A, et al. Evaluation of the effect of aging on retinal nerve fiber layer thickness measured by optical coherence tomography. Ophthalmologica. 2003;217(4):273–8.
Harwerth RS, Wheat JL. Modeling the effects of aging on retinal ganglion cell density and nerve fiber layer thickness. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):305–14.
Acknowledgements
The authors would like to thank all the participants recruited for this study.
Funding
This research has not received any specific grant from any funding body in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
CH.M, CW.C and YT.Z contributed equally to the research. CH.M designed and collected clinical data. CW.C analyzed and interpreted data. CH.M and YT.Z wrote the draft of this manuscript. JY.Y reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patients described in this research. This study was approved by the ethics committee of the first hospital affiliated to Soochow University (Suzhou, China) and conducted according to the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, C., Chen, C., Zhang, Y. et al. Retinal nerve fiber layer thickness and radial peripapillary capillaries density in myopic adults with optical coherence tomography angiography. BMC Ophthalmol 24, 407 (2024). https://doi.org/10.1186/s12886-024-03673-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03673-6