Abstract
Background
Tuberculosis is one the leading causes of death from a single infectious disease, caused by the bacillus mycobacterium tuberculosis. In Ethiopia, even though several primary studies have been conducted on the incidence of tuberculosis among HIV-infected children, the pooled incidence rate of tuberculosis among HIV-infected children (aged 0–14 years) is unknown. Therefore, the main objectives of this systematic review and meta-analysis are to estimate the pooled incidence rate of tuberculosis among HIV-infected children and its predictors in Ethiopia.
Method
International electronic databases such as PubMed, HINARI, Science Direct, Google Scholar, and African Journals Online were searched using different search engines. Quality of primary studies was checked using the Joanna Briggs Institute checklist. The heterogeneity of studies was tested using I-square statistics. Publication bias was tested using a funnel plot and Egger’s test. Forest plots and tables were used to present the results. The random effect model was used to estimate the pooled incidence of tuberculosis among children living with HIV.
Result
A total of 13 studies were included in this systematic review and meta-analysis. The pooled incidence of tuberculosis among HIV-infected children was 3.77 (95% CI: 2.83, 5.02) per 100-person-year observations. Advanced HIV disease (HR: 2.72, 95% CI: 1.9; 3.88), didn’t receive complete vaccination (HR: 4.40, 95% CI: 2.16; 8.82), stunting (HR: 2.34, 95% CI: 1.64, 3.33), underweight (HR: 2.30, 95% CI: 1.61; 3.22), didn’t receive Isoniazid preventive therapy (HR: 3.64, 95% CI: 2.22, 5.96), anemia (HR: 3.04, 95% CI: 2.34; 3.98), fair or poor antiretroviral therapy adherence (HR: 2.50, 95% CI: 1.84; 3.40) and didn’t receive cotrimoxazole preventive therapy (HR: 3.20, 95% CI: 2.26; 4.40) were predictors of tuberculosis coinfection among HIV infected children.
Conclusion
This systematic review and meta-analysis concluded that the overall pooled incidence rate of tuberculosis among HIV-infected children was high in Ethiopia as compared to the END TB strategy targets. Therefore, emphasis has to be given to drug adherence (ART and Isoniazid) and nutritional counseling. Moreover, early diagnosis and treatment of malnutrition and anemia are critical to reduce the risk of TB coinfection.
Registration
Registered in PROSPERO with ID: CRD42023474956.
Similar content being viewed by others
Background
Tuberculosis (TB) is one the leading causes of death from a single infectious, caused by the bacillus mycobacterium tuberculosis [1, 2]. Children living with human immunodeficiency virus (HIV) are at increased risk of acquiring tuberculosis [3]. HIV-infected persons are sixteen times more likely to be co-infected by TB disease as compared to HIV-negative persons [2].
Globally, an estimated 1.3 million children (aged 0–14 years) fell ill with TB in 2022, which covers about 12% of the total TB cases of 10.6 million [2]. HIV and TB confection is lethal, globally, around 214,000 children died from TB disease in 2022; of this, about 31,000 of the deaths were from HIV and TB confected children [4]. The burden of TB infection differs across regions. According to the Global Tuberculosis 2023 report, a higher burden of TB was reported from African and Southeast Asia regions, contributing to about 81% of global TB deaths in 2022 [4]. Africa is home to 17 countries among 30 high TB burden countries [5]. This region alone contributes to 23% of new cases and 31% of TB-related deaths [6,7,8]. Moreover, about one-third (322,000) of the global TB cases of children (aged 0–14 years) were contributed from the African region; with two-thirds being unreported or undiagnosed [6, 9]. In Ethiopia, TB remains a major public health concern. Of the top 30 high TB-burden countries, Ethiopia ranked twelfth [10]. In 2022, about 151,000 people fell ill with TB in Ethiopia and over 19,000 deaths occur each year due to TB [11].
To reduce the burden of TB, the World Health Organization (WHO) adopted “End TB strategies” in 2014, setting a target to reduce the incidence rate and death of TB by 90% ( less than 10 TB cases per 100, 000 population) and 95%, respectively by 2035 compared with 2015 [12]. To achieve this target, an additional intermediate milestone was endorsed for the year 2025, set to reduce the incidence and death of TB by 50% and 75% by 2025, respectively [10, 12]. Despite multiple efforts made, globally, the incidence rate of TB (new cases per 100,000 population per year) has increased by 3.9% between 2020 and 2022 from 128 in 2020 to 133 in 2022 [4]. Moreover, only 19% and 8.7% reductions in TB deaths and TB incidence were achieved between the years 2015 to 2022, respectively [4], which is far from the WHO End TB Strategy milestone of a 50% reduction in new incidence of TB by 2025 and a 75% reduction in number of deaths by 2025 [12]. The burden of TB was more catastrophic in Sub-Saharan African countries where the incidence rate among children and adolescents living with HIV was 2,017 cases per 100,000 patient years [13]. A large multicenter cohort study conducted in South Africa found an incidence rate of 4.0 TB cases per 100 person-year among HIV-positive children receiving ART [14]. In Tanzania, the incidence rate of TB among children living with HIV ranged from 1.67 to 5.2 per 100 person years [15, 16].
In Ethiopia, studies revealed that the incidence of TB among HIV-infected children varies across regions with the highest rate (9.6 per 100 person-years) in the Benshangul Gumz region [17] to the lowest rate (2 per 100 person-years)in the Amhara region [18]. Similarly, other previous primary studies have also reported inconsistent findings regarding the incidence of TB among HIV-positive children [17,18,19,20,21,22,23,24,25,26]. With this discrepancy, the pooled incidence rate of TB for children (aged 0–14 years) living with HIV is not estimated. In Ethiopia, though there is a systematic review and meta-analysis study on the incidence of TB [27], it was conducted for all people living with HIV (including for HIV positive Adults) and yet, not segregated by age. Moreover, among eleven primary studies incorporated in the previous meta-analysis [27], only four of the primary studies were conducted among HIV-positive children and used in subgroup analysis to estimate the pooled incidence of TB among HIV-positive children, which can affect the pooled estimate of TB incidence. Furthermore, since the previous meta-analysis was conducted among all people living with HIV, the predictors were not reported in age-specific manner and predictors such as ART treatment adherence and nutritional status of children have not been investigated. Thus, an age-segregated study is crucial to identify age-specific gaps and predictors of TB among HIV-positive children. Therefore, the main objectives of this systematic review and meta-analysis are to estimate the pooled incidence rate of TB coinfection among HIV-infected children and identify its predictors in Ethiopia. Thereby to develop a comprehensive strategic plan for the identified factors at the national level.
Methods
Search strategy
The Preferred Reporting Items for Systematic Review and Meta-Analysis Statement (PRISMA-2020) guideline was used to report the results [28].International electronic databases such as PubMed, HINARI, Science Direct, Google Scholar, and African Journals Online were searched to obtain relevant studies. Searching was done from September 29, 2023 to back 10 years to provide up-to-date pooled estimates of TB incidences among HIV-positive children. The following terms and phrases such as “Incidence ”, “Tuberculosis”, “opportunistic infection”, “HIV infection”, “ART”, “predictors”, “associated factors”, “risk factors”, “determinants”, “pediatrics”, “children” “under-five children “and “Ethiopia” were used to search studies. The Boolean search operators such as “AND” and “OR” were used separately and in combination during database searching (Additional Table 1).
Eligibility criteria
Inclusion criteria
Studies conducted in Ethiopia, studies that report the incidence rate of TB among children living with HIV, studies that report the number of new TB cases among children living with HIV, studies that report the child person-years, studies published in English languages and studies available at the electronic source in the last 10 years to September 29, 2023 were included in the study.
Exclusion criteria
Studies that report the predictors in other than hazard ratio and citations without abstract and/or full-text, anonymous reports, editorials, and qualitative studies were excluded from the analysis.
Data extraction
After browsing the databases, all the articles were exported to Endnote21 to identify and remove duplication. The data was independently extracted using a standardized extraction form by four authors (GF, ZA, MS, and BB). From each study, the author’s name, publication year, the event of TB (number of TB cases), study region, study design, the total person year, incidence rate per 100 person year, follow-up time, and the predictor of TB with hazard ratios were extracted.
Quality assessment/critical appraisal
The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for cohort study was used to assess the quality of the study [29]. The qualities of the primary studies were independently assessed by two authors (MW and NS). Any discrepancy between the two authors was handled by taking the mean score of the two authors. The tool has Yes, No, Unclear, and Not Applicable options: “1” is given for “Yes” and “0” is given for other options. The scores were summed and changed to percentages. Finally, 13 studies that received a quality score of > 50% were included in this meta-analysis [17,18,19,20,21,22,23,24,25,26, 30,31,32] (Additional Table 2).
Outcome measurement
The first outcome of this systematic review and meta-analysis was the incidence rate of TB among HIV-infected children in Ethiopia. The incidence rate of TB was calculated by dividing the number of children who develop new TB cases by the total child follow-up year and multiplying it by 100. Identifying the predictors of TB coinfection among HIV-infected children was the second outcome of this study. Accordingly, the hazard ratio of predictors with its 95% confidence intervals (CI) was extracted from the original studies to compute the pooled hazard ratio for the predictor of TB coinfection among HIV-positive children.
Advanced HIV disease
Children older than five years whose WHO clinical stages are III and IV. Whereas, children younger than five years living with HIV are considered as having advanced HIV disease, regardless of the clinical stages.
Mild WHO clinical stages
HIV-positive children whose WHO clinical stages are stages I and II [33].
ART adherence
Good (> 95%)—if missed doses is ≤ 2 doses of 30 doses or ≤ 3 doses of 60 doses; Fair: (85– 94%) if missing doses is between 3 and 4 of 30 doses or 4–9 of 60 doses; poor: (< 85%) if missed doses are > 5 doses of 30 doses or 10 and above doses of 60 doses of ART drug [33].
Nutritional status
Underweight : Children with weight for age Z-score < − 2 standard deviation (SD), Stunting: (height for age Z-score < − 2 SD) [34].
Statistical analysis
Data entry was done using Microsoft Excel 2013 and then imported into R software version 4.1.3 for further analysis. Meta-package was used to analyze the data. Heterogeneity was checked using the I-square test [35]. Heterogeneity was declared as low, medium, and high if the I2 value was 25, 50, and 75%, respectively [36]. Subgroup analysis was done using the duration of the follow-up period. To identify the possible source of heterogeneity univariate meta-regression analysis was done considering the sample size and the year of publication. Sensitivity analyses were done by omitting individual studies to detect the contribution of the included study for the final pooled incidence rate of TB. Funnel plot visual inspecting was done to identify publication bias. Finally, the Egger test was done to assess any significant publication bias. Further, the trim-and-fill analysis imputation was done to correct the bias. The forest plot was used to preent the pooled incidence of tuberculosis with its 95% confidence interval. The random effect model was used to estimate the pooled incidence of tuberculosis among children living with HIV.
Results
Characteristics of included studies
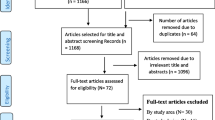
A total of 685 studies were browsed from PubMed, HINARI, Science Direct, Google Scholar, and African Journals Online. Of these, 311 studies were from PubMed, 52 studies were from HINARI, 256 articles were from Science Direct, and the rest 66 studies were searched from Google Scholar and African Journals online. From these studies, 234 articles were excluded due to duplication. From the remaining 451 articles, 425 articles were excluded as not being relevant to the study after reviewing the title and abstract. The rest 26 articles were assessed by reviewing the full text. Finally, a total of 13 studies were eligible and included in the final systematic review and meta-analysis [17,18,19,20,21,22,23,24,25,26, 30,31,32] (Fig. 1). All of the studies were conducted using the retrospective cohort study design. These studies were done from different parts of Ethiopia (Amhara, Oromia, SNNPR (South Nation, Nationalities and People Region), and Bnishangul Gumuz regions) (Table 1).
The pooled incidence rate of TB among HIV‑infected children
In this meta-analysis, a total of 13 studies were used to estimate the pooled incidence rate of TB among HIV-infected children in Ethiopia [17,18,19,20,21,22,23,24,25,26, 30,31,32]. Accordingly, the incidence rate of TB among HIV-infected children in Ethiopia was found to be 3.77 (95% CI: 2.83, 5.02) per 100-person-year observations using a random effect model. There was heterogeneity between studies included in the meta-analysis (I2 = 94%, P-value < 0.01 (Fig. 2). Hence, subgroup analysis was done based on the duration of follow-up time. Accordingly, the incidence rate of TB was 2.76(95% CI: 2.19, 3.47) per 100 person-years among children followed for greater than 60 months and 6.19 (95% CI: 4.55, 8.41) per 100 person-years among children followed for less or equal to 60 months (Fig. 3). Further, meta-analysis was done to identify the possible source of heterogeneity using the publication year and sample size. Accordingly, both the sample size and publication year were identified as the possible source of heterogeneity (Table 2). Sensitivity analysis was done to explore the contribution of each study for the final pooled estimate. Accordingly, except for three studies [17, 25, 30], nearly all studies have equal contributions to the pooled incidence rate of TB in Ethiopia (Fig. 4).
Publication bias
Asymmetric distribution was detected in the funnel plot visual inspection (Fig. 5). The Egger test also shows a statistically significant publication bias with B0 = -2.1598, p-value = 0.03. Due to the presence of statically significant publication bias, meta-trim and fill analysis were done. Accordingly, after filling three studies, the incidence rate of TB among HIV-positive children became 5.49 (95%CI: 3.83, 7.89) per 100 child years using a random effect model (Fig. 6).
Meta-analysis of predictors of TB among HIV-infected children in Ethiopia
A total of eight studies were used to estimate the pooled hazard ratio for predictors of TB coinfection among children living with HIV [17,18,19,20,21,22,23,24]. Accordingly, the hazard of TB coinfection among HIV-infected children was 2.72 times (HR: 2.72, 95% CI: 1.9; 3.88) higher among children with advanced HIV disease as compared to children with mild WHO clinical stages [18, 19, 22, 24]. The hazard of TB co-infection in HIV-infected children was 4.40 times (HR: 4.40, 95% CI: 2.16; 8.82) higher among children who didn’t receive complete vaccination as compared to their counterparts [17, 20, 21]. The likelihood of TB co-infection among HIV-infected children was 2.34 times (HR: 2.34, 95% CI: 1.64, 3.33) higher among children who are stunted as compared to their counterparts [17, 18, 23]. Likewise, the hazard of TB co-infection among HIV-infected children was 2.30 times (HR: 2.30, 95% CI: 1.61; 3.22) higher among children who are underweight as compared to their counterparts. The hazard of TB coinfection among HIV-infected children was 3.64 times (HR: 3.64, 95% CI: 2.22, 5.96) higher among children who didn’t receive Isoniazid preventive therapy as compared to children who received Isoniazid preventive therapy [19, 21, 23]. The likelihood of TB coinfection among HIV-infected children was 3.04 times (HR: 3.04, 95% CI: 2.34; 3.98) higher among children whose hemoglobin level is less or equal to 10 mg/dl) as compared to children whose hemoglobin level is greater than 10 mg/dl [17, 19,20,21, 23, 24]. . The hazard of TB coinfection in HIV-infected children was 2.5 times (HR: 2.50, 95% CI: 1.84, 3.99) higher among children whose adherence level is fair or poor as compared to children with good ART adherence [18, 22, 24]. The likelihood of TB coinfection among HIV-infected children was 3.20 times (HR: 3.20, 95% CI: 2.26; 4.40) higher among children who didn’t receive cotrimoxazole preventive therapy as compared to children who receive cotrimoxazole preventive therapy [17, 19, 21, 23] (Table 3).
Discussion
This systematic review and meta-analysis disclosed the pooled incidence rate of TB among HIV-infected children in Ethiopia, and further, identified its predictors. Accordingly, the incidence rate of TB among children living with HIV was 3.77 (95% CI: 2.83, 5.02) per 100-person-year. The finding is too high and requires immediate attention to achieve the End TB Strategy targets of a 90% reduction in TB incidence rate (less than 10 TB cases per 100, 000 population) by 3035 [12]. The possible elucidation for the high incidence of TB might be due to economic constraints to implement the End TB WHO strategies [37].
This systematic review and meta-analysis revealed that HIV-infected children with advanced HIV disease have a higher hazard of TB coinfection than children with mild WHO clinical stages. The finding is supported by previous studies conducted elsewhere [16, 27, 38]. The possible justification might be children with advanced HIV disease may have compromised body immunity [39]. This may trigger the progression of latent TB to disease stages.
The likelihood of TB co-infection in HIV-infected children was higher among children who are malnourished as compared to children with normal nutritional status. The finding is consistent with studies conducted elsewhere [16, 40, 41]. This is the fact that micro and macronutrients are needed to boost our immunity system [42]. Thus, being malnourished is a golden opportunity for viral replication which further compromises body immunity [43]. Finally, this can increase the incidence of TB-HIV coinfection.
The hazard of TB coinfection in HIV-infected children was higher among children whose hemoglobin level is less or equal to 10 mg/dl as compared to children whose hemoglobin level is greater than 10 mg/dl. The finding is supported by previous studies conducted elsewhere [16, 27, 38]. This could be the fact that anemia can impair the immune response and the bactericidal activity of leucocytes makes them vulnerable to infections, including tuberculosis [42, 44, 45].
This systematic review and meta-analysis revealed that HIV-infected children who didn’t receive Isoniazid preventive therapy have a higher hazard of acquiring TB coinfection than children who receive Isoniazid preventive therapy [38, 46,47,48,49,50]. This could be the fact that Isoniazid preventive therapy will halt the progression of latent TB from the active form of TB disease [51].
The hazard of TB coinfection in HIV-infected children was higher among children who didn’t receive cotrimoxazole preventive therapy as compared to children who received cotrimoxazole preventive therapy. The finding is consistent with previous studies conducted elsewhere [27, 38, 40, 41, 52]. This is the fact that cotrimoxazoles block the biosynthesis of nucleic acid and protein crucial to many opportunistic infections that exacerbate immunosuppression and progression of the disease [53].
In this systematic review and meta-analysis, the likelihood of TB coinfection in HIV-infected children was higher among children whose adherence level is fair or poor than children with good Antiretroviral Therapy (ART) adherence. The finding is synonymous with a previous study conducted in South Africa [54]. This is the fact that ART can halt viral replication and restore immune function and it prevents opportunistic infection, including tuberculosis [55, 56]. Such that, fair or poor ART adherence can create a golden opportunity for viral replication [57]. This can increase the risk of TB coinfection.
Lastly, this systematic review and meta-analysis revealed that HIV-infected children who didn’t receive complete vaccination have a higher hazard of TB coinfection than children who received complete vaccination. The finding is supported by a previous study conducted in Tanzania [58]. This could be that a vaccine is given to produce antibodies that defend against infectious diseases.
The clinical and public health implications of this systematic review and meta-analysis are to take prompt intervention against the identified factors and in turn to reduce the burden of TB coinfection among HIV-infected children, and finally, to reduce HIV-related child mortality. Therefore, researchers, program implementers, and policymakers should consider the aforementioned predictors in health care provision.
Limitations
This systematic review and meta-analysis have the following limitations: In this analysis, articles published only in English were included. Only four regions were included in the analysis, such that other regions may not be represented in the study. Some predictors of TB reported only in one primary article and/or classified differently from the included articles were excluded from the analysis.
Conclusion
This systematic review and meta-analysis concluded that the overall pooled incidence rate of tuberculosis among HIV-infected children was high in Ethiopia as compared to the END TB strategy targets. Advanced WHO clinical staging, didn’t receive complete vaccination, stunting, underweight, didn’t receive Isoniazid preventive therapy, anemia, fair or poor antiretroviral therapy adherence, and didn’t receive cotrimoxazole preventive therapy were predictors of tuberculosis coinfection among HIV infected children. Therefore, emphasis has to be given to drug adherence (ART and Isoniazid) and nutritional counseling. Moreover, early diagnosis and treatment of malnutrition and anemia are critical to reduce the risk of TB coinfection.
Data availability
The data is available at the corresponding author and may be provided upon request.
Abbreviations
- ART:
-
Antiretroviral treatment
- HIV:
-
Human immunodeficiency virus
- BCG:
-
Bacillus Chalmette–Guerin
- PYO:
-
Person-year observation
- TB:
-
Tuberculosis
- WHO:
-
World Health Organization
- SNNPR:
-
South Nation Nationalities and People Region
- JBI:
-
The Joanna Briggs Institute Critical Appraisal Checklist
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis Statement
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
TB Alliance Global Pandemic - TB Alliance. 2021 [cited 2023 October, 3]; https://www.tballiance.org/why-new-tb-drugs/global-pandemic.
World Health Organization. Tuberculosis: Key facts. 2023 7 November 2023 [cited 2023 January, 3]; https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
Centers for Disease Control and Prevention. Groups at High Risk for Developing TB Disease. [cited 2023 october,5]; https://www.cdc.gov/tb/webcourses/tb101/page121.html.
World Health Organazation. Global tuberculosis report 2023. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO 2023.
World Health Organazation: Africa Region. African Union and WHO urge swift action against childhood tuberculosis. 2022 24 August 2022 [cited 2023 october,5]; https://www.afro.who.int/news/african-union-and-who-urge-swift-action-against-childhood-tuberculosi.
World health Organazation. Africa’s TB reduction rate falls short amid slowing global progress. 2023 24 March 2023 [cited 2023 october,4]; https://www.afro.who.int/news/africas-tb-reduction-rate-falls-short-amid-slowing-global-progress.
World Health Organization. Global tuberculosis report 2022. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. 2023.
World Health Organization. Tuberculosis in the WHO African Region: 2023 progress update. Tuberculosis in the WHO African Region: 2023 progress update. 2023
Uwishema O et al. Childhood tuberculosis outbreak in Africa: is it a matter of concern? Int J Surg, 2023: p. 101097.
World Health Organization. Global tuberculosis report 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. 2019.
MINISTER OF HEALTH, E. Sixteenth National Tuberculosis Research Conference. 2022 March 22, 2022 [cited 2023 october,7]; https://www.moh.gov.et/site/node/331.
World Health Organazation. Sixty-seventh World Health Assembly. 2014 [cited 2023 october,3]; https://apps.who.int/gb/ebwha/pdf_files/WHA67-REC1/A67_2014_REC1-en.pdf.
Mandalakas AM, et al. Tuberculosis among children and adolescents at HIV treatment centers in sub-saharan Africa. Emerg Infect Dis. 2020;26(12):2933.
Brennan AMM, Sanne SK, Matthew I. P. Incidence and Risk Factors Associated with Tuberculosis in HIVpositive Children Receiving Antiretroviral Therapy in a Large South African Multicenter Cohort 2013; https://www.heroza.org/wp-content/uploads/2014/10/CROI-2014-TB-Pediatrics1.pdf.
Majigo M, et al. Prevalence and incidence rate of tuberculosis among HIV-infected patients enrolled in HIV care, treatment, and support program in mainland Tanzania. Trop Med Health. 2020;48(1):1–8.
Li N, et al. Incident Tuberculosis and risk factors among HIV-infected children in Tanzania. Aids. 2013;27(8):1273–81.
Kebede F et al. Incidence and Predictors of Tuberculosis and Predictors for Seropositive Children Attending HIV/AIDS Care in Two General Hospitals North West Ethiopia, 2020 2021.
Wondifraw EB, et al. Incidence and predictors of tuberculosis among children on antiretroviral therapy at northeast Ethiopia comprehensive specialized hospitals, 2022; a multicenter retrospective follow-up study. Comput Math Methods Med. 2022;8(12):e12001.
Alemu YM, Andargie G, Gebeye E. High incidence of tuberculosis in the absence of Isoniazid and Cotrimoxazole Preventive Therapy in Children Living with HIV in Northern Ethiopia: a Retrospective Follow-Up study. PLoS ONE. 2016;11(4):e0152941–0152941.
Ayalaw SG, Alene KA, Adane AA. Incidence and predictors of tuberculosis among HIV positive children at University of Gondar Referral Hospital, northwest Ethiopia: a retrospective follow-up study. Int Sch Res Notices. 2015;2015.
Beshir MT et al. Incidence and predictors of tuberculosis among HIV-positive children at Adama Referral Hospital and Medical College, Oromia, Ethiopia: a retrospective follow-up study. Epidemiol Health, 2019. 41.
Endalamaw A, Engeda EH, Tezera N. Incidence of tuberculosis in children on antiretroviral therapy: a retrospective cohort study. BMC Res Notes. 2018;11(1):745–745.
Kebede F et al. Incidence and predictors of pulmonary tuberculosis among children who received antiretroviral therapy (ART), Northwest Ethiopia: a multicenter historical cohorts study 2009–2019. J Trop Med. 2022;2022:9925693–9.
Tekese D, Dawit D. Incidence and predictors of tuberculosis among children receiving antiretroviral therapy in the Wolaita Zone: a retrospective cohort study. 2023. 18(9): p. e0291502.
Tiruneh F, Deyas Y. Effect of highly active antiretroviral treatment on TB incidence among HIV infected children and their clinical profile, retrospective cohort study, South West Ethiopia. Sci Rep. 2020;10(1):21468.
Wondifraw EB, et al. Incidence and predictors of common opportunistic infection among HIV -infected children attending antiretroviral treatment clinic at Northeast Ethiopia, public hospitals 2022: a multicenter retrospective follow-up study. Annals Med Surg. 2022;84:104910–104910.
Azanaw MM, et al. Incidence and predictors of tuberculosis among HIV patients after initiation of antiretroviral treatment in Ethiopia: a systematic review and meta-analysis. Trop Med Health. 2021;49(1):1–11.
PRISMA. PRISMA 2020 statement: PRISMA 2020 flow diagram for new systematic reviews: databases and registers. 2020; https://www.prisma-statement.org/prisma-2020-statement.
Lockwood C et al. Chap. 7: systematic reviews of etiology and risk. JBI Manual for Evidence Synthesis [Internet]. Joanna Briggs Institute, 2017.
Chanie ES, et al. Incidence of advanced opportunistic infection and its predictors among HIV infected children at Debre Tabor referral hospital and University of Gondar Compressive specialized hospitals, Northwest Ethiopia, 2020: a multicenter retrospective follow-up study. Heliyon. 2021;7(4):e06745–06745.
Mekonnen GB, et al. Predictors of a high incidence of opportunistic infections among HIV-infected children receiving antiretroviral therapy at Amhara regional state comprehensive specialized hospitals, Ethiopia: a multicenter institution-based retrospective follow-up study. Front Pead. 2023;11:1107321–1107321.
Melkamu MW et al. Incidence of common opportunistic infections among HIV-infected children on ART at Debre Markos referral hospital, Northwest Ethiopia: a retrospective cohort study. 2020. 20(1): p. 50.
Ethiopia F. National consolidated guidelines for comprehensive HIV prevention, care and treatment. Addis Ababa: Fmoh; 2018. pp. 1–238.
World Health Organazation. Malnutrition in children. [cited. 2023 Feb,28]; https://www.who.int/data/nutrition/nlis/info/malnutrition-in-children.
Rücker G, et al. Undue reliance on I 2 in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:1–9.
Huedo-Medina TB, et al. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychol Methods. 2006;11(2):193.
Assefa DG, et al. Financial burden of tuberculosis diagnosis and treatment for patients in Ethiopia: a systematic review and meta-analysis. BMC Public Health. 2024;24(1):260.
Wondmeneh TG, Mekonnen AT. The incidence rate of tuberculosis and its associated factors among HIV-positive persons in Sub-saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):1–24.
Prabhu S, Harwell JI, Kumarasamy N. Advanced HIV: diagnosis, treatment, and prevention. Volume 6. The lancet HIV; 2019. pp. e540–51. 8.
Anyalechi GE, et al. Tuberculosis prevalence, incidence and prevention in a South African cohort of children living with HIV. J Trop Pediatr. 2022;68(6):fmac084.
Crook AM, et al. Tuberculosis incidence is high in HIV-infected African children but is reduced by co-trimoxazole and time on antiretroviral therapy. BMC Med. 2016;14(1):1–11.
Munteanu C, Schwartz B. The relationship between nutrition and the immune system. Front Nutr. 2022;9:1082500.
Duggal S, Chugh TD, Duggal AK. HIV and malnutrition: effects on immune system. Clin Dev Immunol. 2012;2012.
Hassan TH et al. Impact of iron deficiency anemia on the function of the immune system in children. Medicine, 2016. 95(47).
Ekiz C, et al. The effect of iron deficiency anemia on the function of the immune system. Hematol J. 2005;5(7):579–83.
Yirdaw KD, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS ONE. 2014;9(8):e104557.
Zar HJ, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ. 2007;334(7585):136.
Beshaw MA, Balcha SA, Lakew AM. Effect of isoniazid prophylaxis therapy on the prevention of tuberculosis incidence and associated factors among HIV infected individuals in northwest Ethiopia: retrospective cohort study. HIV/AIDS-Research and Palliative Care; 2021. pp. 617–29.
Ayieko J, et al. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta–analysis. BMC Infect Dis. 2014;14(1):1–10.
Frigati L, et al. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax. 2011;66(6):496–501.
Mølhave M, Wejse C. Historical review of studies on the effect of treating latent tuberculosis. Int J Infect Dis. 2020;92:S31–6.
Ku SWW et al. Cotrimoxazole prophylaxis decreases Tuberculosis risk among Asian patients with HIV. Afr J Reprod Gynaecol Endoscopy, 2019. 22(3).
Pediatric Oncall pediatiric health care. Cotrimoxazole - Mechanism, Indication, Contraindications. [cited 2023 October, 9]; https://www.pediatriconcall.com/drugs/cotrimoxazole/446.
Webb Mazinyo E, et al. Adherence to concurrent tuberculosis treatment and antiretroviral treatment among co-infected persons in South Africa, 2008–2010. PLoS ONE. 2016;11(7):e0159317.
Alemu A, et al. The Effect of Long-Term HAART on the incidence of Tuberculosis among people living with HIV in Addis Ababa, Ethiopia: a matched nested case–control study. Infection and Drug Resistance; 2021. pp. 5189–98.
Zinyakatira N. The impact of antiretroviral therapy on tuberculosis incidence. Faculty of Health Sciences; 2019.
Anito AA et al. Magnitude of viral load suppression and associated factors among clients on antiretroviral therapy in public hospitals of Hawassa City Administration, Ethiopia. HIV AIDS-Res Palliat Care. 2022:529–538.
Faurholt-Jepsen D, et al. BCG protects against Tuberculosis irrespective of HIV status: a matched case-control study in Mwanza, Tanzania. Thorax. 2013;68(3):288–9.
Acknowledgements
We would like to thank all authors for their valuable contributions in their original studies that we used in our systematic review and meta-analysis.
Funding
No fund was offered to conduct this study.
Author information
Authors and Affiliations
Contributions
DG, GF ZA, NS, MW, MS, and BB are involved in the design, selection of articles, data extraction, quality appraisal, and statistical analysis. DG and GF were involved in manuscript writing. All authors read and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Girma, D., Abita, Z., Shifera, N. et al. Incidence rate of tuberculosis among HIV infected children in Ethiopia: systematic review and meta-analysis. BMC Pediatr 24, 363 (2024). https://doi.org/10.1186/s12887-024-04819-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04819-7