Abstract
Background
Bronchopulmonary dysplasia (BPD) is a major complication affecting the survival rate and long-term outcomes of preterm infants. A large, prospective, multicenter cohort study was conducted to evaluate early nutritional support during the first week of life for preterm infants with a gestational age < 32 weeks and to verify nutritional risk factors related to BPD development.
Methods
A prospective multicenter cohort study of very preterm infants was conducted in 40 tertiary neonatal intensive care units across mainland China between January 1, 2020, and December 31, 2021. Preterm infants who were born at a gestational age < 32 weeks, < 72 h after birth and had a respiratory score > 4 were enrolled. Antenatal and postnatal information focusing on nutritional parameters was collected through medical systems. Statistical analyses were also performed to identify BPD risk factors.
Results
The primary outcomes were BPD and severity at 36 weeks postmenstrual age. A total of 1410 preterm infants were enrolled in this study. After applying the exclusion criteria, the remaining 1286 infants were included in this analysis; 614 (47.7%) infants were in the BPD group, and 672 (52.3%) were in the non-BPD group. In multivariate logistic regression model, the following six factors were identified of BPD: birth weight (OR 0.99, 95% CI 0.99–0.99; p = 0.039), day of full enteral nutrition (OR 1.03, 95% CI 1.02–1.04; p < 0.001), parenteral protein > 3.5 g/kg/d during the first week (OR 1.65, 95% CI 1.25–2.17; p < 0.001), feeding type (formula: OR 3.48, 95% CI 2.21–5.49; p < 0.001, mixed feed: OR 1.92, 95% CI 1.36–2.70; p < 0.001; breast milk as reference), hsPDA (OR 1.98, 95% CI 1.44–2.73; p < 0.001), and EUGR ats 36 weeks (OR 1.40, 95% CI 1.02–1.91; p = 0.035).
Conclusions
A longer duration to achieve full enteral nutrition in very preterm infants was associated with increased BPD development. Breastfeeding was demonstrated to have a protective effect against BPD. Early and rapidly progressive enteral nutrition and breastfeeding should be promoted in very preterm infants.
Trial registration
The trial was registered in the Chinese Clinical Trial Registry (No. ChiCTR2000030125 on 24/02/2020) and in www.ncrcch.org (No. ISRCTN84167642 on 25/02/2020).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Due to advancements in perinatal and neonatal care in China, more preterm newborns are receiving treatment and surviving. However, the lung development of preterm infants is susceptible to various injuries, resulting in bronchopulmonary dysplasia (BPD) [1]. The prevalence of BPD in China has risen over the last several decades [2, 3].
BPD is one of the most prevalent and complicated infant sequelae and places a significant burden on newborns, families, and society. Moderate/severe BPD is associated with short- and long-term serious growth restrictions, impaired cardiorespiratory function, and neurodevelopmental disability [4]. The etiology of BPD is multifactorial and is frequently associated with several genetic, prenatal, and postnatal variables modulating lung development and the response to local and systemic inflammation.
Nutrition is crucial for infants with BPD, as sick newborns expend large amounts of energy to compensate for their high metabolic demands and increased work in breathing [5]. Additionally, malnutrition impacts lung development, and early inadequate caloric intake during life is associated with the development of BPD [6].
Therefore, we conducted a multicenter cohort study to evaluate the risk factors for nutritional status during the first week after the progression of BPD in preterm infants with a gestational age (GA) < 32 weeks, < 72 h after birth and a respiratory score > 4.
Methods
Study design and patients
We performed a prospective multicenter cohort study from January 1, 2020, to December 31, 2021. Forty tertiary referral neonatal intensive care units (NICUs) from 21 provinces and cities across mainland China participated in this observational study. Preterm infants with a GA < 32 weeks, < 72 h after birth and a respiratory distress score > 4 at admission were enrolled. This score, evaluated according to the Acute Care of At-Risk Newborns Respiratory Distress Score System, was the sum of the 6 individual scores and is useful for tracking the severity of respiratory distress over time in a baby who is breathing spontaneously (Table 1) [7]. The respiratory score was collapsed into three levels: mild, 0 to 4; moderate, 5 to 8; and severe, > 8 or intubated at admission.
A brief diagnosis can be obtained during physical examination at admission, when there is no blood test requirement, and when there is a greater focus on respiratory diseases [8]. Furthermore, we observed that preterm infants whose scores were less than 4 throughout the first 72 h of life were nearly always normal and rarely progressed to BPD from a clinical standpoint. Therefore, we hypothesized that nutritional support during the first week was associated with the progression of BPD in very preterm infants with a score greater than 4.
The exclusion criteria were major congenital anomalies, including serious congenital heart defects, chromosomal abnormalities, brain malformations, congenital diaphragmatic hernias, digestive tract or kidney anomalies and inborn errors of metabolism or severe heritable disease.
The dropout criterion included death within 14 days after birth or discontinuation of treatment within 36 weeks postmenstrual age (PMA).
The study was approved by the Children Hospital Ethics Committee, Zhejiang University School of Medicine Ethical (2019-IRB-164) on December 24, 2019. Written informed consent was obtained from parents or guardians antenatally or upon NICU admission. The trial was registered in the Chinese Clinical Trial Registry (No. ChiCTR2000030125 on 24/02/2020) and at www.ncrcch.org (No. ISRCTN84167642 on 25/02/2020).
Definitions of the relevant concepts
BPD was diagnosed and graded according to the 2018 National Institute of Child Health and Human Development (NICHD) consensus [9]. Extrauterine growth retardation (EUGR) was described according to the Fenton intrauterine growth curves as infants whose weight was less than the 10th percentile for corrected gestational age [10]. Intrauterine growth retardation (IUGR) was defined as a birth weight below the 10th percentile for gestational age [11]. Hemodynamically significant patent ductus arteriosus (hsPDA) was characterized by a ductus diameter exceeding 1.5 mm, a left atrial inner diameter/aortic root (LA/AO) exceeding 1.4, or a combined left-to-right shunt, as determined by echocardiography. Necrotizing enterocolitis (NEC) was diagnosed based on the modified Bell staging system, clinical features, and radiological evidence with a Bell stage greater than 2 [12].
Data collection
Maternal, perinatal, and neonatal data were obtained from family interviews and from the medical charts or electronic systems of the enrolled infants. Antenatal and neonatal characteristics, including gestational age (GA) at delivery and birth weight (BW), sex, delivery mode, 5-minute Apgar score, intrauterine growth retardation, pregnancy-induced hypertension, maternal diabetes, and premature rupture of membranes, were collected. The following neonatal outcomes and morbidity data were also recorded: respiratory distress scores, invasive mechanical ventilation duration, surfactant replacement, necrotizing enterocolitis, hsPDA, prognosis, EUGR at 36 weeks, and BPD.
Our study focused on nutritional strategies and their related outcomes. Therefore, nutritional data were also collected prospectively, including whether the total fluid intake was > 150 ml/kg/d during the first week, whether the parenteral amino acid intake was > 3.5 g/kg/d, or whether the total energy intake was > 100 kcal/kg/d. Additionally, weight change, the day weight recovered to the birth weight, and when weight loss after birth exceeded 10% birth weight were recorded. In addition to parenteral nutrition (PN), enteral nutrition (EN) is crucial for preterm infants. We focused on the day that the total EN was reached and the feeding type, including exclusive breastmilk, formula, breastmilk > 50%, or exclusive feeding. Additionally, EUGR data at 36 weeks were acquired.
All infants were divided into two groups according to whether their parenteral AA concentration was > 3.5 g/kg/d during the first week compared with the days at which full enteral nutrition was achieved. Additionally, infants were divided into subgroups according to the day at which full EN was reached: 14–28 days, 28–42 days, and > 42 days, compared with the rate of parenteral AA > 3.5 g/kg/d.
All the data were entered into the Resman research manager database of the ChiCTR for subsequent analysis. Only study members had permission to access the database, and a specialist kept all the data to protect patient privacy.
Outcomes
To accomplish an analysis according to the principle of intention to identify risk factors, we defined our primary outcome as BPD at 36 weeks postmenstrual age (PMA). Participants were assessed at 36 ± 1 weeks’ PMA and BPD severity, classified as the 2018 National Institutes of Health Group (NICHD) Workshop, based on modes of respiratory support grades [8]. The secondary outcomes were complications and risk factors for nutritional support during the first week associated with BPD occurrence.
Statistical analysis
First, we conducted a descriptive analysis. For continuous variables, normality was checked. Variables without a normal distribution are presented as medians (interquartile ranges [IQRs]). Categorical variables are presented as numbers and percentages. Second, for the continuous variables, we applied one-way ANOVA to compare the differences across subgroups, and for the categorical variables, we used chi-square tests to compare the differences across subgroups. To further clarify the difference between two groups, we conducted post hoc analysis via one-way ANOVA and chi-square tests. Third, a binary logistic regression model was used to analyze the associations between total fluid intake > 150 ml/kg/d, parenteral amino acid intake > 3.5 g/kg/d, total energy intake > 100 kcal/kg/d during the first week, feeding type, total enteral nutrition day, gestational age, birth weight, hsPDA and the occurrence of BPD.
All the analyses were conducted using SPSS software version 20.0. p < 0.05 indicated statistical significance.
Results
Demographic characteristics and primary outcome
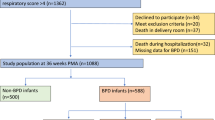
A total of 1410 preterm infants met the inclusion criteria between January 1, 2020, and December 31, 2021. Of these, 124 were excluded according to the exclusion criteria; therefore, 1286 infants were ultimately enrolled in the study. There were 614 preterm infants diagnosed with BPD, and the incidence of BPD in the study population was 47.7% (Fig. 1). Six infants (0.47%) who died before 36 PMA were diagnosed with grade IIIA BPD. Consequently, 345 (56.2%), 174 (28.3%), and 89 (14.5%) infants were diagnosed with grade I, II, and III BPD, respectively. Table 2 shows the demographic and clinical characteristics of infants with BPD and without BPD.
As expected, infants with BPD had a lower gestational age and birth weight (P < 0.001, P < 0.001). Compared to infants without BPD, those with BPD had lower Apgar scores at 5 min. Additionally, hsPDA and NEC were more common among infants with BPD (P < 0.001, P < 0.001). Moreover, infants with BPD had higher respiratory distress scores, more ventilator days, longer hospital stays, and more frequent ventilation use (Table 2). There was no significant difference in maternal history of hypertension or diabetes between infants with BPD and those without BPD. Clinical and demographic characteristics were compared between the BPD subgroup and the non-BPD subgroup and are presented in Supplemental Table 1.
Patterns of nutritional attention
The total energy proportion reaching 100 kcal/kg/d during the first week was lower in the BPD subgroup than in the non-BPD subgroup (P < 0.001) and was lowest in the grade III BPD subgroup. The weight recovery days were significantly longer in the BPD subgroup, especially among those with grade III BPD (P = 0.024). Our study also focused on parenteral protein intake during the first week. The proportions of patients with parenteral AA > 3.5 gram/kg/d differed among the BPD subgroup and the non-BPD subgroup (P < 0.001). The percentages of patients in the BPD I, II and III groups were 62.9%, 71.3% and 52.8%, respectively, which were greater than those in the non-BPD group (Table 3).
Additionally, PN and EN were compared in all infants. The median duration (IQR) for infants without BPD who achieved full EN was 21 (13,32) days, while the average durations for those with BPD grades I, II, and III were 27 (21,42), 30 (21,44), and 45 (29,55) days, respectively. A longer duration was needed to establish full EN for infants with BPD and for those with increased severity.
Among the preterm infants, 735 (57.2%) achieved parenteral AA > 3.5 g/kg/d during the first week, while 551 (42.8%) did not. The duration of full EN achieved was significantly longer in the > 3.5 g/kg/d group during the first week than in the group that did not (28 days vs. 24 days, P < 0.001).
Additionally, infants were divided into groups according to the day at which full EN was reached: 14–28 days, 28–42 days, and > 42 days. The prevalence of parenteral AA > 3.5 g/kg/d during the first week was compared among the non-BPD subgroup and three BPD subgroups. Regardless of the full EN exposure period, the percentage of infants with parenteral AA > 3.5 g/kg/d was much lower among infants without BPD than among those with BPD grade I or II. However, this rate was significantly lower among infants with grade III BPD than among those without BPD, as the duration of full EN was longer (Fig. 2).
Our data demonstrated that feeding type differed among infants with and without BPD. The proportion of exclusively breast-milk-fed infants was 23.5% in the non-BPD group, which was the highest (P < 0.001).
The prevalence of IUGR did not significantly differ between infants with BPD and those without BPD; however, the prevalence of EUGR at 36 weeks PMA was significantly greater in the BPD subgroup than in the non-BPD subgroup. Moreover, the incidence of EUGR increased with BPD severity and was 25.4%, 35.1%, and 54.5% in the BPD I, II, and III; groups, respectively (Table 3).
Conversely, the proportion of infants exceeding 150 ml/kg/d fluid intake was similar among infants with and without BPD. Similarly, weight loss was not significantly different among infants. The results of the comparison of nutritional data between each BPD subgroup and the non-BPD subgroup are presented in Supplemental Table 2.
Nutrition predictor variables for BPD development and secondary outcomes
Variables that were significantly different (at a level of p < 0.05) were used as predictors of BPD severity according to the multivariate logistic regression model. These variables included birthweight, gestational age, hsPDA, day of weight recovery to BW, day of full enteral nutrition, total fluid > 150 ml/kg/d during the first week, parenteral protein > 3.5 g/kg/d during the first week, total energy reaching 100 kcal/kg/d during the first week, feeding type, and EUGR at 36 weeks (PMA). The following six factors were associated with BPD according to odds ratios (ORs):
The inclusion criteria for participants were as follows: birth weight (OR 0.99, 95% CI 0.99–0.99; p = 0.039); day of full enteral nutrition (OR 1.03, 95% CI 1.02–1.04; p < 0.001); parenteral protein > 3.5 g/kg/d during the first week (OR 1.65, 95% CI 1.25–2.17; p < 0.001); feeding type (formula: OR 3.48, 95% CI 2.21–5.49; p < 0.001; mixed feed: OR 1.92, 95% CI 1.36–2.70; p < 0.001; breast milk as reference); hsPDA (OR 1.98, 95% CI 1.44–2.73; p < 0.001); and EUGR at 36 weeks (OR 1.40, 95% CI 1.02–1.91; p = 0.035) (Table 4).
Discussion
Our results demonstrated that nutritional support was associated with the progression of BPD in infants aged < 32 weeks with respiratory distress. A duration of full enteral nutrition was related to BPD occurrence. These findings are consistent with those of previous studies. Retrospective studies by Wemhöner et al. [13] and Uberos et al. [14] revealed that preterm infants who developed BPD received less enteral feed. According to a recent study by Lin et al. [15], a 10% increase in the enteral feeding/total fluid intake ratio dramatically decreased the incidence of BPD by 55.6% during the second week.
Regarding the feed type, compared with an exclusive formula diet and mixed feed, our study suggested that exclusive breastfeeding protected against BPD among very preterm infants. A range of nutrients and physiologically active compounds found in human milk may be crucial for preventing BPD, as they assist neonates in developing their innate immune and antibacterial capabilities [16, 17]. Breast milk contains high tocopherol, carotene, and other antioxidant levels that help preterm infants with oxidative stress and lower their BPD development risk [18, 19].
The relationship between breastfeeding and BPD has also been largely investigated. A systematic review [20] of 17 cohorts and five randomized control trials involving 8661 preterm infants revealed that both exclusive and partial human milk feeding appeared to be associated with a lower BPD risk in preterm infants. A meta-analysis revealed that maternal milk (MOM) may reduce the incidence of BPD [21]; similarly, donor human milk (DHM) has a similar effect as formula [22]. Recent research has indicated that breast milk is not only associated with short-term complications such as BPD but also improves lung function in the long-term. Lavizzari A et al. reported that preterm infants born to ELBW mothers with higher consumption of HMs had significantly less airway obstruction during the first 2 years [23].
Our data showed that hsPDA was associated with an increased risk of BPD, similar to longer full enteral nutrition. hsPDA was a common complication in preterm infants born at < 32 weeks of gestation. A large left-to-right PDA shunt significantly increases the risk of developing BPD or death, as shown in previous studies [24]. When hsPDA is present, the blood flow “steal” from the descending aorta to pulmonary arteries may exceed the physiological compensatory mechanisms aimed at increasing left cardiac output, with subsequent reductions in end-organ perfusion, such as through the gastrointestinal (GI) tract. The development of gastrointestinal complications in preterm infants with hsPDA is a common fear among neonatologists and represents a major challenge for the management of enteral feeds in this delicate population [25]. The presence of hsPDA was associated with a significant delay in enteral feeding introduction and a significantly greater frequency of full enteral feeding (OR 2.80, 95% CI 1.35–5.81; p = 0.006) [26]. The main pharmacological agents used for PDA closure, such as indomethacin, ibuprofen, and paracetamol, also have possible adverse effects on the GI tract, which may influence enteral feeding [27]. Likewise, for preterm infants receiving PDA ligation, full enteral feeding achievement was greatly delayed [28]. Thus, hsPDA may influence the establishment of enteral nutrition, and a longer duration is needed to achieve full enteral nutrition. However, hsPAD and longer full enteral nutrition increased the risk of BPD development.
As nutritional support during the first week of life for very preterm infants, both enteral and parenteral nutrition are crucial and interrelated. Our data showed that infants without BPD reached full EN earlier and that their rate of reaching parenteral protein was lower during the first week. In contrast, the percentage of infants with parenteral protein > 3.5 g/kg/d was greater in infants with BPD due to delayed enteral nutrition. Thus, a parenteral protein concentration > 3.5 g/kg/d was associated with BPD development and was the reason for the longer duration of full enteral nutrition. It was recommended that the amino acid supply in parenteral nutrition start on the first postnatal day at least 1.5 g/kg/d to achieve an anabolic state and between 2.5 g/kg/d and 3.5 g/kg/d and should be accompanied by nonprotein intake > 65 kcal/kg/d and adequate micronutrient intake beginning on day 2 of life according to the ESPGHAN/ESPEN/ESPR guidelines. It has also been shown that parenteral amino acid intake above 3.5 g/kg/d should be administered only as part of clinical trials [29]. In addition to the amount of parenteral protein, it is also necessary to consider the nonprotein energy and the energy–nitrogen ratio. This topic requires further study.
Our results demonstrated that infants with BPD had lower energy intake during the first week, increased EUGR incidence at 36 weeks PMA, and prolonged days for birth weight recovery. Adequate nutrition is required for somatic and lung growth promotion. The effects of postnatal malnutrition on lung development have been studied in animal models. Early postnatal nutritional restriction impairs alveolarization and impacts the bronchiolar epithelium, therefore decreasing epithelial cell division and Clara cell conversion to ciliated cells [30, 31]. According to other animal studies, malnutrition worsens the detrimental effects of hyperoxia on lung alveolarization and extracellular matrix deposition [32, 33]. Consistent with our findings, other studies [34] have reported lower caloric intake among infants with BPD during the first two weeks. However, unlike these studies [14, 34], our study did not confirm that early lower caloric intake after birth was a risk factor for BPD occurrence.
The main strength of our study was that it was the first prospective multicenter observational study across the Chinese mainland with a large sample size exploring the role of early nutritional status in BPD. Nonetheless, our study has several limitations. First, we did not collect specific values for certain nutritional metrics but rather collected information on whether a certain indicator was achieved, such as a total volume > 150 ml/kg/d and total energy > 100 kcal/kg/d. Second, we did not collect data on trace elements, electrolytes, lipids or carbohydrates, which are also important for nutritional support. Despite these limitations, this study explored the outcomes and risk factors associated with early nutritional support for pregnant BPD infants aged < 32 weeks with respiratory distress in China. These findings could lead to further BPD treatment and management.
In conclusion, a longer duration to achieve full enteral nutrition in very preterm infants was associated with increased BPD development. Breastfeeding was demonstrated to have a protective effect against BPD. Early and rapidly progressive EN and breastfeeding should be promoted in very preterm infants.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AA:
-
Amino acid
- BPD:
-
Bronchopulmonary dysplasia
- BW:
-
Birth weight
- ChiCTR:
-
Chinese Clinical Trial Registry
- EN:
-
Enteral nutrition
- EUGR:
-
Extrauterine growth retardation
- GA:
-
Gestational age
- hsPDA:
-
Hemodynamically significant patent ductus arteriosus
- IQRs:
-
Interquartile ranges
- IUGR:
-
Intrauterine growth retardation
- LA/AO:
-
Left atrial inner diameter/aortic root
- NEC:
-
Necrotizing enterocolitis
- NICHD:
-
National Institute of Child Health and Human Development
- NICUs:
-
Neonatal intensive care units
- PMA:
-
Postmenstrual age
- PN:
-
Parenteral nutrition
References
Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–61. https://doi.org/10.1146/annurev.physiol.67.040403.102229.
He W, Zhang L, Feng R, Fang WH, Cao Y, Sun SQ, Shi P, Zhou JG, Tang LF, Zhang XB, Qi YY. Risk factors and machine learning prediction models for bronchopulmonary dysplasia severity in the Chinese population. World J Pediatr. 2023;19(6):568–76. https://doi.org/10.1007/s12519-022-00635-0.
Zhu Z, Yuan L, Wang J, Li Q, Yang C, Gao X, et al. Mortality and morbidity of infants born extremely Preterm at Tertiary Medical Centers in China from 2010 to 2019. JAMA Netw Open. 2021;4(5):e219382. https://doi.org/10.1001/jamanetworkopen.2021.9382.
Gallini F, Coppola M, De Rose DU, Maggio L, Arena R, Romano V, et al. Neurodevelopmental outcomes in very preterm infants: the role of severity of Bronchopulmonary Dysplasia. Early Hum Dev. 2021;152:105275. https://doi.org/10.1016/j.earlhumdev.2020.105275.
Arigliani M, Spinelli AM, Liguoro I, Cogo P. Nutrition and lung growth. Nutrients. 2018;10:919. https://doi.org/10.3390/nu10070919.
Underwood MA, Lakshminrusimha S, Steinhorn RH, Wedgwood S. Malnutrition, poor postnatal growth, intestinal dysbiosis and the developing lung. J Perinatol. 2021;41:1797–810. https://doi.org/10.1038/s41372-020-00858-x.
Vancouver B, A N S. ACoRN Acute Care of at Risk Newborns (First Edition)[Z]. 2005.
Ma XL, Xu XF, Chen C, Yan CY, Liu YM, et al. National Collaborative Study Group for Neonatal Respiratory Distress in late preterm or term infants. Epidemiology of respiratory distress and the illness severity in late preterm or term infants: a prospective multicenter study. Chin Med J (Engl). 2010;123(20):2776–80.
Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300–8. https://doi.org/10.1016/j.jpeds.2018.01.043.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. https://doi.org/10.1186/1471-2431-13-59.
Mandruzzato G, Antsaklis A, Botet F, Chervenak FA, Figueras F, Grunebaum A, et al. Intrauterine restriction (IUGR). J Perinat Med. 2008;36:277–81. https://doi.org/10.1515/JPM.2008.050.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. https://doi.org/10.1097/00000658-197801000-00001.
Wemhöner A, Ortner D, Tschirch E, Strasak A, Rüdiger M. Nutrition of preterm infants in relation to bronchopulmonary dysplasia. BMC Pulm Med. 2011;11:7. https://doi.org/10.1186/1471-2466-11-7.
Uberos J, Jimenez-Montilla S, Molina-Oya M, García-Serrano JL. Early energy restriction in premature infants and bronchopulmonary dysplasia: a cohort study. Br J Nutr. 2020;123:1024–31. https://doi.org/10.1017/S0007114520000240.
Lin B, Xiong X, Lu X, Zhao J, Huang Z, Chen X. Enteral feeding/total fluid intake ratio is associated with risk of bronchopulmonary dysplasia in extremely preterm infants. Front Pediatr. 2022;10:899785. https://doi.org/10.3389/fped.2022.899785.
Munblit D, Treneva M, Peroni DG, Colicino S, Chow LY, Dissanayeke S, et al. Immune components in human milk are associated with early infant immunological health outcomes: a prospective three-country analysis. Nutrients. 2017;9:532. https://doi.org/10.3390/nu9060532.
Trend S, Strunk T, Lloyd ML, Kok CH, Metcalfe J, Geddes DT, et al. Levels of innate immune factors in preterm and term mothers’ breast milk during the 1st month postpartum. Br J Nutr. 2016;115:1178–93. https://doi.org/10.1017/S0007114516000234.
Hylander MA, Strobino DM, Dhanireddy R. Human milk feedings and infection among very low birth weight infants. Pediatrics. 1998;102:E38. https://doi.org/10.1542/peds.102.3.e38.
Spiegler J, Preuß M, Gebauer C, Bendiks M, Herting E, Göpel W, et al. Does breastmilk influence the development of bronchopulmonary dysplasia? J Pediatr. 2016;169:76–e804. https://doi.org/10.1016/j.jpeds.2015.10.080.
Huang J, Zhang L, Tang J, Shi J, Qu Y, Xiong T, et al. Human milk as a protective factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2019;104:F128–36. https://doi.org/10.1136/archdischild-2017-314205.
Villamor-Martínez E, Pierro M, Cavallaro G, Mosca F, Villamor E. Mother’s own milk and bronchopulmonary dysplasia: a systematic review and meta-analysis. Front Pediatr. 2019;7:224. https://doi.org/10.3389/fped.2019.00224.
Villamor-Martínez E, Pierro M, Cavallaro G, Mosca F, Kramer BW, Villamor E. Donor human milk protects against bronchopulmonary dysplasia: a systematic review and meta-analysis. Nutrients. 2018;10:238. https://doi.org/10.3390/nu10020238.
Lavizzari A, Esposito B, Pesenti N, Shaykhova A, Vizzari G, Ophorst M, et al. Dose-dependent impact of human milk feeding on tidal breathing flow-volume loop parameters across the first 2 years of life in extremely low-birth-weight infants: a cohort study. Eur J Pediatr. 2023;182(11):4969–76. https://doi.org/10.1007/s00431-023-05163-1.
Schena F, Francescato G, Cappelleri A, Picciolli I, Mayer A, Mosca F, et al. Association between hemodynamically significant patent ductus arteriosus and bronchopulmonary dysplasia. J Pediatr. 2015;166(6):1488–92. https://doi.org/10.1016/j.jpeds.2015.03.012.
Martini S, Aceti A, Galletti S, Beghetti I, Faldella G, Corvaglia L. To feed or not to feed: a critical overview of Enteral Feeding Management and Gastrointestinal complications in Preterm neonates with a patent Ductus Arteriosus. Nutrients. 2019;27(1):83. https://doi.org/10.3390/nu12010083.
Patole SK, Kumaran V, Travadi JN, Brooks JM, Doherty DA. Does patent Ductus Arteriosus Affect feed Tolerance in Preterm neonates? Arch. Dis Child Fetal Neonatal Ed. 2007;92:F53–5. https://doi.org/10.1136/adc.2006.093708.
Ibrahim MH, Azab AA, Kamal NM, Salama MA, Elshorbagy HH, Abdallah EAA, Hammad A, Sherief LM. Outcomes of early ligation of patent Ductus Arteriosus in Preterms, Multicenter Experience. Med (Baltim). 2015;94:e915. https://doi.org/10.1097/MD.0000000000000915.
van Goudoever JB, Carnielli V, Darmaun D, Sainz de Pipaon M, ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR guidelines on pediatric parenteral nutrition: amino acids. Clin Nutr. 2018;37(6 Pt B):2315–23. https://doi.org/10.1016/j.clnu.2018.06.945.
Gaultier C. Malnutrition and lung growth. Pediatr Pulmonol. 1991;10:278–86. https://doi.org/10.1002/ppul.1950100410.
Massaro GD, Mccoy L, Massaro D. Postnatal undernutrition slows development of bronchiolar epithelium in rats. Am J Physiol. 1988;255:R521–6. https://doi.org/10.1152/ajpregu.1988.255.4.R521.
Mataloun MM, Rebello CM, Mascaretti RS, Dohlnikoff M, Leone CR. Pulmonary responses to nutritional restriction and hyperoxia in premature rabbits. J Pediatr (Rio J). 2006;82:179–85. https://doi.org/10.2223/JPED.1471.
Mataloun MM, Leone CR, Mascaretti RS, Dohlnikoff M, Rebello CM. Effect of postnatal malnutrition on hyperoxia-induced newborn lung development. Braz J Med Biol Res. 2009;42:606–13. https://doi.org/10.1590/s0100-879x2009000700004.
Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, Stoll BJ, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res. 2011;69:522–9. https://doi.org/10.1203/PDR.0b013e318217f4f1.
Klevebro S, Westin V, Stoltz Sjöström E, Norman M, Domellöf M, Edstedt Bonamy AK, et al. Early energy and protein intakes and associations with growth, BPD, and ROP in extremely preterm infants. Clin Nutr. 2019;38:1289–95. https://doi.org/10.1016/j.clnu.2018.05.012.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was supported by the Key Program of The Independent Design Project of National Clinical Research Center for Child and Children’s Hospital, Zhejiang University School of Medicine Health [grant number G20B0007]. The funding agency had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Z C and Lp S, Conceptualization, Methodology, Validation, Project administration; Gn B and Xy H, Formal analysis. Jj G and Xf C, Data curation. Xl M and Lz D, Supervision. Hj L, Original Draft, Writing – Reviewing and Editing. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by the Children Hospital Ethics Committee, Zhejiang University School of Medicine Ethical (2019-IRB-164). Written informed consent was obtained from parents or guardians antenatally or upon NICU admission. The trial was registered in the Chinese Clinical Trial Registry (No. ChiCTR2000030125 on 24/02/202) and at www.ncrcch.org (No. ISRCTN84167642 on 25/02/2020).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, H., Bai, G., Ge, J. et al. Nutritional support during the first week for infants with bronchopulmonary dysplasia and respiratory distress: a multicenter cohort study in China. BMC Pediatr 24, 238 (2024). https://doi.org/10.1186/s12887-024-04675-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04675-5