Abstract
Background
Systematic or targeted screening for developmental delay (DD) is critical to the early identification of developmental disabilities. With limited available information for urban Rwandan children, this study aimed to determine the prevalence of DD and associated risk factors in infants aged 9 to 16 months living in the urban Rwandan city of Kigali.
Methods
A cross-sectional study was conducted in Rwanda from August to November 2019. A convenience sample of 376 Rwandan parents/caregivers and their children attending urban health centers for their routine immunization visits at 9 and 15 months of age was studied. Parents/caregivers completed the official Kinyarwandan version of the Ages and Stages Questionnaire (ASQ-3) and established cutoffs were used to identify DD. Frequency and percentages were used to summarise the data. Logistic regression analysis was used to identify factors associated with DD.
Results
Of the 358 children screened using the ASQ-3, the overall prevalence of DD was 24.6%, with a 27.2% prevalence among 9–10-month old children and 22.4% prevalence among 15–16-month old children. Delays in the combined group among the domains of gross motor, communication, fine motor, personal social, and problem solving were 12.8%, 2.5%, 8.4%, 1.7% and 7.5%, respectively. Gestational age at delivery and district of origin were most highly associated with DD, with preterm children at significantly higher risk of having DD compared to term children (Adjusted Odd Ratio AOR = 8.3; 95% CI = 2.5–27.4) and children from Nyarugenge District at high risk of DD compared to children from Gasabo district (AOR = 2.15; 95% CI = 1.2–3.9).
Conclusions
The prevalence of ASQ-detectable DD among urban Rwandan children between 9 and 16 months of age was 24.6%, with a high correlation to a history of prematurity and district of origin. This study demonstrates the need for thoughtful health planning regarding integrated developmental surveillance for children, particularly those at high risk, to allow for earlier identification and intervention in the urban area of Kigali, Rwanda.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Developmental delay (DD) is defined as any delay in reaching thinking, social, language, or motor development skills compared to peers from the same population [1]. Early childhood is the best time to identify, attempt to prevent adverse events, and intervene in the areas that are responsible for a child’s delay, which has potential negative consequences for the ability to function across the lifespan [2]. Many modifiable risk factors for DD have been identified and they are more prevalent in low and middle-income countries (LMICs) [3]. The World Bank’s 2018 report recognises that optimising the wellbeing of people in the early years of life is an essential component of the social and functional development of countries and it challenges partners to increase investments in early childhood programmes. Despite this endorsement, only one-quarter of the 250 million children under five have access to preschool [4, 5].
It is estimated that more than 200 million children in LMICs do not develop to their full potential [6] and these countries often have weak or absent systems of universal preventive primary care that would identify developmental delay. Meanwhile, the health and education systems in high-income countries (HICs) offer multiple opportunities to prevent, identify and manage early childhood developmental problems [7]. The capacity to duplicate such systems in LMICs is challenging. The World Health Organisation (WHO) highlights the fact that in LMIC regions the lower global health expenditure per person [8] leads to more out-of-pocket payments as the main health financing source. This naturally limits access to care for poor populations. Esmat Nemati et al., in their systematic review on out-of-pocket money payments in LMICs documented a high mean of out-of-pocket money payments of up to 67% as a percentage of households final consumption expenditure [9], in contrast to 1.3% in Turkey and 5.8% in Switzerland [10]. Additionally, there is a tremendous disparity in the healthcare provider-patient ratio, with medical doctors per 10,000 population ratios of 0.23 in LMICs and 84.27 in HICs [11]. This significant disparity in universal comprehensive preventive health care draws attention to the nature and impact of socioeconomic gradients found both within HICs and LMICs, and between nations. Children, particularly those with delays and disadvantaged by lack of access to adequate health and interventional services, perform less well at school, earn less as adults, have higher fertility rates, and are less able to economically provide for their children, leading to intergenerational poverty transmission [12, 13].
Sapna V. Kumar et al. in their systematic review evaluating home based or clinical based interventions on developmental difficulties examined parental practices in nutrition, stimulation, play and communication, documented improved cognition, language and motor development in the intervention group compared to the control group [14]. PK Maulik et al. in their systematic review, identified low-resource, effective, and simple community-based interventions targeting 0–3 years that can be implemented in the LMICs, including reading, play, tactile stimulation, and music [15]. Developmental interventions in LMICs for children identified with delays will have to rely on these lower-cost strategies, much like existing programs to combat iron deficiency and malnutrition, psychosocial stimulation training for home caregivers of high-risk infants, and establishing community rehabilitation programs [16]. The positive impact of such early childhood intervention programs on youth school performance, adult functioning, and economic power has been previously described [17]. Thus, a country like Rwanda, which declares its intention to dramatically improve the lives of its citizens, can find support in the literature to invest in early childhood programs with a high likelihood of economic return [18, 19].
Limited information on DD is available for Rwandan urban children. Two investigations have been conducted in rural Rwandan areas: one studied prior preterm children at 1–3 years of age which showed a prevalence of 67.4% [20]; the second was part of an Early Childhood Development and Family Baseline Evaluation by UNICEF, which demonstrated that children from the poorest families have higher rates of DD than children from wealthier families [21]. Therefore, this present study aimed to determine the prevalence of DD and associated risk factors among infants aged 9 to 16 months living in the urban Rwandan city of Kigali in order to provide a more representative picture of developmental delay. Our results should help to inform policymakers and program planners working in the area of child health.
Methods
Study design and period
This is a cross-sectional study of infants coming to the health center for their standard 9th and 15th month vaccinations over a period of 3 months, from August 2019 to November 2019.
Study setting
Rwanda is a lower-income developing country with a total surface area of 2630 km2 and a population of 13,246,394 in August 2022 [22]. The study took place in the most urbanised city of Rwanda, Kigali, the capital city of Rwanda, in its different health centers located in three administrative districts (Nyarugenge, Gasabo and Kicukiro), where one large health center was selected from each district except in Nyarugenge, which has many health centers, and, thus, two large health centers were selected. Data collection took place as children were brought for their routine immunizations: in Nyarugenge district this occurred at Nyiranuma and Muhima health centers, in Gasabo district at Kimironko health center, and in Kicukiro district at Kicukiro health center.
Study population
All children aged 9–10 months and 15–16 months who were brought to the selected health centers for their routine national-based childhood vaccinations at 9 and 15 months during the study period were included. Children were excluded if their family care provider declined to participate or was not present, or if they fell outside of the specific age categories selected.
Sample size and sampling procedure
There are limited available data on DD in LMICs with which to compute the sample size. One study using the same measurement tool in Rwanda documented a high prevalence of 67.4% among high-risk prior premature infants aged 1–3 years from a very rural area [20]. We chose to use a prevalence of 37% based on a study from Chile with 8 months old infants since it more likely reflects a similar general population in a LMIC country [23]. Our single population proportion sample size calculation used a prevalence of 37%, a 95% confidence interval, and a 5% margin of error [24] resulting in an initial sample size of 358 which ,estimating a 5% non-response rate, was adjusted to the final sample size of 376.
Since the vast majority of Rwandan families (> 95%) bring their children to government health centers for their vaccinations, we surmised that we could capture a broad representation of families across the economic strata by conducting the sampling in the major health centers distributed around the greater Kigali metropolitan area.
Variables
The outcome variable of interest in this study is developmental delay, which is defined as any single developmental domain (communication: child’s verbal and non verbal communication skills; gross motor: child’s ability to stand, walk and run; fine motor: child’s hands and finger movement; problem solving: child’s ability to play with toys and solve problems; and personal social skills: child’s self help skills and interactions with others) score falling below the ASQ-3 standardised lower cutoff points [25].
The ASQ-3 is a standardized screening tool used throughout the world: it consists of 30 age-specific questions in five domains that is completed by caregivers and resulting in a score reflecting the child’s status of being either on-track, needing monitoring due to potential concern (up to 1 standard deviation below the mean), or below cutoff, which is indicative of need for professional evaluation due to the high possibility of disability based on scores two or more standard deviations below the mean. In this study, children in the on-track and monitoring groups are considered normally developing while those below the cutoff are defined as having developmental delay [25].
Explanatory variables include Economic Categories I, II, and III which are Rwandan specific levels of household income and economic status (ubudehe category), where those in Level I earn less than those in Level III [29]. A caregiver is considered married if the child’s parent live with the partner, and single if the parent lives alone with the child. Children coming from Kigali city had their district of origin recorded, while children from outside Kigali have been classified in the “other” category.
The continuous variables of height, current weight, and head circumference have been measured on the day of DD screening by the data collectors and plotted on the standard WHO growth charts. The terms stunted or microcephalic refer to measurements with less than 2 standard deviations below the mean for age and sex [26]. We use the WHO definition of low birth weight (LBW) as a birth weight less than 2.5 kg, and preterm as a delivery at gestational age (GA) less than 37 weeks. The mother’s age in this study was the age of the mother on the day of the screening.
Data collection process
Developmental data were collected using an official Kinyarwanda translation of the ASQ-3. The tool has gone through a validation process with the publisher to adapt the screening tool to Rwanda, and it has been purchased from Brookes Publishing Co., Inc. There are 21 age-specific ASQ-3 forms, and in this study, the 10 month form was used with 9–10 months old children and the 16 month form with those 15–16 months old. The questions were completed by parents upon entry to the health center and after consent was obtained, assisted by data collectors and the PI if a parent requested help, and were analysed immediately by the PI and entered into the database.
Anthropometric measures of weight, length, head circumference and middle upper arm circumference (MUAC) as well as the demographic and medical information were conducted by three trained data collectors and entered into the Google form database by the PI. Parents of children with suspected developmental delay or other medical comorbidities who might benefit from a pediatric visit were referred from the health center to pediatric consultants at the local district hospital.
Data processing and analysis
Data collected on Google forms were migrated to EpiData V3.1 and then exported to SPSS 25 (IBM, New York, United States) for analysis. Descriptive analysis and categorical data are presented using frequency and percentages. Binary logistic regression was used to study the association between the outcome and possible risk factors or predictors. Factors with a p-value < 0.2 have been included in a multivariable logistic regression analysis to minimize confounding factors and to determine more accurate levels of association with developmental delay. Crude and AOR with a 95% confidence interval (CI) were computed. Multicollinearity and the Hosmer-Lemeshow test for model fitness have been performed after multivariable logistic regression and the Mean VIF (variance inflation factor) was 1.18 with a non-statistically significant p-value of 0.490 after the goodness-of-fit test. A p-value less than 0.05 was considered statistically significant.
Data quality assurance
The principle investigator, a paediatric resident, provided training to the data collectors and monitored the data entry daily. The questionnaire and methodology were successfully pre-tested on 19 mother/infant pairs at the Nyiranuma Health Center, with no need to modify the process or further train the collectors. The ASQ-3 in Kinyarwanda is the copywritten, official and tested version purchased from Brookes [20].
Results
376 mothers/family care givers and their children were invited to participate in this study, and 358 adequately completed ASQ forms were analysed (Fig. 1).
A slight majority of children were male in both age groups, with 84 (51.5%) at 9–10 and 101 (51.5%) at 15–16 months. A majority of participants had a normal birthweight ≥ 2.5 kg with 146 (90.1%) at 9–10 and 185 (94.4%) at 15–16 months (Table 1).
Table 2 demonstrates that the majority of mothers were married 263 (73.5%), half of them were interviewed at the single Gasabo District center 188 (52.5%), almost all 321 (89.7%) had medical insurance, half were in the Rwandan middle class (economic category II) 190 (53.1%), a minority 37 (10.3%) had university degrees, very few were HIV positive 15 (4.2%) or smokers 3 (0.8%), and 37 (10%) used an undefined amount of alcohol at some time in pregnancy.
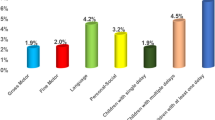
In the combined group of 358 children, the percentage of children with suspected DD in any one of the five domains varied between a low of 1.7% for personal social and 12.8% for gross motor. There existed a greater spread in those screening at-risk at 9 months (1.9–20.4%) compared to the older 15 month cohort (1.5–11.2%), reflecting the rapid improvement in gross motor skills (20–12.8%). The problem-solving domain was the most challenging for the older children at 11.2%, while communication scores were consistent between the groups (Table 3). Some children scored below the cut-off in multiple areas, and the overall prevalence of infants falling into the below-cutoff category in one or more domains was 27.2% at 9 months and 22.4% at 15 months with an overall prevalence of 24.6%, 95% CI (20.1, 29.1) (Fig. 2).
Bivariate analysis demonstrates a strong association between suspected DD and three variables. Prior preterm infants were more likely to screen at high risk of having DD compared to term infants (OR = 8.52; 95% CI = 2.60–27.90); prior low birth weight infants of < 2.5 kg were at higher risk for DD compared with normal birth weight children (OR = 2.68; 95% CI = 1.20–5.99); and children from Nyarugenge district had an increased risk of developmental delay compared to children from Gasabo district (OR = 2.19; 95% CI = 1.24–3.90). Interestingly, there was no demonstrated association between prenatal alcohol exposure and delay (Table 4).
Multivariable analysis (Table 5) of the variables that showed p < 0.2 significance in the binary logistic regression (birth weight, gestational age at delivery, current weight, marital status of the mother and district of origin) resulted in gestational age and district of origin being factors associated with DD. Consistent with the literature, preterm children were at higher risk of being screened below the cutoff compared to term children (AOR = 8.60; 95% CI = 2.70–28.50). Children from Nyarugenge District have an increased risk of DD compared to children from Gasabo district (OR: 2.15; 95% CI = 1.20–3.87).
Discussion
This study successfully screened young children from three districts of the capital city of Rwanda and our data reveal that 24.6% (95% CI = 20.1–29.1) of children between 9 and 16 months of age screened positive for DD in at least one of the five critical areas of development examined. This 24.6% prevalence is lower than the 2019 Rwandan demographic health survey prevalence of 33% among urban Rwandan children aged 3–5 years old using a different tool and geographic settings [27]. It is significantly lower than the 67.4% prevalence using the same ASQ-3 tool with children at high risk of DD between 1 and 3 years old who were ex-premature babies in a rural Rwandan Neonatology Intensive Care Unit follow-up clinic [20]. There are few comparable studies in Sub-Saharan Africa: this Rwandan prevalence is lower than the 44.6% in rural Ghanaian children under 5 years of age, and higher than the 11.7% in a rural Malawian preschool setting using different screening tools [28,29,30].
Similar studies have been conducted on other continents and our 24.6% is far lower than the 51% found among 4–24-month Indian children, where 40% of them were identified as high risk [31]. A comparable prevalence of 28.8% was found among 9–30 month Chilean children using a similar screening tool [23].
The subcategories of delay, or domains, are also important to consider. In our combined cohort, the prevalence of delay in different domains ranged between1.7% and 12.8%. A 2014 UNICEF baseline evaluation that studied rural Rwandan children as part of an early child development and family service (ECD + F) study demonstrated delays of 10–35% in a younger 0–11 month cohort and 13–33% in an older 24–45 month cohort. This higher prevalence and range compared to our urban study participants may reflect the family conflict, poverty, and higher proportion of illiterate primary care providers that was noted in their study population [21].
There is a striking similarity between our urban cohort and the rural UNICEF ECD + F group regarding rapid improvement of gross motor skills as children age: 20.4% of our younger cohort fell below the gross motor cutoff and that dropped to 6.6% in the older group. A similar large improvement in motor skills was seen in the rural ECD + F children and may reflect the fact that younger Rwandan children are carried alomost constantly up to 9 or 10 months of age and then strongly encouraged to stand and walk.
Problem solving (11.2%) was the domain that challenged the majority of our older age group. Interestingly, communication scores were very similar at both ages and are similar to a South African study in which young infants scored equally well as children in western countries. The South African authors suggested that it is due to the day-to-day practices of African mothers who carry their babies on the mother’s body, feeding on demand, immediately pacifying babies when they cry, co-sleeping, and the exposure of babies to a wide social network of kin and family [32]. Likewise, similar findings were reported in an Iranian study where their children scored well compared to children in HIC in the areas of communication and problem solving during the first 3 years of life [33].
Two factors emerge in this study as prominent predictors of falling below the ASQ-3 cutoff and indicative of delay. The first is prematurity and is consistent with findings in Rwanda, Chile, Canada, and Norway and is proposed to be due to stresses on the immature brain, perinatal risk factors, and high susceptibility to environmental exposures [34, 20, 35,36,37]. These findings lend urgency to the importance of implementing screening and intervention programs while concurrently improving neonatal services in Rwanda.
The second predictive factor is place of residence, with our study highlighting the difference in levels of developmental delay between children from different parts of the city. Further study is needed to elucidate which factors contribute to the children in Nyarugenge screening for delay at twice the rate of those in Gasabo: The Rwandan demographic and health survey hints at greater numbers of less educated adults, more infant stunting, higher teenage pregnancy, and less insurance coverage in Nyarugenge compared to other neighbouring districts [38]. In contrast to the strong association with prematurity, birth weight did not appear to be associated with delay after multivariate analysis, which surprised us since a systematic review of African children had earlier made that association [39].
Limitation of the study
Rwanda is a largely rural country and these results, generated from studying urban children, many of whom come from families in the middle economic level, may not be generalizable to the broader population. Caregiver recall of gestational age at delivery and birth weight is not consistent as evidenced by some mothers referring to the vaccination card in order to remind themselves of the birth weight. We do not feel that a data collector helping a caregiver to read and understand questions is a limitation since that occurred in the development of the ASQ-3 and is employed as a routine in many clinical settings when there are literacy barriers. Assigning the label of developmental delay based on a single screening is not something that the ASQ-3 user’s guide endorses but for the purposes of this study it provides us a rough estimate and not a clinically accurate diagnosis.
Study strength
Regardless of the limitations, this study contributes valuable data and methodology for health planners in Rwanda and neighbouring East African nations by utilising an internationally validated tool that has been used around the world.
Conclusion
This study demonstrates a strong association between prematurity and poorer districts in the Kigali metropolitan area with developmental delay in 9–16-month urban Rwandan children. Improvements in neonatal care throughout Rwanda and other LMICs must be paired with improved developmental surveillance and interventions for the greater number of higher risk children surviving. Further work will be needed to identify which factors in Kigali’s poorer districts lead to increased rates of delay so that scarce resources are prioritised where they are most needed. This study demonstrates that the need exists and that it will take effort to devise even targeted intervention programs that are culturally appropriate and economically feasible and respond to the aforementioned call to action by the World Bank.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASQ-3:
-
Ages and Stages Questionnaire
- CMHS IRB:
-
College of Medicine and Health Sciences Institutional Review Board
- RDHS:
-
Rwanda Demographic Health Survay
- DD:
-
Developmental Delay
- UNICEF:
-
United Nations Children’s Fund
References
Khan I, Bennett, Leventhal L, Affiliations. Developmental Delay. In 2021.
Daelmans B, Black MM, Lombardi J, Lucas J, Richter L, Silver K et al. Effective interventions and strategies for improving early child development. BMJ. 2015;h4029.
Ertem IO, World Health Organization. Developmental difficulties in early childhood: prevention, early identification, assessment and intervention in low- and middle-income countries: a review [Internet]. Geneva: World Health Organization; 2012. 112 p. Available from: https://apps.who.int/iris/handle/10665/97942.
World bank. The world bank annual report 2018. available at: https://documents1.worldbank.org/curated/en/630671538158537244/pdf/The-World-Bank-Annual-Report-2018.pdf. accessed in 2021.
World Bank. World Bank Support to Early Childhood Development. 2015;194.
Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, et al. Developmental potential in the first 5 years for children in developing countries. Lancet Lond Engl. 2007;369(9555):60–70.
Canadian T. Recommendations on screening for developmental delay. 2016;188(8):579–87.
World Health Organization. Global Health Expenditure Database [Internet]. Available from: https://apps.who.int/nha/database.
Nemati E, Nosratnejad S, Doshmangir L, Zarea V. The out of pocket payments in low and middle-income countries and the affecting factors: a systematic review and meta-analysis. Bali Med J. 2019;8:733.
Organization for Economic Cooperation and Development(OECD). Out-of-pocket spending as share of final household consumption, 2019 (or nearest year) [Internet]. Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2021_ae3016b9-en.
World Health Organization. Medical doctors (per 10 000 population) [Internet]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/medical-doctors-%28per-10-000-population%29.
Grantham-McGregor S, Cheung YB, Cueto S, et al. Series, child development in developing countries. Developmental potential in the first 5 years for children in developing countries. Child Care Health Dev. 2007;33(4):502–2.
Lu C, Cuartas J, Fink G, Mccoy D, Liu K, Li Z et al. Inequalities in early childhood care and income development in low / middle- countries: 2010–2018. 2020;2010–8.
Kumar SV, Narayan S, Malo PK, Bhaskarapillai B, Thippeswamy H, Desai G, et al. A systematic review and meta-analysis of early childhood intervention programs for developmental difficulties in low-and-middle-income countries. Asian J Psychiatry. 2022;70:103026.
Maulik PK, Darmstadt GL. Community-based interventions to optimize early childhood development in low resource settings. J Perinatol. 2009;29(8):531–42.
Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E et al. Child development in developing countries 2 child development: risk factors for adverse outcomes in developing countries. 2007;145–57.
World Bank. Later Impacts of Early Childhood Interventions: A Systematic Review [Internet]. Available from: https://ieg.worldbankgroup.org/evaluations/later-impacts-early-childhood-interventions.
Heckman JJ, Masterov DV. The Productivity Argument for Investing in Young Children.
Shonkoff J, Richter L, Gaag J, Bhutta Z. An Integrated Scientific Framework for child survival and early Childhood Development. Pediatrics. 2012;129:e460–72.
Kirk CM, et al. Health, nutrition, and development of children born preterm and low birth weight in rural Rwanda: a cross-sectional study. BMC Pediatr. 2017;17(1):1–9.
Kirk C, Rwanda U. Early Childhood Development and Family Services: Baseline Evaluation in 20 Sites in Rwanda. 2016.
National Institute of Statistics of Rwanda(NISR). Rwanda census 2022 pdf [Internet]. Available from: https://statistics.gov.rw/datasource/171.
Schonhaut L, Armijo I, Schonstedt M, Alvarez J, Cordero M. Validity of the Ages and Stages Questionnaires in Term and Preterm Infants. Pediatrics. 2013;131(5):e1468–74.
Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. 2013;6(1):14–7.
ASQ-For-Parents. -Packet.pdf [Internet]. [cited 2023 Sep 3]. Available from: https://agesandstages.com/wp-content/uploads/2018/12/ASQ-For-Parents-Packet.pdf.
The WHO Child Growth Standards [Internet]. Available from: https://www.who.int/tools/child-growth-standards.
National Institute of Statistics of Rwanda. In: Program RDHS, editor. Rwanda demographic and health survey, 2014-15: final report. Kigali, Rwanda: Rockville, Maryland, USA: National Institute of Statistics of Rwanda, Ministry of Finance and Economic Planning. Ministry of Health ; The DHS Program, ICF International; 2016. p. 615.
Murphy R, Jolley E, Lynch P, Mankhwazi M, Mbukwa Ngwira J, Bechange S et al. Estimated prevalence of disability and developmental delay among pre-school children in rural Malawi: findings from ‘Tikule Limodzi’, a cross‐sectional survey. Child Care Health Dev. 2020;46.
Bello AI, Quartey JNA, Appiah LA. Screening for developmental delay among children attending a rural community welfare clinic in Ghana. 2013.
Gladstone M, Lancaster GA, Umar E, Nyirenda M, Kayira E, van den Broek NR, World Health Organization. Ertem IO,. Developmental difficulties in early childhood: prevention, early identification, assessment and intervention in low- and middle-income. PLoS Med. 2010;7(5):e1000273.
Juneja M, Mohanty M, Jain R, Ramji S. Ages and Stages Questionnaire as a screening tool for developmental delay in indian children. Indian Pediatr. 2012;49(6):457–61.
Heerden A, Van, Hsiao C, Matafwali B, Louw J, Richter L. Support for the feasibility of the ages and stages questionnaire as a developmental screening tool: a cross-sectional study of South African and Zambian children aged 2–60 months. 2017;1–9.
Vameghi R, Sajedi F, Mojembari AK. Cross-cultural adaptation, validation and standardization of Ages and Stages Questionnaire (ASQ) in iranian children. 2013;42(5):522–8.
Hee Chung E, Chou J, Brown KA. Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl Pediatr. 2020;9(Suppl 1):3–8.
Schonhaut L, Armijo I, Pérez M. Gestational Age and Developmental Risk in Moderately and Late Preterm and Early Term Infants. 2020;135(4).
TROUDE, Pénélope SQUIRES, Jane L’HELIAS. Ages and Stages Questionnaires: feasibility of postal surveys for child follow-up. Early Hum Dev. 2011;87(10):671–6.
Valla L, Wentzel-larsen T, Hofoss D, Slinning K. Prevalence of suspected developmental delays in early infancy: results from a regional population-based longitudinal study. BMC Pediatr. 2015;1–8.
National Institute of Statistics Rwanda. Demographic and Health Survey. (2019/20) [Internet]. Available from: https://www.statistics.gov.rw/datasource/demographic-and-health-survey-201920.
Tchamo ME, Prista A, Leandro CG. Low birth weight, very low birth weight and extremely low birth weight in african children aged between 0 and 5 years old: a systematic review. J Dev Orig Health Dis. 2016;7(4):408–15.
Acknowledgements
We acknowledge the help from Health Center staff and thank the parents who remained long after their child got their immunizations. Gratitude to Dr. C Marquez for teaching us the use of Google forms.
Funding
The authors didn’t receive any financial support.
Author information
Authors and Affiliations
Contributions
Victoire Tuyisenge: conceptualized and designed the study, carried out the literature search, oversaw the research proposal, data collection and analysis, wrote the initial draft of the manuscript and reviewed and revised the final manuscript.Cliff O’Callahan: conceptualized and designed the study, carried out the literature search, oversaw data analysis, wrote the initial draft of the manuscript and revised the final drafts of the manuscript.Febronie Mushimiyomana: conceptualized and designed the study, oversaw data analysis, revised the initial and final manuscript.Aimable Kanyamuhunga: Oversaw data analysis, reviewed multiple drafts of the original Master’s thesis and revised and reviewed the initial and final manuscript.Jean Paul Rukabyarwema: reviewed multiple drafts of the original Master’s thesis and later versions of the publication manuscript.Archana A. Patel: Oversaw the data analysis, reviewed multiple drafts of the original Master’s thesis and later versions of the publication manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical consideration
This study hasbeen performed according to the 1964 declaration of Helsinki. Ethical clearance and approval was obtained from the Ethical Scientific Committee of the University of Rwanda (IRB: No 365/CMHS IRB/2019). Permission was purchased to use the ASQ-3 assessment tool from the publisher. Official letters were written to each respective health facilities and permission to conduct the study was obtained from the responsible authorities. Written informed consent was obtained from the mothers/caretakers and confidentially was ensured. Respondents had the right to not participate in, or withdraw from the study at any stage, and subjects did not receive any incentive for this study.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tuyisenge, V., Mushimiyimana, F., Kanyamuhunga, A. et al. Screening for developmental delay in urban Rwandan children: a cross sectional study. BMC Pediatr 23, 522 (2023). https://doi.org/10.1186/s12887-023-04332-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04332-3