Abstract
Background
Swimming pools are places for practicing sports, recreation, relaxation, and socialization. However, swimming pools can expose swimmers to physicochemical and microbiological risks. Accordingly, we studied the environmental health aspects and microbial infections for such recreational water aiming to disclose the possible risks they pose on swimmers.
Methods
26 pools in Alexandria, Egypt were checked for water quality; 13 pools were checked in winter then summer, and other 13 pools were checked in summer only. Water was collected from both the top and the bottom of each pool; a total of 78 samples were collected in sterile containers. Each sample was divided into three parts; the first part was used for assessing the bacteriological quality of water. They were tested for total colony count (TCC), total coliform (TC), fecal coliform, and E. coli. The second part was used for chemical analysis. The third part was checked for parasitological study.
Results
Obtained data showed that only 7.7%, 78.2%, and 100% of the examined water samples have been found to fulfill the Egyptian standards for TCC, TC, and E. coli, respectively. Moreover, parasitic infection (PI) was noticed in 73.1% of the collected water samples; mainly Cyclospra and Isospora (37.2% each), followed by Cryptosporidium spp., Giradia lamblia, Microsporidia spp., and Blastocystis spp. (34.6%, 21.8%, 15.4%, and 14.1%, respectively). Acanthameba spp. was detected but at a lower rate (5.1%). The frequency of cleaning the swimming pools, flow rate, Cl2, and total dissolved solids are significantly affected PI, independently.
Conclusion
The tested water samples don’t meet Egyptian bacteriological criteria. High parasitic contamination despite high residual chlorine level mainly intestinal coccidia, G. lamblia, microsporidia, and Blastocystis spp. Thus, monitoring pool’s water quality and improving the disinfection system are mandatory. Consequently, Health education regarding hygienic behaviors before and during swimming should be included in governmental programs.
Similar content being viewed by others
Introduction
Swimming is considered a healthy activity, and swimming pools are places for practicing sports, recreation, relaxation, and socialization [1]. However, swimming pools can also expose swimmers to several physical, chemical, and microbiological risks [2]. Poor management of swimming pools poses a high risk of microbial infections due to fecal and non-fecal sources [3]. Fecal contamination of the water is the main microbiological risk of bathing in recreational water which may be due to accidental release of remnants of fecal matter that could remain on skin of children, old and immune-compromised people especially after improper post-defecation cleansing. So, exposure to such poor microbiological water quality may pose health risks for swimmers, causing gastro-enteritis as a result of infections with bacteria, viruses, or parasites of fecal origin [4, 5]. Centers for Disease Control and Prevention (CDC) recorded 134 outbreaks of recreational water infections in the USA from 2007 to 2008 resulting in 25,000 cases [5]. In 2017, Rice and his colleagues highlighted the importance of bacteriological indicators including total coliform (TC), fecal coliform (FC) and total colony count (TCC); for determining the microbiological quality of swimming pools [6]. Moreover, fecal contamination is one of the most important factors for measuring the microbiological quality of water in swimming pools [7]. Detecting enteric pathogens, such as Salmonella typhi, Salmonella paratyphi, Shigella dysenteriae and Vibrio cholerae is difficult and costly [8]. Therefore, they are usually replaced by detecting Escherichia coli (E. coli), a strong fecal indicator [2, 9]. Tiwari et al. (2021) reported that coupling of pathogen indicators with microbial source tracking process for targeting different fecal sources, may further strengthen the process of tracking bathing waters and deliver better understanding to protect the health of bathers [10].
Moreover, at least 208 outbreaks associated with recreational water occurred In the United States during 2015–2019 [11]. Most of these outbreaks were caused by Cryptosporidium spp., mainly in swimming pools, and by Legionella, mainly during the period from June to August [9]. In 2017, CDC revealed that at least 32 outbreaks were caused by Cryptosporidium spp. in the United States compared to 16 outbreaks in 2014 [12]. It is typically common that when some people swim while having diarrhea definitely will put others at risk of swallowing contaminated water [13], and accidently causing chronic persistent diarrhea among immunocompromised group of people [14]. Although Microsporidia spp. are currently uncommon causes of recreational waterborne disease, their role as emerging pathogens is increasingly recognized. Their small size makes them difficult to be removed using conventional water filtration techniques [15]. Additionally, recreational water could be contaminated by other parasites including G. lamblia and free-living amebae (FLA). G. lamblia causes gastrointestinal infections with certain complications including malabsorption, steatorrhea, and growth retardation in children [16]. Pathogenic and opportunistic FLA can cause serious infections in humans [17], whereas Acanthameba spp. can produce corneal keratitis as well as chronic granulomatous amebic encephalitis [18].
Various trace chemical contaminants have been reported to occur in swimming pools [19]. Possible sources of these chemical contaminants include the water sources, bather-derived chemicals, and pool maintenance chemicals [20]. Hence, it is necessary to frequently measure the chemical quality of pool water. The American National Standard Institute has developed certain procedures to measure the water quality in public pools, including levels of total alkalinity, calcium and magnesium hardness, total dissolved solids (TDS), pH, and free chlorine contents [21]. The noncompliance of alkalinity and calcium hardness to recommended standards poses risks. Accordingly highlight the significance of these parameters in maintaining pH, sanitizer efficacy, water balance, and clarity [21]. Furthermore, filtration and disinfection are useless without proper turnover rates; so, pathogens could not be eliminated [22]. Few types of research were conducted on pool water quality, and most of them revealed poor compliance with pool water standards, especially in Alexandria; consequently, there was a strong need to improve disinfection and cleaning procedures [23]. Therefore, the current comprehensive study was carried out to investigate the operations of most of swimming pools found in Alexandria, Egypt, by collecting water samples from the top and the bottom of the swimming pools during wet and dry seasons. Then, investigating and correlating different parameters such as physicochemical, bacteriological, parasitological, and fecal indicators. So, aiming to scrutinize health and hygienic aspects of these pools, disclose the possible risks they pose on swimmers and try to find best ways for improvement.
Materials and methods
Study setting
Twenty-six pools (governmental and private) from 14 clubs in Alexandria, Egypt, were examined during the period from November 2020 to August 2021. Thirteen pools were double-checked, once in winter and the second time in summer while the remaining 13 pools were examined in summer only. The study was approved by the ethics committee of the High Institute of Public Health, Alexandria University in Alexandria. (HIPH556).
Physicochemical aspects
The data of physicochemical aspects of each pool examined were obtained from pool maintenance office. Data included: the type of disinfectant used, filter capacity and cycling, turnover, pH, temperature, Pool length, water volume, work duration, and bathers’ load.
Sample collection
Two water samples were collected from each pool during each examination: one from the top (50 cm below the surface) and one from the bottom (50 cm above the floor). So, a total of 78 water samples were collected in sterile containers.
Water sampling for chemical analysis
Samples were collected in sterile containers (100 ml each). The samples were transferred to the Drug Research Center in Pharos University in Alexandria to be processed.
Water sampling for bacteriological analysis
Samples were collected in sterile well-labeled one-liter ground glass stoppered bottles. These bottles contained sodium thiosulfate (100 mg/l) (Na2S2O3, sodium thiosulfate pentahydrate, 106509, Merck KGaA, Darmstadt, Germany) to dechlorinate water samples. The samples were transported within an hour using insulated coolers (4°C) to the Microbiology laboratory at the High Institute of Public Health, Alexandria University. Samples were processed immediately after arrival at the laboratory.
Water sampling for parasitological analysis
Each sample was collected in two 5L containers. The samples were transferred to the High Institute of Public Health, Alexandria University, for immediate processing.
Water analysis
All methods were performed in accordance with the Declaration of Helsinki.
Physicochemical analysis
pH, total alkalinity, total dissolved solids (TDS), total hardness (TH), and chloride were analyzed physicochemically according to conventional techniques to determine the water quality.
The pH was measured using a standard pH meter HANNA instrument; total alkalinity using methyl orange, TDS using standard methods [24] Martini instrument (Mi 170 Bench Meter), while total hardness (Ca and Mg) by EDTA titrimetric method [25], and chloride content by Mohr’s method [26].
Biological tests
Bacteriological analysis
Each sample was vigorously shaken and mixed before bacteriological examination.
Total aerobic colony count (TCC)
The TCC agar media was prepared according to the manufacturer’s instructions and poured into petri dishes. It was then incubated at 37°C for 18 hrs. to assure its sterility. The samples were 10-fold serially diluted in sterilized peptone water. Then, 100 µl of each sample and/or appropriate dilutions were plated onto the TCC agar and surface spread using a sterilized glass spreader for uniform inoculation. Next, the plates were incubated at 37°C for 48 hrs. Following an appropriate period of incubation, all visible colonies were counted, and the results were calculated by multiplying the number of colonies on each plate by the reciprocal of the dilution factor, and then multiplied by 10, which were reported as colony-forming units per milliliter [27].
Parasitological analysis
Water samples transferred to the Tropical Health Laboratory were filtered through sterile 0.45 µm membrane filters (Sartorius Stedim Biotech, Germany) within 2 hrs of collection. Two membrane filters were used for each sample. Three thin smears were prepared from each first membrane filter then leftovers, air-dried, and fixed. Smears were stained with Modified Ziehl Neelsen (MZN) [24], quick hot Gram chromotrope [28] and trichrome stains [29] for detecting intestinal coccidia, Microsporidia spp. and intestinal protozoa, respectively. Stained smears were examined under a light microscope using 1000× magnification. Afterward, the same filter membrane was covered by 10 ml formalin (10%) and stirred; dissolved formalin residues were collected and centrifuged [24], to be examined under a light microscope using 400× magnification
The second membrane filters were inverted on 1.5% non-nutrient agar seeded with inactivated E. coli in glass petri dishes for detecting Acanthameba spp. Monoxenically [30]. The culture plates were incubated at room temperature and monitored for trophozoites and/or cysts daily for up to two weeks by inverted phase-contrast microscope (CK; Olympus, Tokyo, Japan) using 10×and 40×objectives lens. The confirmation of Acanthameba, based on size and the presence of acanthopodia and a large central karyosome, was conducted using trichrome-stained smears which were examined microscopically at 1000× magnification under a light microscope [31].
Fecal indicators
Determination of total coliform (TC), fecal coliform (FC), and E. coli by the multiple tube fermentation technique as follow:
Presumptive phase
A multiple tube dilution method was conducted for the TC and FC using lauryl sulphate tryptose broth (LST). Three rows of five Durham fermenting tubes were used. Each tube of the first raw had a 10 ml double-strength LST medium, whereas the other tubes contained a 10 ml single-strength LST medium. The samples were vigorously shaken and each fermentation tube of the first, second and third raw was inoculated with 10, 1, and 0.1 ml of the sample, respectively. The tubes were gently shaken and incubated at 35°C ± 0.5°C. After 24 ± 2hrs., the tubes were examined for the presence of gas and turbidity. Negative tubes were re-incubated for another 24 hrs. at 35°C ± 0.5°C. All positive presumptive tubes showing gas and turbidity within 48 ± 2hrs were submitted to the confirmed phase [27].
Confirmed phase
Two sets of fermentation tubes containing brilliant green lactose bile broth were racked in the same manner as the positive presumptive tubes. The positive tubes were gently shaken, and two loopfuls from each tube were transferred into its corresponding confirmed tubes using a sterile metal loop. One set of tubes was incubated at 35°C ± 0.5°C, whereas the other was incubated at 44°C ± 0.5°C in a covered water bath. The tubes that showed gas and turbidity at any time within 48 hrs. were considered positive for TC (first set) or FC (second set). The most probable number (MPN) was calculated from the MPN tables and recorded as MPN/100 confirmed TC or FC, respectively [27].
Completed phase
Finally, a complete test for E. coli was conducted. Positively confirmed tubes for FC were streaked on eosin methylene blue plates and incubated for 24 hrs. at 37°C. Typical dark-centered nucleated colonies were subjected to Gram stain, triple sugar iron (TSI), and indole, methyl red, Voges Proskauer (VP), and Simmon citrate (IMViC) tests. E. coli was identified as gram-negative non spore forming rods. The TSI result of E. coli was acid slant/acid with gas but without H2S. The IMViC results for E. coli were positive for indole and MR, but negative for VP and citrate tests [27].
Statistical analysis
Data were entered, verified, and analyzed using SPSS version 25.0 (IBM, Armonk, USA). Differences and associations were tested using Pearson’s chi-squared or Fisher’s exact test, and the odds ratio (OR) with its corresponding 95% confidence interval (CI) was calculated to identify infection or association predictors. Multivariate logistic regression was conducted for establishing the relationship between independent variables (physicochemical parameters) and dependent factors (parasitic or bacterial water contaminants) to predict categorical variables.
Results
Physicochemical analysis
Table 1 shows the physical and chemical aspects of water samples collected from the swimming pools, presented as mean ± SD. Residual chlorine was 2.68 ± 0.107 ppm, TDS was 1304.78 ± 48.87 ppm, pH was 6.026 ± 0.0835, alkalinity was 206.56 ± 9.34 mg/l, TH was 457.69 ± 10.40 mg/l, and Ca and Mg were 302.08 ± 6.86 g/l and 155.62 ± 3.538 g/l, respectively.
Bacteriological analysis and chemical parameters compared to egyptian standards
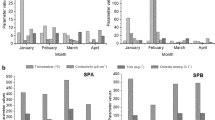
The number of water samples that complied with the bacteriological and chemical Egyptian standards is demonstrated in Table 2. Regarding the bacteriological parameters, 7.7%, 78.2%, and 100% of the surveyed samples complied with the Egyptian standard of TCC (< 100CFU/ml), TC (0 CFU /100 ml), and E. coli (0 CFU /100 ml), respectively. However, only 28.2% and 11.5% of these samples were complying with the residual chlorine (1-1.5ppm) and pH levels (7.2–7.8), as determined by the Egyptian standards, respectively. Table 3 shows no difference was noted in the percentage of positive TCC (≥ 100CFU/ml) and TC (≥ 1CFU/100 ml) regarding different chlorine levels. This result shows that with residual chlorine above the Egyptian standard (> 1.5 ppm), TCC (≥ 100CFU/100 ml) and TC (≥ 1 CFU/100ml) represented 72.2% and 76.5%, respectively, compared with 27.8% and 23.5% with a standard chlorine level of 1-1.5 ppm. Table 4 shows that TCC and TC have a positive correlation between the number of swimmers and temperature; but only TCC shows a significant correlation with pH. TCC and TC were negatively correlated with residual chlorine levels; however, this was not statistically significant. Figures 1 shows that collected samples were positive for TCC in dry and wet seasons [(100% and 38.6%, respectively) and for TC in dry and wet seasons (82.4% and0.0%) respectively] with no statistically significant difference.
Parasitic infection (PI) and fecal indicators
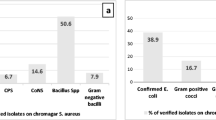
Table 5 shows a total PI rate of 73.1% in water samples of the studied swimming pools. It also, shows no statistically significant differences between dry and wet seasons; however, the dry season was a possible risk factor for PI, two times more than to the wet season ([79.5% vs. 64.7%] [OR = 2.121, 95% CI = 0.768–5.856]). Additionally, the odd’s ratio of PI in pools with heavy daily bather’s load (250–500 bathers /h) was twice compared to daily load of 100- <250 bathers/h ([83.3% vs. 66.7%] [OR = 2.500, 95% CI = 0.806–7.757]). Similarly, higher water temperatures (≥ 27oC) were two times at higher risk to harbor parasites compared to cooler water (< 27o C (77.6% vs 60.0%, OR = 2.308, 95% CI = 0.778–6.842). Longer durations of swimming pool work (> 10 h/day) were almost 3 times more likely related to PI than durations of ≤ 10 hrs/day ([84.4% vs. 65.2%] [OR = 2.880, 95% CI = 0.930–8.923]). The turnover of 6 hrs was two times more likely correlated with PI that turnover of < 6 h ([81.8% vs. 69.6%] [OR = 1.962, 95% CI = 0.577–6.671]). Abnormal pH and alkalinity levels could be possible risk factors for PI compared with the normal ones ([73.9% vs. 66.7%, for pH] [OR = 1.417, 95% CI = 0.320–6.26]) and ([75.5% vs. 68.0%, for alkalinity] [OR = 1.448, 95% CI = 0.508–4.128]), respectively. On the other hand, superficially collected samples were at significant higher risk for PI compared with the deeply collected ones ([84.6% vs. 61.5%] [OR = 3.437, 95% CI = 1.164–10.152]). Regarding the frequency of cleaning the pools, it was found that the PI increased with decreasing the frequency of cleaning with a statistically significant difference (P = 0.030). Meanwhile, samples from highly circulated water (≥ 80 m3/min) had a high rate of PI compared to those circulated at < 80 m3/min (88.9% vs. 37.5%), and the difference was statistically significant (P < 0.001). Cl2 > 1.5 ppm was approximately nine times of higher risk for PI than Cl2 of 1 -1.5 ppm [(85.7% vs. 40.9) (OR = 8.667, 95% CI = 2.793–26.896)]. Similarly, TDS ≥ 1500 ppm and TH > 400 mg/l were risk factors for PI compared with TDS < 1500 ppm and TH < 400 mg/l, respectively, with a statistically significant difference. The multivariate logistic regression study for physicochemical parameters to predict the values of categorical variables affecting pools’ water contamination by parasites, showed that the frequency of cleaning the swimming pools, flow rate, Cl2, and TDS were significantly affecting PI independently (Table 6). As regards types of parasites, intestinal coccidia were the most prevalent parasites in samples examined (Cyclospra & Isospora 37.2%, and Cryptosporidium spp., 34.6%). Acanthameba spp. rate amounted to 5.1% while E. coli and E. histolytica showed the lowest rates (2.6% and 1.3% respectively) (Fig. 2).
Discussion
Physicochemical parameters including Cl2, TDS, pH, alkalinity, TH, Ca, and Mg showed great fluctuations in all the investigated swimming pools. Most samples violated the 1995 Egyptian Ministry of Health Decree no 418 standards. This was in accordance with a previous study done [23] in Alexandria in 2012 concluded that none of the surveyed swimming pools was met the Egyptian Standards for Swimming Pool Water no. 418/1995. Iranian research [32] recorded higher rates of pH, decreased Ca, temperature, and alkalinity all of them met Iranian standard. However free residual chlorine was lower and the mean TH was higher than the standard.
Most of the swimming pool water samples (92.3%) did not meet Egyptian bacteriological criteria. This conclusion is comparable with those of other studies in Alexandria, where 92.2–94.4 percent of water samples were bacteriologically unacceptable [3]. In Ghana, Amman, and Palestine, all water tests didn’t meet bacteriological requirements [33,34,35]. Noncompliance was due to poor maintenance, operator training, disinfection, and evaluation. In other studies, lower percentages were reported in Alexandria (43.3%), Italy (34.2%), and Greece (32.9%) [23, 36, 37]. In a study conducted a year before and during the Olympic Games in Athens 2004, swimming pool noncompliance was found to be reduced from 16% in 2003 to 0% in August 2004. This study’s outcome emphasizes the necessity of regular pool inspection [38].
Regarding the bacteriological parameters, the current study showed that 7.7%, 78.2%, and 100% of the surveyed samples complied with the Egyptian standard of TCC (< 100CFU/ml), TC (0 CFU /100 ml), and E. coli (0 CFU /100 ml), respectively. However, only 28.2% and 11.5% of these samples were complying with the residual chlorine (1-1.5ppm) and pH levels (7.2–7.8), as determined by the Egyptian standards, respectively. The high bacteriologically unaccepted water samples in the present study were attributed to the contamination by TCC > 100 CFU/ml, since only 7.7% of the examined swimming pool water samples complied with the Egyptian standards regarding TCC (< 100 CFU/ml). Some of the swimming pools in Alexandria showed better percentages (30.6–68%) [3].
Also, in the present study, 78.2% of water samples did not exceed the Egyptian limit for TC (0 CFU/100 ml). Lower rates were recorded in studies done in Alexandria (47%-57%) and Amman (43%) [3, 34] as well as in Iran (9.0%) and Greece (32.9%) [32, 37]. In Alexandria (2012), TC was also found in all samples and MPN/100 ml > 3.0 in 43.3% of water samples [23]. Different sample sizes, swimming pools, and disinfection techniques may explain these varying percentages.
In this study, none of the water samples examined revealed E. coli. However, other studies detected E. coli in 2.6% -8% of the samples inspected [3, 36]. In Iranian pools, the proportion was 18.2% [39]. An extremely high fecal contamination percentage was detected in Amman swimming pools, where fecal coliforms were detected in 94.7% of the samples containing TC. This shooting result was explained by a lack of pre-swimming baths, foot disinfection, and poor disinfection by untrained pool employees [34].
No difference was noted in the present study regarding the percentage of positive TCC (≥ 100CFU/ml) and TC (≥ 1CFU/100 ml) associated with different chlorine levels. This result shows that with residual chlorine above the Egyptian standard (> 1.5 ppm), TCC (≥ 100CFU/100 ml) and TC (≥ 1 CFU/100ml) represented 72.2% and 76.5%, respectively, compared to 27.8% and 23.5% with a standard chlorine level of 1-1.5 ppm. The current study revealed that TCC and TC have a positive correlation between the number of swimmers and temperature; but only TCC shows a significant correlation with pH. For years, chlorine has been the most applicable disinfectant for swimming pools. However, it must reach a certain level to be effective as a disinfectant. The accepted range of residual chlorine is 1 -1.5 ppm according to the Egyptian standard adopted by the Egyptian Ministry of Health Decree no. 418/1995. This study demonstrated that only 28.2% of the studied swimming pools’ water samples complied with the Egyptian standard regarding chlorine level, whereas 71.8% had unacceptable chlorine levels. This result was similar to those previously reported in Alexandria, where 80% -88.4% of the pools had unacceptable levels of chlorine [23, 40]. However, lower percentages (56% -61.1%) of noncomplying water samples were also reported in Alexandria by Masoud in 2016 [3]. All unacceptable chlorine levels in this study were above the permissible level (> 1.5 ppm), with a mean of 2.68 ppm. This was close to that reported previously by Hamid et al. (1993) [41], who measured the chlorine level in different swimming pools in Alexandria and found that the mean residual chlorine was 2.8 mg/l. The high chlorine level recorded in this study may explain the absence of chlorine sensitive organisms, such as E. coli from all water samples. Different results were reported in other studies showing lower chlorine levels among unaccepted pools’ water in Egypt and Iraq [23, 42]. Additionally, other studies have demonstrated higher and lower residual chlorine levels among noncomplying pools [34, 40]. This high variability of recorded residual chlorine levels indicates the variable amount of chlorine added to the pools (operator dependent) and lack of continuous monitoring and maintenance of pools by the authority.
However, despite high residual chlorine levels, high TCC (> 100 CFU/ml) was demonstrated in 72.2% of the studied water samples. This result may be explained by the presence of chlorine resistant bacteria (CRB). CRB are frequently described as bacteria that exhibit high resistance to chlorine disinfection or bacteria which may persist or regrow within the residual chlorine. Therefore, complete control of CRB infectivity cannot be achieved through chlorine disinfection. Another study focused on CRB pathogenicity and antibiotic resistance. Researchers found that Mycobacterium, Bacillus, Legionella, Pseudomonas and Sphingomonas are among the commonest pathogenic CRB genera, which are mainly pathogenic. However, nonpathogenic CRB were overlooked by other researchers [43, 44]. In a previous study, there was an overexpression of antibiotic resistant genes in some CRB which tolerates different chlorine levels, indicating a chlorine-associated induction of antibiotic resistance in the pathogen [43].
Mucoid Pseudomonas aeruginosa strains survive and regenerate better in chlorinated pool water than nonmucoid cultures. This was explained by overexpression of the extracellular polysaccharide layer that acts as a barrier against the disinfecting effect of chlorine [45]. In addition, Jin et al. (2020) [46] showed that chlorinated water improved plasmid transfer through genetic transformation. These transfer organelles were unaffected by chlorination, resulting in antibiotic-resistant bacteria. Environmental pollutants, CODMn, NH4+–N, and metal ions accelerated this reaction. The present study showed that 100% and 38.6% of the collected samples during the dry season, were positive for TCC and TC, respectively, whereas 82.4% and none of the samples collected in the wet season were positive for TCC and TC, respectively, with no statistically significant difference.
Globally, bathing in swimming pools is a channel for infection transmission; therefore, evaluation of parasitic infections in relation to physicochemical parameters pools’ water is mandatory [47]. It had been shown that mismanaged swimming pools may result in waterborne diseases [32]. The contamination of pools with pathogenic protozoa poses a serious threat to bathers [48]. In the current study, a PI rate of 73.1% was recorded in the water of studied swimming pools. Intestinal coccidia showed the highest rate of infections (Cyclospra & Isospora 37.2%, and Cryptosporidium spp., 34.6%), which may be attributed to their abilities to survive for months at ambient temperatures in moist environments [49]. This was followed by G. lamblia, 21.8%; Blastocystis spp., 14.1%; and Microsporidia spp., 15.4%. Lower rates of infections (≤ 2.6%) were reported for E. coli, and E. histolytica. Meanwhile, Acanthameba spp., rate was 5.1%.
High rates of Cryptosporidium spp. were detected in swimming pools in Italy and the United States (40% and 55.6%, respectively) [50, 51]. In contrast, other studies done in Italy and Netherlands revealed lower rates (28.6% and 4.6%, respectively) [52, 53]. Recently, Chalmers et al. [54] detected Cryptosporidium oocysts in 12/59 (20%) of pool water samples and increased to 8/12 (66%) in pool samples collected in August when bather loads were highest. The study done by Abd El-Salam in 2012 reported a low rate of both Cryptosporidium and G. lamblia (10%) in the five swimming pools selected randomly from different districts in Alexandria [23]. Meanwhile, these two parasites were detected with rates of (16.7% and 15.0%, respectively) in the 60 water samples collected from 35 swimming pools in Beijing, China [55]. Lower rates of G. lamblia were even detected in the Netherlands and United States (5.9% and 5.6%, respectively) [51, 53]. Contrarily, two studies done in Italy in 2004 and 2006 recorded high G. lamblia infection rates (40% and 28.6%, respectively) [50, 52]. The high percentage of contamination might be attributed to high bathers’ load/day, young-aged swimmers, and not having a shower before swimming, all these factors contributed to shedding of pathogens in the water [50].
Fournier et al. [56] reported Cryptosporidia spp. and Microsporidia spp. from one out of the 48 samples collected from six swimming pools in Paris, France, while giardiasis wasn’t detected. Additionally, Galván et al. (2013) [57] estimated a rate of 9% of Cyclospra in the different water sources examined including recreational water.
Several studies in Iran detected higher rates of Acanthameba spp. Than the rate reported by the present study. In 2017, Mafi [58] reported 24% as a rate for Acanthameba spp. in pool water and amusement parks’ pool; meanwhile Solgi et al. and Sarmadian et al. [59, 60], revealed percentages of 20% and 16.7% respectively, in swimming pools. The difference in these studies was attributed to an increase in the water temperature of hot springs.
The present work showed that dry season, heavy daily bathers’ load/h (250–500 bathers/h), higher temperatures (27C), longer duration of swimming pool work (> 10 h/day), turnover of 6 hrs, abnormal pH and alkalinity are associated with higher rate of water contamination by parasites. Superficially collected samples, decreased cleaning frequency, highly circulated water samples (≥ 80 m3/min), Cl2 > 1.5 ppm, TDS ≥ 1500 ppm, and TH > 400 mg/l were proved to be significant risk factors for parasitic contamination of pools’ water. Multivariate logistic regression evaluating physicochemical parameters linked with parasite water contamination revealed that the highest risk variables were pool cleaning frequency, water flow rate, Cl2 and TDS. The increased bather load, extended pool work hours, and infrequent cleaning, altogether, increase both fecal and nonfecal water contamination resulting in high rated of PI [61]. Zangiabadi et al. (2011) [61] reported that the simultaneous use of pools by bathers is associated with microbial contamination risk and subsequent diseases. In 2008, WHO reported that as the temperature increases to more than 27°C, microbial activity would increase accordingly [62]. Reduction in the turnover rate results in increasing the flow rate, which subsequently do not remove particles leading to lower filtration efficiency [63, 64]. In China, it was reported that the rate of Cryptosporidium was higher in August than in May (24.2% and 7.4%, respectively) [55]. In the done in UK, 2021, oocysts were detected in 12/59 (20%) pool water samples, 66% of them were detected in August [54]. Similar data was reported by Sheilds et al., [65] and they attributed it to a high density of bathers due to the hot weather in August in Beijing. The same was found in USA and Urmia, with higher protozoan and fungal contamination recorded during summer for the same reasons, in addition to the high temperature and humidity in this season [66, 67].
Concerning chemical parameters, this study revealed a high parasitic contamination of water in spite of the achieved desired residual chlorine level, and this could be attributed to parasitic resistance to chlorination [61, 62, 68, 69]. Hence, the desirability of physicochemical parameters could be ineffective in removing the parasites even if they were in the standard level, and this agreed with Rabi et al. [34]. A high level of TDS could reduce the chlorine efficacy and could cause dulling in pools’ water clarity [70].
In conclusion, the current comprehensive study revealed that the tested water samples don’t meet the Egyptian bacteriological criteria and about 73% of samples examined were contaminated with parasites in spite that desired chlorine level was achieved. The most common pathogens recorded were intestinal coccidia, G. lamblia, Microsporidia spp., and Blastocystis spp. Acanthameba spp. was also detected but at a low rate. Thus, monitoring the swimming pool’s water quality and improving the disinfection system are both mandatory. Government safety programs should incorporate health education about swimming hygiene.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Abbreviations
- CDC:
-
Centers for Disease Control and Prevention
- CFU:
-
Colony forming unit
- CRB:
-
Chlorine resistant bacteria
- E. coli :
-
Escherichia coli
- FC:
-
Fecal coliform
- G. lamblia :
-
Giradia lamblia
- HIV:
-
Human immunodeficiency virus
- Hrs:
-
Hours
- IMViC:
-
Indole, methyl red, Voges Proskauer (VP), and Simmon citrate
- LST:
-
lauryl sulphate tryptose
- MPN:
-
The most probable number
- MZN:
-
Modified Ziehl Neelsen
- Na2S2O3:
-
Sodium thiosulfate
- OR:
-
Odds ratio
- PI:
-
Parasitic infection
- Ppm:
-
Part per million
- SPSS:
-
Statistical Package for the Social Sciences
- TC:
-
Total coliform
- TCC:
-
Total colony count
- TDS:
-
Total dissolved solids
- TH:
-
Total hardness
- TSI:
-
Triple sugar iron
References
Kamioka H, et al. Effectiveness of aquatic exercise and balneotherapy: a summary of systematic reviews based on randomized controlled trials of water immersion therapies. J Epidemiol. 2010;20:2–12.
Fantuzzi G, et al. Airborne trichloramine (NCl(3)) levels and self-reported health symptoms in indoor swimming pool workers: dose-response relationships. J Expo Sci Environ Epidemiol. 2013;23:88–93.
Masoud G, Abbass A, Abaza A, Hazzah W. Bacteriological quality of some swimming pools in Alexandria with special reference to Staphylococcus aureus. Environ Monit Assess. 2016;188:412.
Bwire G, Mwesawina M, Baluku Y, Kanyanda SS, Orach CG. Cross-border cholera outbreaks in Sub-Saharan Africa, the mystery behind the silent illness: what needs to be done? PLoS ONE. 2016;11:e0156674.
Hlavsa MC, et al. Surveillance for waterborne disease outbreaks and other health events associated with recreational water - United States, 2007–2008. Morbidity and mortality weekly report. Surveillance summaries. 2011;60:1–32.
Baird R, Bridgewater L. Standard methods for the examination of water and wastewater. 23rd edition. Washington, D.C.: American Public Health Association; 2017.
Korajkic A, McMinn BR, Harwood VJ. Relationships between Microbial Indicators and Pathogens in Recreational Water Settings. Int J Environ Res Public Health. 2018;15(12):2842.
Nga TV, et al. The sensitivity of real-time PCR amplification targeting invasive Salmonella serovars in biological specimens. BMC Infect Dis. 2010;10:125.
Tiwari A, Niemela SI, Vepsalainen A, Rapala J, Kalso S, Pikanen T. Comparison of Colilert-18 with miniaturized most probable number method for monitoring of Escherichia coli in bathing water. J Water Health. 2016;14(1):121–31.
Tiwari A, Oliver DM, Bivins A, Sherchan SP, Pitkanen T. Bathing water quality monitoring practices in Europe and the United States. Int J Environ Res Public Health. 2021;18(11):5513.
Hlavsa MC, Aluko SK, Miller AD, Person J, Gerdes ME, Lee S, Laco JP, Hannapel EJ, Hill VR. Outbreaks associated with treated recreational water - United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70(20):733–8.
Centers for Disease Control and Prevention (CDC Newsroom). Crypto outbreaks linked to swimming have doubled since 2014. Centers for Disease Control and Prevention. 2017.
Centers for Disease Control and Prevention (CDC Newsroom). 1 in 3 swimming-related disease outbreaks occur at hotels. Centers for Disease Control and Prevention. 2018.
Gedle D, et al. Intestinal parasitic infections and its association with undernutrition and CD4 T cell levels among HIV/AIDS patients on HAART in Butajira, Ethiopia. J Health Popul Nutr. 2017;36:15.
World Health Organization (WHO). Water Recreation and Disease. Plausibility of Associated Infections: Acute Effects, Sequelae and Mortality. Geneva: Switzerland: WHO; 2005.
Kumar T, et al. Presence of Cryptosporidium parvum and Giardia lamblia in water samples from Southeast Asia: towards an integrated water detection system. Infect Dis Poverty. 2016;5:3.
Schuster FL, Visvesvara GS. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updates. 2004;7:41–51.
Lorenzo-Morales J, et al. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol. 2013;29:181–7.
Teo TL, Coleman HM, Khan SJ. Chemical contaminants in swimming pools: occurrence, implications and control. Environ Int. 2015;76:16–31.
Esinulo AC, Ogbuagu DH. Quality assessment of selected public swimming pools in Owerri Metropolis, Nigeria. Int J Innovative Environ Stud Res. 2016;4:28.
American National Standards Institute (ANSI). American national standard for FPGA Mezzanine Card (FMC) Standard”. ANSI Standard ANSI/VITA. 2018.
Pennsylvania Department of Health. Public swimming and bathing places (aquatic facilities): operational and biological contamination protocol recommendations. Bureau of Community Health Systems. 2016:1–13.
Abd El-Salam MM. Assessment of water quality of some swimming pools: a case study in Alexandria, Egypt. Environmental Monitoring and Assessment. 2012;184:7395–406.
Garcia LS, Bruckner DA. Diagnostic Medical Parasitology. Washington: ASM Press; 2016.
Lewis LL, Melnick LM. Determination of calcium and magnesium with (ethylenedinitrilo) tetraacetic acid. Studies in accuracy. Anal Chem. 1960;3:38–42.
Belcher R, Macdonald AMG, Parry E. On mohr’s method for the determination of chlorides. Anal Chim Acta. 1957;16:524–9.
Rice E, Eaton A, Clesceri L. Standard methods for the examination of water and wastewater (22nd ed.).American Public Health Association (APHA). 2012.
Weber R, et al. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. The enteric opportunistic infections Working Group. New Engl J Med (NEJM). 1992;326:161–6.
John DT, Petri WA, and Martin G . Markell and Voge's Medical Parasitology - Elsevier eBook on VitalSource, 9th Edition. 2006.
Schuster FL. Cultivation of pathogenic and opportunistic free-living amebas. Clin Microbiol Rev. 2002;15(3):342–54.
Dendana F, et al. Free-living amoebae (FLA): detection, morphological and molecular identification of Acanthamoeba genus in the hydraulic system of an hemodialysis unit in Tunisia. Parasite. 2008;15:137–42.
Fadaei A, Amiri M. Comparison of chemical, biological and physical quality assessment of indoor swimming pools in Shahrekord City, Iran in 2013. Global J Health Sci. 2015;7:240–8.
Osei-Adjei G, Sarpong SK, Laryea E, Tagoe E. Bacteriological quality assessment of swimming pools in the Osu-Labadi Area, Accra. J Nat Sci Res. 2014;4:126–9.
Rabi A, Khader Y, Alkafajei A, Aqoulah AA. Sanitary conditions of public swimming pools in Amman, Jordan. Int J Environ Res Public Health (International Journal of Environmental Research and Public Health Editorial Office International Journal of Environmental Research and Public Health. 2008;5:152–7.
Al-Khatib IA, Salah S. Bacteriological and chemical quality of swimming pools water in developing countries: a case study in the West Bank of Palestine. Int J Environ Health Res. 2003;13:17–22.
Leoni E, Legnani P, Guberti E, Masotti A. Risk of infection associated with microbiological quality of public swimming pools in Bologna, Italy. Public Health. 1999;113:227–32.
Papadopoulou C, et al. Microbiological quality of indoor and outdoor swimming pools in Greece: investigation of the antibiotic resistance of the bacterial isolates. Int J Hyg Environ Health. 2008;211:385–97.
Hadjichristodoulou C, et al. Management of environmental health issues for the 2004 Athens Olympic Games: is enhanced integrated environmental health surveillance needed in every day routine operation? BMC Public Health. 2006;6:306.
Hajjartabar M. Poor-quality water in swimming pools associated with a substantial risk of otitis externa due to Pseudomonas aeruginosa. Water Sci Technol. 2004;50:63–7.
Abdou MH, Akel MM, El-Shal WI, El-Naggar AS. Study of the environmental health aspects of swimming pools in Alexandria City. J Egypt Public Health Assoc. 2005;80(1–2):263–96.
Hamid AAA, Farag HFM, Ahmed HY. Study of some health problems among school aged swimming pool swimmers. Bull High Inst Public Health. 1993;23:387–401.
Khalaf SH, Talec AY, Al-Bahawi NE. Water hygiene of swimming pools in Mosul City, Iraq. J Environ Sci Health Part A. 1992;27:1021–31.
Karumathi DP, Yin HB, Kollanoor-Johny A, Venkitanarayanan K. Effect of chlorine exposure on the survival and antibiotic gene expression of multidrug resistant Acinetobacter baumannii in water. Int J Environ Res Public Health. 2014;11:1844–54.
Luo LW, et al. Evaluating method and potential risks of chlorine-resistant bacteria (CRB). Rev Water Res. 2020;27:116474.
Grobe S, Wingende J, Flemming HC. Capability of mucoid Pseudomonas aeruginosa to survive in chlorinated water. Int J Hyg Environ Health. 2001;204:139–42.
Jin M, et al. Chlorine disinfection promotes the exchange of antibiotic resistance genes across bacterial genera by natural transformation. Int Soc Microb Ecol J. 2020;14:1847–56.
Borgmann-Strahsen R. Comparative assessment of different biocides in swimming pool water. Int Biodeterior Biodegrad. 2003;51:291–7.
Cotruvo JA, et al. (2004). Waterborne zoonoses : identification, causes, and control / edited by J. A. Cotruvo ... [et al.]. World Health Organization. 2004. https://apps.who.int/iris/handle/10665/42977.
Chalmers RM, McCann R, Lowe G, Modi AK, Stiff RE, Quigley C, et al. Guidance for the investigation of Cryptosporidium linked to swimming pools. 2011.
Bonadonna L, Briancesco R, Magini V, Orsini M. Romano-Spica, V. A preliminary investigation on the occurrence of protozoa in swimming pools in Italy. Ann Ig. 2004;16:709–19.
Dziuban EJ, et al. Surveillance for waterborne disease and outbreaks associated with recreational water–United States, 2003–2004. Morbidity and mortality weekly report. Surveillance summaries. (Washington D C. 2006;55:1–30.
Oliveri R, et al. Occurrence of Giardia cysts and Cryptosporidium oocysts in swimming pools in the province of Palermo, Italy. Ann Ig. 2006;18:367–74.
Schets FM, Engels GB, Evers EG. Cryptosporidium and Giardia in swimming pools in the Netherlands. J Water Health. 2004;2:191–200.
Chalmers RM, et al. Occurrence of Cryptosporidium Oocysts in leisure pools in the UK, 2017, and modelling of Oocyst contamination events. Water. 2021;13:1503.
Xiao S, Yin P, Zhang Y, Hu S. Occurrence of Cryptosporidium and Giardia and the relationship between protozoa and water quality indicators in swimming pools. Korean J Parasitol. 2017;55:129–35.
Fournier S, et al. Detection of Microsporidia, Cryptosporidia and Giardia in swimming pools: a one-year prospective study. FEMS. Med Microbiol Immunol. 2002;33:209–13.
Galván AL, et al. Molecular characterization of human-pathogenic microsporidia and cyclospora cayetanensis isolated from various water sources in Spain: a year-long longitudinal study. Appl Environ Microbiol. 2013;79:449–59.
Mafi M, Niyyati M, Haghighi A, Lasjerdi Z. Contamination of swimming pools and park ponds with free living amoebae in Tehran. Med J Tabriz Univ Med Sci. 2017;38:60–7.
Solgi R, et al. Thermotolerant Acanthamoeba spp. isolated from therapeutic hot springs in Northwestern Iran. J Water Health. 2012;10:650–6.
Sarmadian H, et al. Fungal and parasitic contamination of indoor public swimming pools in Arak, Iran. J Egypt Public Health Assoc. 2020;95:8.
Zangiabadi AL, Aali R, Zarei A, Ghanbari R. Spatial analysis and health grading of swimming pools. Health Syst Res. 2011;6:802–9.
World Health Organization (WHO). Guidelines for safe recreational water environments. Swimming pools and similar environment. Geneva: Switzerland: WHO; 2008.
World Health Organization (WHO). Guidelines for safe recreational-water environments final draft for Consultation Vol. 2: swimming pools, spas and similar recreational-water environments. Geneva: Switzerland: WHO; 2000.
Alansari A, Amburgey J, Madding N. A quantitative analysis of swimming pool recirculation system efficiency. J Water Health. 2018;16:449–59.
Sun Z, Xu W, Song G. Analysis on sanitary conditions of swimming pool water in Dongcheng District of Beijing in 2013. Occup Health. 2015;31:656–7.
Shields JM, Gleim ER, Beach MJ. Prevalence of Cryptosporidium spp. and Giardia intestinalis in swimming pools, Atlanta, Georgia. Emerg Infect Dis. 2008;14:948–50.
Rasti S, et al. Evaluation of parasitic and fungal contamination and physicochemical parameters of indoor public swimming pools in Kashan during 2008-9. Feyz. J Kashan Univ Med Sci. 2011;15:74–80.
Viessman W, Hammer MJ. Water Supply & Pollution Control (6th ed.). Addison-Wesley. 1998.
Baruth EE. Water treatment plant design (4th ed.). Mcgraw-Hill; 2005.
Sorvillo FJ, et al. Swimming-associated cryptosporidiosis. Am J Public Health. 1992;82:742–4.
Acknowledgements
We would like to thank the coaches of the swimming pools in Alexandria for their help. Our thanks also extend to the divers who helped us to collect water from the swimming pools depth.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors report no financial interest.
Author information
Authors and Affiliations
Contributions
F.H.: Concept, design, literature search, interviewing questionnaire, sample collection, parasitological analysis analysis, data entry and analysis through SPSS version 25, then drafting the manuscript, revising it critically for important intellectual content, the integrity of the work from inception to the published article and should be designated as ‘guarantor’ and have given final approval of the manuscript; I.M.M.: sample collection, chemical analysis, chemical data entry and analysis through SPSS version 25analysis , manuscript preparation, manuscript editing and revising it critically for important intellectual content. M.M.F.: bacteriological analysis, drafting the manuscript, and revising it critically for important intellectual content; M. S. A.: Study design, data analysis, manuscript editing and proofreading; H. A. S.: design, interviewing questionnaire, sample collection, and proof reading; M.S.: design, interviewing questionnaire, and proof reading; A. I. S.: revising tables, proofreading and manuscript editing. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical committee of medical research (High Institute of Public Health-Alexandria University) has reviewed the proposal and found that it has fulfilled the guarantees and safeguards for the medical research ethics and that the proposal is in compliance with the policy of the committee. All methods were performed in accordance with the Declaration of Helsinki”.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hassanein, F., Masoud, I.M., Fekry, M.M. et al. Environmental health aspects and microbial infections of the recreational water. BMC Public Health 23, 302 (2023). https://doi.org/10.1186/s12889-023-15183-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-15183-z