Abstract
Background
Non-adherence to tuberculosis treatment increases the risk of poor treatment outcomes. Digital adherence technologies (DATs), including the smart pillbox (EvriMED), aim to improve treatment adherence and are being widely evaluated. As part of the Adherence Support Coalition to End TB (ASCENT) project we analysed data from a cluster-randomised trial of DATs and differentiated care in Ethiopia to examine individual-factors for poor engagement with the smart pillbox.
Methods
Data were obtained from a cohort of trial participants with drug-sensitive tuberculosis (DS-TB) whose treatment started between 1 December 2020 and 1 May 2022, and who were using the smart pillbox. Poor engagement with the pillbox was defined as (i) > 20% days with no digital confirmation and (ii) the count of days with no digital confirmation, and calculated over a two evaluation periods (56-days and 168-days). Logistic random effects regression was used to model > 20% days with no digital confirmation and negative binomial random effects regression to model counts of days with no digital confirmation, both accounting for clustering of individuals at the facility-level.
Results
Among 1262 participants, 10.8% (133/1262) over 56-days and 15.8% (200/1262) over 168-days had > 20% days with no digital confirmation. The odds of poor engagement was less among participants in the higher stratum of socio-economic position (SEP) over 56-days. Overall, 4,689/67,315 expected doses over 56-days and 18,042/199,133 expected doses over 168-days were not digitally confirmed. Compared to participants in the poorest SEP stratum, participants in the wealthiest stratum had lower rates of days not digitally confirmed over 168-days (adjusted rate ratio [RRa]:0.79; 95% confidence interval [CI]: 0.65, 0.96). In both evaluation periods (56-days and 168-days), HIV-positive status (RRa:1.29; 95%CI: 1.02, 1.63 and RRa:1.28; 95%CI: 1.07, 1.53), single/living independent (RRa:1.31; 95%CI: 1.03, 1.67 and RRa:1.38; 95%CI: 1.16, 1.64) and separated/widowed (RRa:1.40; 95%CI: 1.04, 1.90 and RRa:1.26; 95%CI: 1.00, 1.58) had higher rates of counts of days with no digital confirmation.
Conclusion
Poorest SEP stratum, HIV-positive status, single/living independent and separated/ widowed were associated with poor engagement with smart pillbox among people with DS-TB in Ethiopia. Differentiated care for these sub-groups may reduce risk of non-adherence to TB treatment.
Similar content being viewed by others
Background
Non-adherence to treatment remains to be one of the challenges that tuberculosis programmes face. It can result in increased risk of poor treatment outcomes, development of drug resistant strains, transmission of TB in the community, increased morbidity and mortality [1].
The literature has documented several factors that are known to affect treatment adherence. These include individual factors such as age, sex, treatment phase, HIV co-infection, stigma, low income, lower education level, lack of support; provider factors such as health care workers’ skills, training, and attitudes; and health system factors including patient load and health care staffing [2,3,4,5,6,7,8,9,10]. Digital adherence technologies (DATs), such as the smart pillbox, short message service (SMS) or video supported therapy, aim to improve treatment adherence, ideally resulting in better treatment outcomes and reducing treatment recurrence [11]. These interventions are based on patient engagement with the technology, such as a pillbox opening, considered a proxy for treatment adherence. Real-time monitoring of DAT engagement by health care workers allows additional support to be offered to patients who appear to have issues with treatment adherence [11].
The Adherence Support Coalition to End TB (ASCENT) project is conducting cluster-randomised trials (CRTs) in five countries with varied epidemiology of TB and HIV, to evaluate the effectiveness of DATs with differentiated care in improving treatment outcomes [12, 13]. In this context, understanding engagement with the DAT with respect to individual-level and facility-level factors is of interest in order to understand intervention delivery. Furthermore, in resource limited settings, identifying factors that contribute to poor engagement with DAT could facilitate optimal implementation of adherence interventions and promote patient-centred differentiated care to improve TB treatment adherence. Hence, good engagement with the DAT is important for showing potential effectiveness of these interventions.
Ethiopia is among the 30 high TB burden countries in the world with an estimated TB incidence rate of 143 per 100,000 population and mortality rate of 18 per 100,000 population [14]. Studies from Ethiopia have variable and inconsistent findings regarding non-adherence to treatment for tuberculosis ranging between 9 and 36% [5, 10, 15, 16]. Early detection of non-adherence as measured through poor engagement with the DAT and information on contributing factors are needed to understand how interventions, including smart pill boxes, can improve adherence through person-centred care.
This analysis examined the individual-factors associated with poor engagement with the DAT among adults with pulmonary drug-sensitive TB (DS-TB) using the smart pillbox participating in the ASCENT project in Ethiopia. Our findings will inform a pragmatic approach of measuring treatment adherence using smart pillbox to identify and support people with TB at risk of poor medication adherence.
Methods
Study design/setting
The study area is in Addis Ababa city and Oromia region in Ethiopia. The ASCENT trial is a pragmatic three-arm cluster randomised trial (CRT), with health facility as the unit of randomisation. Facilities were randomised, in a ratio of 1:1:1, to either a DAT intervention (smart pillbox or medication labels) with daily monitoring of adherence and differentiated care, or standard of care. Adults (≥ 18 years) with pulmonary DS-TB initiating treatment in the health facilities participating in the trial were offered enrolment. Following informed consent, participants received the intervention allocated to the facility they enrolled from (Trial registration: PACTR202008776694999). Details of the trial design are described elsewhere [12]. Those in the medication labels arm facilities who did not have access to a phone were offered a pillbox.
Participants using the smart pillbox, received the box with dosing instruction and anti-TB drugs were placed were inside. Based on the participants’ preference, the boxes were configured with an audio-visual alert to remind patients to take their medication at a pre-defined time. Each time participants opened the box to take their medication, their “daily dose” was automatically logged on to the Everwell Hub web-based platform (https://www.everwell.org) via a built-in mobile internet connection. For days where the box was not opened, the platform flagged a missed daily dose. This platform allowed TB care providers to access and evaluate real-time dosing data of each participant and offer differentiated care, including SMS reminders, phone calls and home visits, as appropriate.
Study population
The study population for this analysis was nested within an ongoing pragmatic CRT of smart pillbox and medications labels, and included adult patients, who started treatment for DS-TB between 1 December 2020 and 1 May 2022, initiating on the pillbox within 14 days of treatment start. Participants with completely missing baseline or dosing data were excluded, as were those switching to MDR-TB treatment, missing treatment outcomes, or whose diagnosis changed to not TB. Engagement with technology was based on digital logging of the box being opened, over the first 56 and 168 days of treatment, representing the intensive phase of treatment and full course of treatment for TB, respectively.
Measures
To investigate the effect of risk factors on poor engagement with the technology among adults with DS-TB, individual-level factors known to affect adherence to treatment for tuberculosis were assessed. These factors were collected at study enrolment by interview or abstracted from the TB register and categorised as socio-demographic or clinical factors. The socio-demographic factors were sex, age, education, marital status, and wealth index. The clinical factors were HIV status, previous TB status and type of TB (whether the TB was diagnosed bacteriologically or not). A wealth index was created representing household socioeconomic position (SEP) using principal component analysis [17, 18]. Using a polychoric analysis of household assets (livestock, land, and house ownership), characteristics (cooking fuel type, toilet/sewage facilities, source of drinking water, number of rooms per person, frequency of income), and consumer goods (bicycle, truck, cart, motorcycle, bed, refrigerator, lamp, mattress, mitad, mobile, ratio, sofa, television), we constructed a dual-component wealth index. This allows an additional dimension of wealth, expressed by the second component, which helps to limit the introduction of urban bias through a better representation of rural patterns of wealth [19]. This index was subsequently grouped into quintiles, measuring household SEP. A facility-level (health-system) measure of the percentage of poor treatment outcome (death, lost to follow-up or treatment failure) among adults with DS-TB, evaluated in a cohort prior the start of the trial, was calculated. This percentage was grouped into terciles based on the distribution at the cluster-level to account for baseline health facility-level characteristics.
The main outcome for this study was poor engagement with smart pillbox and measured using two approaches: (i) a binary response defined as > 20% days with no digital confirmation and (ii) the count of days with no digital confirmation. Both outcomes were measured over the two time periods of 56-days (intensive phase) and 168-days (full course of 6 month TB treatment). For the binary outcome the denominator was defined as the number of days from the start date of the DAT to the earliest of (i) treatment stop or (ii) analysis time period (day 56 and 168 from treatment start). Treatment stop was either at the time of (i) transfer to another facility, completion of treatment, on-treatment death, lost to follow-up or treatment failure, or (ii) participant withdrawal from the intervention either due to the participant wanting to stop or technical issues with the device. The count outcome was measured as the number of days with no recorded digital pillbox opening. Digital confirmation on a treatment-day refers to the participant opening the pillbox on the day, and is assumed to be a proxy for a dose taken.
Statistical analysis
Descriptive statistics, including proportions, standard deviations and ranges, were computed to describe age and sex distributions, HIV prevalence and poor engagement with the smart pillbox. Logistic random effects regression was used to model the binary outcome of at least > 20% days with no digital confirmation. Negative binomial random effects regression was used to model the count of days with no digital confirmation, and the denominator, as described previously, used as an offset. Both models accounted for clustering of individuals at the facility-level. Risk factors investigated included age, sex, previous TB, bacteriological confirmation, HIV status, level of education, marital status, and SEP. Changes in the magnitude of estimates between crude and (age and sex) adjusted measures of effect were evaluated for each potential risk factor. Following age- and sex-adjustment three variables were identified to investigate further in multivariable analysis, based on confidence intervals excluding one for either outcome or time period. Separate, fully-adjusted models, were constructed for these variables where adjustment was guided by a conceptual framework, avoiding adjustment for intermediate variables. All available data based on our inclusion criteria for this study were used in the analysis and no formal sample size calculation was conducted. Precision of estimates were judged by reviewing the 95% confidence intervals. To avoid issues of data sparsity for the binary outcome model adjustment was restricted to a minimum of 10 outcomes per variable [20, 21]. Complete case analyses were conducted as the percentage of the total sample size with missing data was less than 2.5%. Data analysis was conducted using Stata 17.
Written informed consent was obtained from all participants in the ASCENT trial. The trial has approval from London School of Hygiene & Tropical Medicine Ethics Committee (19,120), United Kingdom; WHO Ethical Review Committee (ERC.0003297), Switzerland; Addis Ababa City Administration Health Bureau Public Emergency and Health Research Directorate Institutional Review Board (AA16238/227), and Oromia Regional Health Bureau Public Emergency and Health Research Directorate Institutional Review Board, Ethiopia (BEFO/HBTFH/1–16/10415).
Results
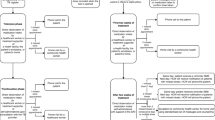
Between 1 December 2020–1 May 2022, 2,430 adults with DS-TB were enrolled across the three arms of the parent study, excluding those who had switched to an MDR regimen, or diagnosis changed to not TB or TB outcome unknown. A total of 1,000 participants were in the standard of care arm or started medication labels, and a further 87 started the pillbox > 14 days after treatment start, leaving 1,343 participants. Of these, 76 had no available baseline or DAT engagement data and five had other data errors that could not be resolved. This resulted in a total of 1262 participants (94%) included in this analysis (Fig. 1). These participants were enrolled from 43 facilities; 26 facilities randomised to the pillbox arm and 17 randomised to the medication labels arm, for whom patients with no mobile phone access used the pillbox. The mean number of participants per facility was 29.3 (standard deviation [SD] 27.3) with a range of 1 to 135. The mean age was 33.9 years (SD 13.8, range 18 to 99), 59.2% (747/1262) were male and 40.8% (515/1262) were female, and prevalence of HIV was 12.5% (157/1257). Half (50.9%, 643/1262) of the participants had no or less than primary level education and 66.5% (839/1261) had bacteriologically confirmed TB (See Table 1).
Over the intensive phase and full course of TB treatment, 10.5% (133/1262) and 15.8% (200/1262) of participants had > 20% of days with no digital confirmation, respectively. Table 2 displays the crude odds ratio [OR] (and 95% confidence interval [CI]) of poor engagement with technology for the risk factors explored, as well as the corresponding age-and-sex-adjusted ORs (and 95%CI). Participants in the fourth stratum of SEP had a 51% lower (95%CI 6% to 75%) odds of poor engagement with technology over intensive phase – compared to those in the poorest stratum; and that participants in the wealthiest stratum of SEP had a 41% (95%CI 0% to 65%) lower odds of poor engagement with technology over full course of TB treatment – compared to those in the poorest stratum. Over intensive phase, being clinically diagnosed was associated with a 55% increase in odds of poor engagement, though this effect was not observed over full course of TB treatment.
When poor engagement was measured using counts of days with no digital confirmation, a total of 4,686 (6.9%) doses were not digitally confirmed (out of 67,315 doses required) over the intensive phase of treatment and 18,042 (9.0%) were not digitally confirmed (out of 199,133 required) over the full course of TB treatment (Table 3). Participants in the wealthiest stratum of SEP had a 21% (95%CI 4% to 35%) reduction in the rate of days not digitally confirmed over full course of TB treatment compared to the poorest stratum, after adjusting for age and sex. No such pattern was observed over intensive phase. HIV-positive participants had a 29% higher rate of no digital confirmation over intensive phase (95%CI 2% to 63%) and a 28% higher rate of doses missed over full course of TB treatment (95%CI 7% to 53%) compared to HIV negative participants, after adjusting for age and sex. There was also some evidence of higher rates of no digital confirmation among those single/ living independent and those separated/widowed compared to single participants living with parents. The age-and-sex adjusted rate ratio (RR) for those single/living independent was 1.31 (95%CI 1.03 to 1.67) over intensive phase and 1.38 (95%CI 1.16 to 1.64) over full course of TB treatment; for those separated/widowed the corresponding RR were 1.40 (95%CI 1.04 to 1.90) and 1.26 (95%CI 1.00 to 1.58).
Table 4 displays the results of the multivariable models assessing HIV status, SEP and marital status as risk factors for poor engagement with technology, using the two outcome measures. After adjusting for age, sex, previous TB, level of education, marital status and SEP, HIV-positive participants had 27% higher rates of no digital confirmation over the intensive phase (95%CI 0% to 62%) compared to HIV negative participants and 29% higher rates (95%CI 7% to 55%) when the outcome was assessed over full course of TB treatment. The association was not observed for the binary poor engagement outcome over intensive phase or full course of TB treatment. For SEP, after adjusting for age, sex, previous TB, level of education and marital status, the wealthiest stratum had 18% lower rates of no digital confirmation over full course of TB treatment (95%CI 0% to 34%), with similar patterns for the binary outcome, albeit with confidence intervals including one. In the analysis of count of no digital confirmation over full course of TB treatment, being single/living independent, and separated/widowed showed higher rates of poor engagement. The facility-level measure showed no association with either poor engagement outcome.
Discussion
This study analysed individual and facility factors associated with poor engagement with the smart pillbox in a population of 1262 adults with DS-TB receiving treatment in a predominantly urban setting in Ethiopia. Over the intensive phase of treatment, 7.0% of doses were not digitally confirmed and 10.5% of participants had > 20% of treatment days with no digital confirmation. These became, 9.1% and 15.8% respectively when the outcome was evaluated over full course of TB treatment. We found some evidence that higher relative SEP was associated with better engagement with technology particularly over full course of treatment for TB. Rates of no digital confirmation were higher in HIV positive compared to HIV negative participants, over the intensive phase and full course of treatment for TB. Finally, those single/ living independent or separated/widowed had higher poor engagement for both outcomes, particularly over full course of treatment for TB. Rather than showing different factors for intensive versus the full course of treatment period, our results were generally consistent across the two time periods.
Engagement with the smart pillbox over the entire 6-month treatment period has been reported in recent trials, with results comparable to our observations. In a cluster-randomised trial from China with every other day dosing, in the pillbox arm, 13.9% of treatment days were not digitally confirmed and 17% of participants had at least 20% days with no digital confirmation [22]. In the TB Mate study from South Africa, among participants in the pillbox arm, 11.5% of days were not digitally confirmed and 18.5% of participants had at least 20% of treatment days without digital confirmation [23].
Comparison of our results to the literature on factors associated with poor engagement is limited to what is known about factors associated with more direct measures of adherence, as little is reported on DAT engagement. The relationship between lower SEP and increased risk of general poor health has been well explored in the literature [24,25,26]. People with low SEP, and particularly many people with TB, are burdened with more complex life issues, including lack of access to nutritious food, lack of access to health care, be overworked or stressed and less privacy at home due to overcrowding [5, 10, 27,28,29,30,31]. This may make them prioritise basic life needs rather than their overall health and/ or treatment care, be less conversant with TB treatment and ultimately lead to poor engagement to the technology that was provided to support their TB treatment.
In our study, participants with TB/HIV co-infection were more likely to miss doses of their anti-TB treatment medication, based on the proxy of poor engagement with the DAT, measured by counts of days with no digital confirmation. This finding was consistent with previous studies conducted in Sub Saharan African countries, including Ethiopia. Pill burden, adverse effects of anti-TB medication and fear of stigma among people with TB/HIV co-infection have been documented to be more common compared to people with TB only [6, 27, 32,33,34]. Furthermore, less motivation to take medication among people with TB/HIV co-infection has been reported in studies conducted in Sub-Saharan Africa [35, 36]. These stressors may likely exacerbate forgetfulness, psychological distress, and non-disclosure of HIV status to family, which in turn could negatively influence their medication adherence and engagement to DAT. Therefore, our study may highlight the relevance of social relationships in improving adherence to treatment, especially in resource limited settings.
We found participants who were single/ living independent and separated/ widowed had higher likelihood of missing doses as evidenced by poor engagement with the DAT, after adjustment for age and other factors. This could reflect lower social capital in this subgroup. Although we did not capture a more direct measure of social capital defined as psychosocial assets which shape health, it includes the support from household- or community members that individuals with TB receive in their treatment [17]. Social factors for non-adherence such as stigma and limited knowledge of TB may have a pronounced detrimental effect in the livelihood of these sub-groups and deprive them of social network that could have addressed the social barriers to TB treatment adherence. Previous studies have shown that providing social support that could involve financial incentives, emotional support, and informational provision from one’s social network, can improve adherence to TB treatment [37,38,39,40,41]. Another pathway for those single could be through having to travel longer periods for work and not taking pillbox due to the inconvenience [5].
A facility-level measure of percentage of people with TB with poor treatment outcomes prior to our study, aiming to reflect facility and individual-level engagement with care, was not found to be associated with participant engagement with the DAT. More detailed data on facility-level measures such facility infrastructure, staff leadership, engagement with the interventions, and patient–provider relationship would be required to have a better understanding of the role of facility-level factors in DAT engagement [42].
Our study aimed to understand engagement with the DAT among people with TB. Comparing studies evaluating treatment adherence is often challenging without a gold standard comparator test, such as urine isoniazid testing. Some studies have suggested good sensitivity and/ or specificity but others have shown poor agreement, particularly with a SMS-based DAT. Our study included participants from 43 health facilities in two regions in Ethiopia, though mainly limited to more urban areas. We used two measures of DAT poor engagement, measured over two time periods—a binary variable reflecting > 20% of treatment days with no digital confirmation, aligning with commonly used categorisation of adherence in the literature [22, 43,44,45,46,47] and a count of treatment days with no digital confirmation. The binary measure is likely to have resulted in reduced power to detect differences by subgroups. We constructed a measurement of SEP using two principal components, rather than the first one, as proposed by Martel et al. (2021). This allows incorporation of the additional dimension of wealth thereby limiting the introduction of urban bias through a better representation of rural patterns of wealth. Measures of stigma and several dimensions social capital which may influence DAT engagement were not collected as part of the study. Our study sample is representative of the trial population and missing data were minimal. Results from the two outcome approaches are broadly similar, reflecting consistency of baseline effects on poor engagement. Despite careful adjustment for confounders in the regression models guided by conceptual frameworks, one limitation of the study is we cannot discount residual confounding due to unmeasured confounders.
Conclusion
Participants’ poor engagement with smart pillbox and rates of days with no digital confirmation increased over TB treatment time. Poorest SEP stratum, HIV-positive status, single/ living independent and separated/ widowed were individual-level factors associated with poor engagement with the DAT among adults with DS-TB receiving treatment in Ethiopia. Additional support, such as counselling, psychosocial and peer support to these sub-groups is recommended to reduce risk of non-adherence to TB treatment. Provider and facility level factors associated with poor engagement during the treatment course warrants further study.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to pending publication of main trial results which were used under license for the current study but available from the corresponding author on reasonable request.
Abbreviations
- ASCENT:
-
Adherence Support Coalition to End TB
- CI:
-
Confidence interval
- CRT:
-
Cluster randomised trial
- DAT:
-
Digital adherence technology
- DS-TB:
-
Drug-sensitive tuberculosis
- HIV:
-
Human Immunodeficiency Virus
- MDR-TB:
-
Multidrug resistant tuberculosis
- ORc :
-
Crude odds ratio
- ORa :
-
Adjusted odds ratio
- RRc :
-
Crude rate ratio
- RRa :
-
Adjusted rate ratio
- SEP:
-
Socioeconomic position
- SMS:
-
Short message service
- TB:
-
Tuberculosis
- WHO:
-
World Health Organisation
References
WHO, World Health Organisation. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003.
Ali AOA, Prins MH. Patient characteristics associated with non-adherence to tuberculosis treatment: a systematic review. J Tuberc Res. 2020;08(02):73–92.
Dogah E, et al. Factors influencing adherence to tuberculosis treatment in the Ketu North District of the Volta Region. Ghana Tuberc Res Treat. 2021;2021:6685039.
Fang XH, et al. Prevalence of and factors influencing anti-tuberculosis treatment non-adherence among patients with pulmonary tuberculosis: a cross-sectional study in Anhui Province. Eastern China Med Sci Monit. 2019;25:1928–35.
Gashu KD, Gelaye KA, Tilahun B. Adherence to TB treatment remains low during continuation phase among adult patients in Northwest Ethiopia. BMC Infect Dis. 2021;21(1):725.
Nezenega ZS, Perimal-Lewis L, and Maeder AJ. Factors influencing patient adherence to tuberculosis treatment in Ethiopia: a literature review. Int J Environ Res Public Health. 2020; 17(15).
Ruru Y, et al. Factors associated with non-adherence during tuberculosis treatment among patients treated with DOTS strategy in Jayapura, Papua Province, Indonesia. Glob Health Action. 2018;11(1):1510592.
Stagg HR, et al. Temporal factors and missed doses of tuberculosis treatment. A causal associations approach to analyses of digital adherence data. Ann Am Thorac Soc. 2020; 17(4):438–449.
Suliman Q, et al. Risk factors for early TB treatment interruption among newly diagnosed patients in Malaysia. Sci Rep. 2022;12(1):745.
Zegeye A, et al. Prevalence and determinants of anti-tuberculosis treatment non-adherence in Ethiopia: a systematic review and meta-analysis. PLOS ONE. 2019;14(1):e0210422.
WHO. Handbook for the use of digital technologies to support tuberculosis medication adherence. 2017, Geneva: World Health Organization.
Tadesse AW, et al. Evaluation of implementation and effectiveness of digital adherence technology with differentiated care to support tuberculosis treatment adherence and improve treatment outcomes in Ethiopia: a study protocol for a cluster randomised trial. BMC Infect Dis. 2021;21(1):1149.
Jerene D, et al. Effectiveness of digital adherence technologies in improving tuberculosis treatment outcomes in four countries: a pragmatic cluster randomised trial protocol. BMJ Open. 2023;13(3): e068685.
Global tuberculosis report 2022. Geneva: World Health Organization. 2022. Licence: CC BY-NC-SA 3.0 IGO.
Gebremariam RB, Wolde M, Beyene A. Determinants of adherence to anti-TB treatment and associated factors among adult TB patients in Gondar city administration, Northwest, Ethiopia: based on health belief model perspective. J Health Popul Nutr. 2021;40(1):49.
Mekonnen HS, Azagew AW. Non-adherence to anti-tuberculosis treatment, reasons and associated factors among TB patients attending at Gondar town health centers, Northwest Ethiopia. BMC Res Notes. 2018;11(1):691.
Krieger N. Theories for social epidemiology in the 21st century: an ecosocial perspective. Int J Epidemiol. 2001;30(4):668–77.
Atkinson AB, et al. Measuring Poverty around the World. Micklewright J, Brandolini A, editors. Princeton University Press; 2019.
Martel P, Mbofana F, Cousens S. The polychoric dual-component wealth index as an alternative to the DHS index: Addressing the urban bias. J Glob Health. 2021;11:04003.
Peduzzi P, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9.
Stoltzfus JC. Logistic regression: a brief primer. Acad Emerg Med. 2011;18(10):1099–104.
Liu X, et al. Effectiveness of Electronic Reminders to Improve Medication Adherence in Tuberculosis Patients: A Cluster-Randomised Trial. PLoS Med. 2015;12(9): e1001876.
Charalambous S, et al. TB treatment adherence amongst drug-susceptible TB persons using medication monitor and differentiated care approach versus standard of care in South Africa. in IJTLD. 2022. EP-27–870.
Lynch J, et al. Is income inequality a determinant of population health? Part 1. A systematic review Milbank Q. 2004;82(1):5–99.
Pickett KE, Wilkinson RG. Income inequality and health: a causal review. Soc Sci Med. 2015;128:316–26.
Wilkinson RG, Pickett KE. Income inequality and population health: a review and explanation of the evidence. Soc Sci Med. 2006;62(7):1768–84.
Gebremariam MK, Bjune GA, Frich JC. Barriers and facilitators of adherence to TB treatment in patients on concomitant TB and HIV treatment: a qualitative study. BMC Public Health. 2010;10:651.
Jaeggi AV, et al. Do wealth and inequality associate with health in a small-scale subsistence society? Elife. 2021;10:e59437
Lonnroth K, et al. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68(12):2240–6.
Whitehead M, Dahlgren G, Evans T. Equity and health sector reforms: can low-income countries escape the medical poverty trap? Lancet. 2001;358(9284):833–6.
Hailemichael Y, et al. Mental health problems and socioeconomic disadvantage: a controlled household study in rural Ethiopia. Int J Equity Health. 2019;18(1):121.
Awofeso N. Anti-tuberculosis medication side-effects constitute major factor for poor adherence to tuberculosis treatment. Bull World Health Organ. 2008; 86(3): p. B-D.
Mindachew M, et al. Perceived barriers to the implementation of Isoniazid preventive therapy for people living with HIV in resource constrained settings: a qualitative study. Pan Afr Med J. 2014;17:26.
Zewude SB, Ajebe TM. Magnitude of optimal adherence and predictors for a low level of adherence among HIV/AIDS-infected adults in South Gondar zone, Northwest Ethiopia: a multifacility cross-sectional study. BMJ Open. 2022;12(1): e056009.
Govender S, Mash R. What are the reasons for patients not adhering to their anti-TB treatment in a South African district hospital? South African Family Practice. 2014;51(6):512–6.
Muture BN, et al. Factors associated with default from treatment among tuberculosis patients in Nairobi province, Kenya: a case control study. BMC Public Health. 2011;11:696.
Deshmukh RD, et al. Social support a key factor for adherence to multidrug-resistant tuberculosis treatment. Indian J Tuberc. 2018;65(1):41–7.
Fetensa G, et al. Magnitude and determinants of delay in diagnosis of tuberculosis patients in Ethiopia: a systematic review and meta-analysis: 2020. Arch Public Health. 2022;80(1):78.
Li X, et al. Effectiveness of comprehensive social support interventions among elderly patients with tuberculosis in communities in China: a community-based trial. J Epidemiol Community Health. 2018;72(5):369–75.
Musiimenta A, et al. Mobile health technologies may be acceptable tools for providing social support to tuberculosis patients in rural Uganda: a parallel mixed-method study. Tuberc Res Treat. 2020;2020:7401045.
Tadesse S. Stigma against Tuberculosis Patients in Addis Ababa, Ethiopia. PLOS ONE. 2016;11(4):e0152900.
Charalambous S, et al. Clinic-level factors influencing patient outcomes on antiretroviral therapy in primary health clinics in South Africa. AIDS. 2016;30(7):1099–109.
Paterson DL, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30.
Bastard M, et al. Effects of treatment interruption patterns on treatment success among patients with multidrug-resistant tuberculosis in Armenia and Abkhazia. J Infect Dis. 2015;211(10):1607–15.
Menzies D, et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: a randomized trial. Ann Intern Med. 2008;149(10):689–97.
Nackers F, et al. Adherence to Self-Administered Tuberculosis Treatment in a High HIV-Prevalence Setting: a Cross-Sectional Survey in Homa Bay, Kenya. PLOS ONE. 2012;7(3):e32140.
Van der Kop ML, et al. The effect of weekly text-message communication on treatment completion among patients with latent tuberculosis infection: study protocol for a randomised controlled trial (WelTel LTBI). BMJ Open. 2014;4(4): e004362.
Acknowledgements
We would like to thank participants who have consented to take part in this study, the National Tuberculosis Program at Ministry of Health Ethiopia, the Disease Prevention and Control team at regional health bureau of Addis Ababa city and Oromia regional state, zonal, town, sub-city and woreda health offices, and the TB focal persons at each facility in implementing the ASCENT project.
Funding
The trial is funded by Unitaid (grant agreement number:2019-33-ASCENT) through the Adherence Support Coalition to End TB (ASCENT) project (https://www.digitaladherence.org/)
Author information
Authors and Affiliations
Contributions
AWT, MC, DJ, KLF conceptualised the study idea. KLF and AWT contributed to study design. KLF provided statistical expertise in the analysis. AWT, GTW, TA, MS, DA, HW, DG, MB, AS, JvR and AB were responsible for implementing the study. The first draft of the manuscript was written by AWT, MC, NF and KLF. All authors contributed to feedback to previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial has approval from London School of Hygiene & Tropical Medicine Ethics Committee (19120), United Kingdom; WHO Ethical Review Committee (ERC.0003297), Switzerland; Addis Ababa City Administration Health Bureau Public Emergency and Health Research Directorate Institutional Review Board (AA16238/227), and Oromia Regional Health Bureau Public Emergency and Health Research Directorate Institutional Review Board (BEFO/HBTFH/1–16/10415), Ethiopia. All methods were carried out in accordance with relevant guidelines and regulations and written informed consent was sought from all participants in the study.
Consent for publication
Not applicable.
Competing interest
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tadesse, A.W., Cusinato, M., Weldemichael, G.T. et al. Risk factors for poor engagement with a smart pillbox adherence intervention among persons on tuberculosis treatment in Ethiopia. BMC Public Health 23, 2006 (2023). https://doi.org/10.1186/s12889-023-16905-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-16905-z