Abstract
Background
Asthma is a common non-communicable disease in children, and airway inflammation is the main pathological change of asthma. Tobacco smoke exposure (TSE) can cause systematic inflammation and oxidative stress, which may further aggravate the progression of asthma. Dietary antioxidants can relieve the inflammation and oxidative stress in human body. This study aims to assess the effect of overall antioxidant capacity of dietary intake, evaluating by dietary antioxidant quality score (DAQS), in the association between TSE and childhood asthma.
Methods
Data of this cross-sectional study were extracted from the National Health and Nutrition Examination Surveys (NHANES) 2007–2018. DAQS was calculated based on the daily dietary intake of selenium, zinc, magnesium, vitamin A, C and E. TSE was measured by serum cotinine concentration. The weighted univariate and multivariate logistic regression models were employed to evaluate the role of DAQS in the association between TSE and asthma among children and adolescents. Subgroup analysis was conducted to further evaluate the association based on gender.
Results
Totally 11,026 children and adolescents were included, of whom 1,244 (11.28%) had asthma. After adjusted all covariates, TSE was associated with the high odds of childhood asthma (OR = 1.26, 95%CI = 1.05–1.52). Among children exposed to tobacco smoke, those with higher DAQS level (OR = 1.15, 95%CI: 0.88–1.50) had a reduced risk of asthma compared with those children with lower DAQS level (OR = 1.43, 1.08–1.89), especially among girls (OR = 1.42, 95%CI: 0.93–2.17).
Conclusion
High DAQS may have a moderating effect on asthma in children; that is, the higher DAQS, the lower the odds of asthma in children who exposed to tobacco smoke. Our study provides a reference for developing more targeted strategies for prevention and treatment of asthma in children.
Similar content being viewed by others
Background
Asthma, a common chronic non-communicable disease in children, is featured by airway obstruction due to airway inflammation and increased mucus secretion production [1]. It is estimated by the US Centers for Disease Control and Prevention (CDC) that 1 in 12 children between ages of 0–17 have asthma [2]. Although the majority of children with asthma can be controlled with inhaled corticosteroids, a part of children may experience frequent and severe asthma attacks which will lead to deterioration of lung function [3]. Control of asthma in children is becoming a major challenge in primary health care.
The most important pathological change in asthma is chronic inflammation of the airways and clinical studies have shown that airway inflammation occurred even in mild asthma [4]. Environment exposure and dietary intake are two modifiable factors affecting airway inflammation and asthma [5,6,7,8]. Tobacco smoke exposure (TSE), a major source of indoor air pollution, has been shown to directly cause airway inflammation and is associated to poorer asthma control [9]. Cotinine, as a direct metabolite of nicotine, is a specific and sensitive marker of TSE [10]. Clinical studies suggested that high serum cotinine concentrations are independently related to the high risk of asthma in children [11]. Moreover, dietary antioxidants play an anti-inflammatory role by participating in oxidative stress and clearing oxidative free radicals, thereby reducing the risk of asthma [12, 13]. It is thought that evaluating the overall antioxidant quality of an individual’s diet rather than a single antioxidant dietary factor might provide a more comprehensive picture of the relationship between dietary antioxidants health outcome [14]. Hence, dietary antioxidant quality score (DAQS) is proposed, which represents the overall dietary antioxidant capacity by adding the common dietary antioxidant: vitamin A, C, E, magnesium (Mg), zinc (Zn) and selenium (Se) [15]. Luu et al. [16] reported a significant inverse correlation between DAQS and the levels of oxidative stress and inflammatory markers in vivo. Mendes et al. [17] found the quality of diet might affect the relationship between indoor air pollution and asthma in children. Compared to the anti-inflammatory diet, children exposed to particulate matter 2.5/10 and with the inflammatory diet have a high risk of asthma.
Based on the above studies about relationships between dietary antioxidants, environmental exposure and asthma, we hypothesized that high DAQS may have a protective effect on the risk of asthma in children who exposed to tobacco smoke. Herein, the study was to explore the effect of DAQS on TSE and asthma in the general U.S. children and adolescents.
Methods
Study design and population
Data of this cross-sectional study were extracted from the National Health and Nutrition Examination Survey (NHANES) 2007–2018. NAHNES is a major project conducted by National Center for Health Statistics (NCHS), a part of CDC and aimed to evaluate the health and nutrient status of noninstitutionalized U.S. population [18]. This survey uses complex, multistage, probability sampling methods based on broad population distribution. NHANES protocols are approved by the NCHS Ethics Board of the US CDC. All individuals provided written informed consent during the survey. According to the Ethics Review Board of First Hospital Affiliated to Fujian Medical University Hospital, cross-sectional studies have been exempted from the ethical review.
The included criteria were: (1) participants aged 1–17 years old; (2) participants with complete dietary intake information; (3) participants with the serum cotinine measurement. The excluded criteria were: (1) missing body mass index (BMI) data; (2) missing the asthma assessment information.
DAQS assessment
In this study, dietary intake information was obtained through two days 24-h dietary recall interviews. The first 24-h dietary recall interview was administered during the examination at the mobile examination center (MEC). In this interview, all foods and nutritional supplements consumed in the 24-h prior to the interview, the quality of food reported, and a detailed description of the food. The second 24-h interview was administered by telephone 3–4 day after the MEC exam [19]. The dietary intake was calculated as the total intake of dietary and nutritional supplements.
DAQS was obtained from some vitamins and minerals that have antioxidant function including vitamin A, C, E, zinc, Mg and selenium [20]. We compared each above six vitamins or minerals to their recommended daily intake (RDI) for US adults 2015-2020_Dietary_Guidelines.pdf (health.gov). Each of the vitamins or minerals was assessed and then we allocated a value of 0 or 1, respectably. When the intake was lower than 2/3 of the RDI, it was assigned a value of 0. Similarity, when the vitamins or minerals was higher than 2/3 of the RDI, it was assigned a value of 1. Finally, the total DAQS ranged from 0 (poor quality) to 6 (high quality). In present study, the DAQS was then classified into the two groups by media level of population included in our study: < 5 (low quality) and ≥ 5 (high quality).
TSE assessment
Serum cotinine is a primary nicotine metabolite and its short-term level was used as a marker of active smoking and as an indicator of exposure to secondhand smoke [21]. Isotope dilution high-performance liquid chromatography and atmospheric pressure chemical ionization tandem mass spectrometry were used to measure the serum cotinine levels. This assay has good accuracy, with mean values within 9% of theoretical values at all levels expect the lower limit of quantification, where it was within 14% of the theoretical values. All samples were analyzed at the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention in Atlanta, Georgia. In present study, serum cotinine levels ≥ 0.05 ng/mL were considered as TSE, and < 0.05 ng/mL were considered as non-TSE [22].
Definition of current asthma
Current asthma was defined using self-reported questionnaire responses. Participants were defined to have asthma if they answered the question “Has a doctor or other health professional ever told you that you have asthma?” positivity. For asthma patients, the following question were asked for further analysis: (1) “During the past 12 months, have you had an episode of asthma or an asthma attack?” (2) “During the past 12 months, have you had to visit an emergency room or urgent care center because of asthma?” If participants answered the question “don’t know”, s/he was not considered as current asthma patient and was excluded from this study [23]. For children aged 1–6 years, all of these questions were answered by their guardians; subjects aged 7–11 years old were accompanied by their guardian to assist in responding, while subjects aged 12–17 years old were responded by themselves.
Potential covariates
Demographic information, physical information, laboratory values and dietary intake were extracted from the NHANES database. Age was divided into three groups: 1–5 years old, 6–11 years old and 12–17 years old. Physical activity levels of children aged 2–11 years old were assessed by the question “During the past 7 days, on how many days was physical active for a total of least 60 min per day?” Then, add up all the time spent in any kind of physical activity that increased him/her heart rate and made him/her breathe hard some of the time. For adolescent aged 12–17 years old, physical activity was expressed as the metabolic equivalent task (MET) and calculate as follows: physical activity (met·min) = recommended MET \(\times\) exercise time for corresponding activities (min/day) \(\times\) the number of exercise days per week day (day) [24]. Ideal physical activity was defined as ≥ 180 met·min/day for 12–17 years old or ≥ 60 met·min/in/day for 2–11 years old. Sedentary time for children aged 2–11 years old was the sum of time spent watching television or videos and using computer per day; while sedentary time for adolescent aged 12–17 years old, sedentary time was assessed by the NHANES through the individual’s daily hours of television, video, or computer use according to the in-person interview data and was divided into three categories: < 3, 3–6 h, and ≥ 6 h [25]. Hay fever and family history of asthma were assessed by medical conditions questionnaire. Hay fever was assessed by the question “During the past 12 months, have you had an episode of hay fever?” (yes/no). Family history of asthma was assessed by the question “Including living and deceased, were any of your close biological that is, blood relatives including father, mother, sisters or brothers, ever told by a health professional that they had asthma?” (yes/no). BMI was converted to a BMI Z-score accounting for age and gender using recommended CDC percentiles. A BMI Z-score of ≥ 85th percentile and < 95th percentile indicates overweight status, and a BMI Z-score of ≥ 95th percentile indicates obesity [26]. Smoking during pregnancy was assessed by the question “Did (participant’s name) biological mother smoke at any time while she was pregnant with him/her?” (yes/no).
Statistical analysis
Continuous data were expressed as mean and standard error (S.E.), and the weighted t-test was used for comparison between groups. Categorical variables were described as the number and percentage [N (%)], and comparisons between groups used the weighted Rao-Scott χ2 test. The weighted univariate and multivariate logistic regression models were used to utilized to explore the association between the role of DAQS in the association between TSE and asthma among children, with odds ratios (ORs) and 95% confidence intervals (CIs). Model 1 was a crude model without adjusting any covariates. Model 2 adjusted age, gender, family history of asthma, energy and BMI. Subgroup analysis were conducted to further explore the association based on gender. All statistical analyzes were performed using R v 4.20 (R Foundation for Statistical Computing, Vienna, Austria) and SAS v 9.4 (SAS Institute, Cary, North Carolina) software. Two-sided P-value < 0.05 was considered statistically significant.
Results
Characteristics of study children
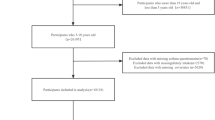
The flow chart of population screening was shown in Fig. 1. Totally, 31,321 children were screened. Among them, 11,583 subjects missing serum cotinine measurement data, 3,211 subjects missing dietary two-day intake information, 4 subjects missing DAQS calculation information, 87 subjects missing BMI data, and 5,410 subjects missing asthma diagnosis information were excluded. Table 1 shown the characteristics of the study population. Totally 11,026 eligible children were included, of whom, 1,244 (11.28%) had asthma. The proportion of higher DAQS in children and adolescents with asthma was lower than in non-asthma group (58.97% vs. 62.98%). Children and adolescents with asthma had higher rates of exposure to tobacco than those non-asthma (47.35 vs. 39.21). Difference was found in age, the level of energy intake, sedentary time and BMI, and the history of family asthma and hay fevers between two groups (all P < 0.05).
Association between DAQS and TSE with current asthma
We explored two weighted logical regression models to explore the association of DAQS and TSE with current asthma in children and adolescents, as presented in Table 2. After adjusted age, gender, family history of asthma, energy and BMI, TSE was associated with the high odds of asthma in children (OR = 1.26, 95%CI: 1.05–1.52, P = 0.015). However, no significant association was found in DAQS and asthma in our study population (P > 0.05).
Effect of DAQS on the association between TSE and current asthma
The effect of DAQS on the association between TSE and current asthma in children and adolescents was shown in Table 3. In low quality of DAQS group, children and adolescents who exposed to tobacco smoke had a high odds of asthma (OR = 1.43, 95%CI: 1.08–1.89, P = 0.013). While in high quality of DAQS group, no significant effect of ADQS on the association between TSE and current asthma was observed (P > 0.05). Taken together, high quality of DAQS may has a moderating effect on the association between TSE and asthma in children and adolescents.
Moderating effect of DAQS on the association between TSE and current asthma in children and adolescents based on gender
To further explore the moderating effect of DAQS on the TSE and current asthma in children and adolescents, subgroup analysis based on gender was conducted, as presented in Fig. 2. After adjusted age, gender, family history of asthma, energy and BMI in model 2, we found that the moderating effect of DAQS on the association of TSE and childhood asthma remains robust, especially in girls (OR = 1.42, 95%CI: 0.93–2.17).
Discussion
In the present study, using the moderating effect analysis, we found that there was a moderating effect of DAQS to the association between TSE and current asthma among children and adolescents, especially in girls. That was, the higher the DAQS, the smaller effect of TSE on the risk of asthma in children and adolescents. Our study provides a reference for developing more targeted strategies for prevention and treatment of current asthma in children and adolescents.
TSE is associated with lots of health hazards. While the proportion of adults who smoke and children exposed to tobacco smoke continues to decline, the proportion of TSE children was still substantial at about 50%. Previous, various evidence suggested that individual who exposed to tobacco smoke had a higher risk of asthma [11, 27,28,29]. Akinbami et al. [11] focused on the environmental tobacco smoke and asthma in children suggested that 53.3% of children aged 6–9 years old suffer from asthma were TSE exposed. Even TSE resulting in low serum cotinine concentrations was related to risks for children with asthma. An analyses from Phase Three of the ISAAC programme reported that children exposed to tobacco smoke had a higher risk of asthma in both age groups of 6–7 and 13–14 years old, and there was a clear dose relationship between maternal smoking and asthma symptoms [27]. Wang et al. [28] concluded that TSE associated with the more severe asthma attacks. Children with asthma and TSE were twice as likely to be hospitalized for asthma exacerbation and are more likely to have the bad outcome of pulmonary function. Similar results were also reported by Andrews et al. [29] that there was a significantly related to length of stay at both institutions among children hospitalized for asthma. Cohen et al. [30] showed that in utero TSE increased age-related airway hyper responsiveness as well as reduced the efficacy of inhaled corticosteroids among asthmatic children. Moreover, a study from Israel examined the association between cotinine concentration in serum, urine and saliva and the severity of asthma. Based on the Global Initiative for Asthma (GINA) classification, percentage of severe asthmatic patients was significantly higher in passive smoker group. In passive smoker group, the concentration of cotinine in serum, urine and saliva were higher than moderate ad mild asthma [31]. These findings gave support to the results of our study, which observed that children exposed to tobacco smoke had a higher risk of asthma. The potential mechanism for the relationship between TSE and asthma might be Inflammatory response and oxidative stress. Oxidative stress is an important feature of the pathophysiology of asthma, and chemicals such as nicotine in cigarettes can cause elevated levels of reactive oxygen species (ROS) in vivo [32]. The accumulation of ROS further enhances the oxidative stress response, which can damage cellular and subcellular targets such as lipids, proteins and nucleic acids, thereby inducing airflow obstruction, airway hyperresponsiveness and remodeling, and ultimately promoting the occurrence of asthma [33].
Dietary supplements are widely acknowledged to offer the potential to improve health if appropriately targeted to those populations in need [34]. Airways in asthmatic patients have specific inflammatory abnormalities associated with increased generation of ROS and the tissue damage by free radicals [35]. Dietary antioxidants such as vitamin A, C and E may protect the respiratory system from oxidants. Several studies have reported an association between selected dietary antioxidants and asthma. A latent class analysis showed that dietary inflammatory index (DII), an index indicated an individual’s diet on a continuum, from the most anti-inflammatory to the most pro-inflammatory, was associated with the high-burden asthma [36]. In a Swedish birth cohort study, Rosenlund et al. [12] showed that Mg intake was inversely related to asthma. A cross-sectional study from the NHANES database found that children with high intakes of vitamin C and E may be related to a reduced prevalence of asthma [13]. A study in urban and rural Saudi Arabia reported that after adjustment for all covariates, the lowest intakes of vitamin E, Mg and sodium related significantly and independently to the risk of asthma [37]. Recently, a study included 501 children from 20 public schools located in Portugal showed that after adjustment, the exposure effect of PM2.5/10 levels on children with asthma was higher for those having a pro-inflammatory diet, highlighting the relevance of children’s diet as a potential protective factor to pollutant exposure in children with asthma [16]. In our study, we also observed the moderating effect of DAQS on the association between TSE and asthma in children. Among children exposed to tobacco smoke, the risk of asthma in children with high DAQS level was reduced from 43 to 15% compared with children with low DAQS levels. TSE can induce oxidative stress and inflammation on the respiratory airways, and antioxidants plays a vital role in relieving the severity and controlling asthma. Possible pathways by which dietary antioxidants modulate the association between TSE and childhood asthma was through reducing oxidative stress and inflammation in the airway caused by TSE.
Herein, we provided reference for the prevention of childhood asthma and increase social well-being by exploring the moderating effect of DAQS, a comprehensive index reflecting the antioxidant capacity of dietary antioxidants, on the risk of asthma in children exposed to tobacco smoke. Previous study has shown that parental food habits and feeding strategies are the most dominant determinants of a child’s eating behaviors and food choices [38]. Our study highlights the benefits of a healthy diet rich in dietary antioxidants in reducing the risk of asthma in children and provides a reference for parents and pediatricians to manage a healthy diet for children and adolescents. Nevertheless, several limitations need caution in interpreting our findings. First, this was a cross-sectional study, only the moderating effect of DAQS on the relationship between tobacco smoke exposure and the odds of asthma in children and adolescents could be found, and causal association could not be inferred. Second, the information on physical activity time and family income was self-reported by the study children, which may be affected by recall bias. Further large-scale prospective cohort studies are needed to conduct to explore the moderating effect of DAQS on the association between tobacco smoke exposure and asthma in children and adolescents.
Conclusion
This study explored the effect of DAQS on the association between asthma and TSE among children and adolescents. The results found that DAQS has a moderating effect on the relationship between TSE and the risk of asthma in children and adolescents, especially among girls. The findings suggested that children and adolescents exposed to tobacco smoke are recommended to keep healthier diet to decrease their risk of asthma.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.
Abbreviations
- CDC:
-
Centers for Disease Control and Prevention
- DAQS:
-
Dietary antioxidant quality score
- Mg:
-
Magnesium
- Zn:
-
Zinc
- Se:
-
Selenium
- NHANES:
-
National Health and Nutrition Examination Survey
- NCHS:
-
National Center for Health Statistics
- BMI:
-
Body mass index
- MEC:
-
Mobile examination center
- MET:
-
Metabolic equivalent task
- S.E.:
-
Standard error
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
References
Banno A, Reddy AT, Lakshmi SP, Reddy RC. Bidirectional interaction of airway epithelial remodeling and inflammation in asthma. Clin Sci (Lond). 2020;134:1063–79.
Patel SJ, Teach SJ. Asthma. Pediatr Rev. 2019;40:549–67.
Abrams EM, Szefler SJ, Becker AB. What Is the Role of Increasing Inhaled Corticosteroid Therapy in Worsening Asthma in Children? J Allergy Clin Immunol Pract. 2019;7:842–7.
Moraes-Ferreira R, Brandao-Rangel MAR, Gibson-Alves TG, Silva-Reis A, Souza-Palmeira VH, Aquino-Santos HC, et al. Physical Training Reduces Chronic Airway Inflammation and Mediators of Remodeling in Asthma. Oxid Med Cell Longev. 2022;2022:5037553.
Cibella F, Cuttitta G, Della Maggiore R, Ruggieri S, Panunzi S, De Gaetano A, et al. Effect of indoor nitrogen dioxide on lung function in urban environment. Environ Res. 2015;138:8–16.
Aithal SS, Gill S, Satia I, Tyagi SK, Bolton CE, Kurmi OP. The Effects of Household Air Pollution (HAP) on Lung Function in Children: A Systematic Review. Int J Environ Res Public Health. 2021;18(22):11973. https://doi.org/10.3390/ijerph182211973.
Garcia-Larsen V, Del Giacco SR, Moreira A, Bonini M, Charles D, Reeves T, et al. Asthma and dietary intake: an overview of systematic reviews. Allergy. 2016;71:433–42.
Garcia-Marcos L, Castro-Rodriguez JA, Weinmayr G, Panagiotakos DB, Priftis KN, Nagel G. Influence of Mediterranean diet on asthma in children: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2013;24:330–8.
Vanker A, Gie RP, Zar HJ. The association between environmental tobacco smoke exposure and childhood respiratory disease: a review. Expert Rev Respir Med. 2017;11:661–73.
Lang JE, Dozor AJ, Holbrook JT, Mougey E, Krishnan S, Sweeten S, et al. Biologic mechanisms of environmental tobacco smoke in children with poorly controlled asthma: results from a multicenter clinical trial. J Allergy Clin Immunol Pract. 2013;1:172–80.
Akinbami LJ, Kit BK, Simon AE. Impact of environmental tobacco smoke on children with asthma, United States, 2003–2010. Acad Pediatr. 2013;13:508–16.
Rosenlund H, Magnusson J, Kull I, Håkansson N, Wolk A, Pershagen G, et al. Antioxidant intake and allergic disease in children. Clin Exp Allergy. 2012;42:1491–500.
Nakamura K, Wada K, Sahashi Y, Tamai Y, Tsuji M, Watanabe K, et al. Associations of intake of antioxidant vitamins and fatty acids with asthma in pre-school children. Public Health Nutr. 2013;16:2040–5.
Zhong Y, Zhang Z, Hu Y. The combined effects of overweight/obesity and dietary antioxidant quality score on hypertension in children and adolescents. BMC Pediatr. 2023;23(1):584. https://doi.org/10.1186/s12887-023-04397-0.
Nikrad N, Shakarami A, Tousi AZ, Farhangi MA, Ardekani AM, Jafarzadeh F. Dietary Antioxidant Quality Score (DAQS), serum lipids, markers of glucose homeostasis, blood pressure and anthropometric features among apparently metabolically healthy obese adults in two metropolises of Iran (Tabriz and Tehran): a cross-sectional study. BMC Endocr Disord. 2023;23:157.
Luu HN, Wen W, Li H, Dai Q, Yang G, Cai Q, et al. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid Redox Signal. 2015;22:951–9.
de Castro MF, Paciência I, Cavaleiro Rufo J, Silva D, Cunha P, Farraia M, et al. The inflammatory potential of diet impacts the association between air pollution and childhood asthma. Pediatr Allergy Immunol. 2020;31:290–6.
Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1. 2013;(56):1–37.
Li C, Richter P, Cobb LK, Kuiper HC, Seymour J, Vesper HW. Dietary Sources of Plasma trans Fatty Acids among Adults in the United States: NHANES 2009–2010. Curr Dev Nutr. 2021;5:nzab063.
Tur JA, Serra-Majem L, Romaguera D, Pons A. Does the diet of the Balearic population, a Mediterranean type diet, still provide adequate antioxidant nutrient intakes? Eur J Nutr. 2005;44:204–13.
Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. https://doi.org/10.1093/oxfordjournals.epirev.a017925.
Espenship MF, Silva LK, Smith MM, Capella KM, Reese CM, Rasio JP, et al. Nitromethane Exposure from Tobacco Smoke and Diet in the U.S. Population: NHANES, 2007–2012. Environ Sci Technol. 2019;53:2134–40.
Chin HL, Cheong KK. Association between asthma and headache: Findings from the NHANES 2001–2004. Clin Respir J. 2023;17:799–804.
Mendes MA, da Silva I, Ramires V, Reichert F, Martins R, Ferreira R, et al. Metabolic equivalent of task (METs) thresholds as an indicator of physical activity intensity. PLoS ONE. 2018;13:e0200701.
Jia G, Wu CC, Su CH. Dietary inflammatory index and metabolic syndrome in US children and adolescents: evidence from NHANES 2001–2018. Nutr Metab (Lond). 2022;19:39.
Whooten RC, Perkins ME, Gerber MW, Taveras EM. Effects of Before-School Physical Activity on Obesity Prevention and Wellness. Am J Prev Med. 2018;54:510–8.
Mitchell EA, Beasley R, Keil U, Montefort S, Odhiambo J. The association between tobacco and the risk of asthma, rhinoconjunctivitis and eczema in children and adolescents: analyses from Phase Three of the ISAAC programme. Thorax. 2012;67:941–9.
Wang Z, May SM, Charoenlap S, Pyle R, Ott NL, Mohammed K, et al. Effects of secondhand smoke exposure on asthma morbidity and health care utilization in children: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2015;115:396-401.e2.
Andrews AL, Shirley N, Ojukwu E, Robinson M, Torok M, Wilson KM. Is secondhand smoke exposure associated with increased exacerbation severity among children hospitalized for asthma? Hosp Pediatr. 2015;5:249–55.
Cohen RT, Raby BA, Van Steen K, Fuhlbrigge AL, Celedón JC, Rosner BA, et al. In utero smoke exposure and impaired response to inhaled corticosteroids in children with asthma. J Allergy Clin Immunol. 2010;126:491–7.
Hassanzad M, Khalilzadeh S, Eslampanah Nobari S, Bloursaz M, Sharifi H, Mohajerani SA, et al. Cotinine level is associated with asthma severity in passive smoker children. Iran J Allergy Asthma Immunol. 2015;14:67–73.
Ramalingam A, Budin SB, Mohd Fauzi N, Ritchie RH, Zainalabidin S. Targeting mitochondrial reactive oxygen species-mediated oxidative stress attenuates nicotine-induced cardiac remodeling and dysfunction. Sci Rep. 2021;11(1):13845. https://doi.org/10.1038/s41598-021-93234-4. Published 2021 Jul 5.
Michaeloudes C, Abubakar-Waziri H, Lakhdar R, et al. Molecular mechanisms of oxidative stress in asthma. Mol Aspects Med. 2022;85: 101026. https://doi.org/10.1016/j.mam.2021.101026.
Rautiainen S, Manson JE, Lichtenstein AH, Sesso HD. Dietary supplements and disease prevention - a global overview. Nat Rev Endocrinol. 2016;12:407–20.
Riccioni G, Barbara M, Bucciarelli T, di Ilio C, D’Orazio N. Antioxidant vitamin supplementation in asthma. Ann Clin Lab Sci. 2007;37:96–101.
Cilluffo G, Han YY, Ferrante G, Dello Russo M, Lauria F, Fasola S, et al. The Dietary Inflammatory Index and asthma burden in children: A latent class analysis. Pediatr Allergy Immunol. 2022;33:e13667.
Hijazi N, Abalkhail B, Seaton A. Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax. 2000;55:775–9.
Mahmood L, Flores-Barrantes P, Moreno LA, Manios Y, Gonzalez-Gil EM. The Influence of Parental Dietary Behaviors and Practices on Children's Eating Habits. Nutrients. 2021;13(4):1138. https://doi.org/10.3390/nu13041138.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Wei Lin designed the study, Jinliang Lin wrote the manuscript, Fuhuang Lai and Jiaqiang Shi collected, analyzed and interpreted the data, Wei Lin critically reviewed the manuscript, all authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The requirement of ethical approval for this was waived by the Institutional Review Board of Longyan First Hospital Affiliated to Fujian Medical University, because the data was accessed from NHANES (a publicly available database). The need for written informed consent was waived by the Institutional Review Board of Longyan First Hospital Affiliated to Fujian Medical University due to retrospective nature of the study. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, W., Lin, J., Lai, F. et al. Effect of dietary antioxidant quality score on tobacco smoke exposure and asthma in children and adolescents: a cross-sectional study from the NHANES database. BMC Pediatr 24, 535 (2024). https://doi.org/10.1186/s12887-024-05009-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-05009-1