Abstract
Background
The prevalence of non-alcoholic fatty liver disease (NAFLD) keeps increasing annually worldwide. Non-invasive assessment tools for evaluating the risk and severity of the disease are still limited. Insulin resistance (IR) and abdominal obesity (ABO) are closely related to NAFLD.
Methods
A retrospective large-scale, population-based study was conducted based on the data from the 2017–2018 cycle of the National Health and Nutrition Examination Survey (NHANES). Three ABO indices, namely lipid accumulation product (LAP), visceral obesity index (VAI), waist circumference-triglyceride index (WTI), and three IR indices, including triglyceride glucose index (TyG), homeostasis model assessment of insulin resistance (HOMA-IR) and metabolic score for insulin resistance (METS-IR), were analyzed and compared for their relationships with NAFLD based on weighted multivariable logistic regression, spearman correlation heatmap, smooth curve fittings. The area under the curve (AUC) of receiver-operating characteristic (ROC) curve was used to evaluate the diagnostic capability of these indices for NAFLD. Differences among the AUCs were calculated and compared by Delong test.
Results
In total, 3095 participants were included in our study among which 1368 adults were diagnosed with NAFLD. All six indices presented positive associations with NAFLD. There was a claw-shaped curve between HOMA-IR, VAI, LAP and NAFLD while a smooth semi-bell curve was observed in TyG, METS-IR and WTI. LAP and HOMA-IR had the best diagnostic capability for NAFLD (LAP: AUC = 0.8, Youden index = 0.48; HOMA-IR: AUC = 0.798, Youden index = 0.472) while VAI (AUC = 0.728, Youden index = 0.361) showed the lowest predictive value. The correlation heat map indicated positive correlations between all six indices and liver function, hepatic steatosis and fibrosis severity. In the NAFLD group, IR indicators presented a stronger association with alanine aminotransferase (ALT) compared with ABO indices.
Conclusions
All six indices can screen NAFLD withLAP and HOMA-IR being possibly optimal predictors. IR indices may be more sensitive to identify acute hepatic injury in NAFLD patients than ABO indices.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) was defined as idiopathic hepatic steatosis confirmed by image or histology, in the absence of secondary causes of hepatic fat accumulation including alcohol, drug and virus [1]. It has emerged as a major public health issue globally with the morbidity tightly associated with metabolic diseases such as obesity and type 2 diabetes mellitus (T2DM) [2]. The prevalence of NAFLD is estimated to be 25–34% in developed countries and 29.2% in China [3, 4]. One meta-analysis discovered a moderately increased risk of extrahepatic cancers in NAFLD [5]. NAFLD can be categorized into nonalcoholic fatty liver (NAFL) or nonalcoholic steatohepatitis (NASH). During natural history, a fraction of NAFLD patients may progress from NASH to cirrhosis [3]. Based on a long-term follow-up study, the researchers found approximately 20% of NASH patients progressed to cirrhosis with an attributable mortality rate of 8% [6]. As recommended by the authoritative guidance, pharmacological treatments should be limited to those with biopsy-proven NASH and fibrosis [1], emphasizing the importance of prevention and early diagnosis for most NAFLD patients. Thus, reliable and non-invasive diagnostic tools are urgently needed to screen NAFLD. The association between NAFLD and metabolic syndrome (MetS) has been well established [1]. Insulin resistance(IR), the core mechanism underlying MetS, induces lipolysis in adipocytes and overproduction of free fatty acids causing ectopic lipid deposition which is the main pathology of NAFLD [7]. Visceral obesity, characterized by excess intra-abdominal adipose tissue accumulation [8], and IR are both important factors in NAFLD [9]. Hence, we conducted this study to explore and compare the associations between IR indicators and ABO indices with NAFLD based on the data from the NHANES database. Although the relationship between metabolic disorders and NAFLD is well known, few studies have compared the predictive value of IR indicators and ABO indicators for NAFLD.
Methods
Data from the 2017–2018 cycle of the National Health and Nutrition Examination Survey(NHANES) were analyzed. NHANES is a public and well-designed program of studies assessing the health and nutritional status of adults and children in the United States. It began in the early 1960s and was conducted as a series of surveys focusing on different populations or health topics. The survey selects a nationally representative sample of about 5,000 persons per year via a complex, stratified, multistage probability cluster sampling design. These persons are located in counties across the country which ensures the randomness and representativeness of the data. The survey protocol was approved by the National Center for Health Statistics Research Ethics Review Board and informed consent was obtained from each participant. The whole survey consists of two parts: a structured interview performed at home and a standardized health checkup at a mobile examination center (MEC). The raw data were processed through data cleaning based on exclusion conditions to obtain eligible data. The detailed procedure for participant enrollment was described in Fig. 1.
Analysis of sample
There were totally 9254 individuals participating in 2017–2018 NHANES cycle. Following exclusion criteria were used to select eligible participants: lack of MEC data; younger than 18 years old; viral hepatitis induced by hepatitis C virus (HCV) and hepatitis B virus (HBV), excessive alcohol intake or usage of steatogenic medication; incomplete vibration controlled transient elastography data. Finally, 3095 participants with available data were included, with 1368 diagnosed with NAFLD (Fig. 1).
Excessive alcohol consumption was defined as > 3 drinks per day for male and > 2 drinks per day for female [1]. HCV infection was confirmed by positive antibody test or presence of HCV RNA, and HBV infection was diagnosed by positive HBV surface antigen. Steatogenic medications include corticosteroid, tamoxifen, valproate, amiodarone, methotrexate, and antiretroviral drugs [1].
Demographic data and laboratory tests
Age, gender and race were acquired from demographic questionnaires conducted by trained interviewers using the Computer-Assisted Personal Interview system. Weight (kg), height (cm), and waist circumference (cm) were measured by NHANES staff during the MEC visit. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Weight grade was categorized as normal weight, overweight and obesity based on BMI values. BMI < 25 kg/m2 was regarded as normal weight, overweight was defined as BMI: 25–29.9 kg/m2, and obesity was confirmed when BMI ≥ 30 kg/m2 [10]. T2DM was diagnosed with any of the following evidences [11]: (1) Fasting plasma glucose (FPG) ≥ 126 mg/dl (7 mmol/L); (2) Random plasma glucose ≥ 200 mg/dl (11.1 mmol/L); (3) glycated hemoglobin level ≥ 6.5% (48 mmol/mol); (4) Self-reported diabetes; (5) Using antidiabetic medicines. Hypertension was diagnosed as blood pressure of ≥ 140/90 mmHg on at least two office visits [12].
Full methodology of laboratory tests, such as standard biochemistry profile, plasma fasting glucose, glycohemoglobin, lipid profile and so on, can be available on the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
Measurements of the six indices
High-density lipoprotein cholesterol (HDL-c) (mg/dL), waist circumference (WC) (cm), triglyceride (TG) (mg/dL), BMI (kg/m2), FPG and fasting serum insulin (µU/ml) were collected to calculated the indices.
HOMA-IR, LAP, VAI, TyG, METS-IR and WTI were calculated by using the following formulas [13,14,15,16,17,18]:
IR indicators as follows:
HOMA-IR = fasting serum insulin *FPG (mmol/l)/22.5
METS-IR = Ln [BMI* (TG + 2PG)]/Ln (HDL − c)
TyG = Ln [TG * FPG (mg/dL)/2]
ABO indicators as follows:
VAI = \(\frac{WC}{(39.68+(1.88^\ast BMI))}\times\frac{TG}{1.03}\times\frac{1.31}{(HDL-c)}\) for male and \(\frac{WC}{(36.58+(1.89^\ast BMI))}\times\frac{TG}{0.81}\times\frac{1.52}{(HDL-c)}\) for female
WTI = Ln [TG * WC/2]
LAP = (WC-65) * TG for male and (WC-58) * TG for female
Vibration-controlled transient elastography
Vibration-controlled transient elastography was used to assess hepatic steatosis and fibrosis severity depending on the controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) values respectively [19]. The FibroScan® model 502 V2 Touch (Echosens, Paris, France) equipped with medium and extra-large probes was used by NHANES technicians to perform this examination. Data were regarded as complete only if at least 10 LSMs were obtained after a fasting duration of at least three hours, with an interquartile range / median < 30%.
NAFLD was defined as follows: (1) evidence of hepatic steatosis, while in our study, we regarded patients with CAP value ≥ 274 dB/m as hepatic steatosis [20]. (2) lack of secondary causes of hepatic fat accumulation such as long-term use of a steatogenic medication, excessive alcohol consumption, or monogenic hereditary disorders.
Fibrosis stages were defined by median LSM value: no evidence of fibrosis (F0) as < 8.0 kPa, fibrotic non-alcoholic steatohepatitis (F2) as 8.0 to < 12.0 kPa, advanced fibrosis (F3) as 12.0 to < 20.0 kPa, and cirrhosis (F4) as ≥ 20 kPa [19].
Statistical analysis
Weighted analysis was performed for the complex survey using the appropriate subsample weights, strata, and primary sampling units as recommended by the NCHS. SPSS Statistics 26/25 software (IBM, Armonk, NY, USA), software R (R-4.3.1 for Windows), EmpowerStats4.0 (http://www.empowerstats.com), GraphPad Prism 8.0.1, Adobe Illustrator 2022 and Microsoft Excel were used to analyze data and build charts or figures. Categorical variables were expressed as weighted proportions (± Standard Error (SE)) and weighted means (95% Confidence interval (CI)) for continuous variables.
The multivariable logistic regression analysis was used to assess the relationship between six indices(as continuous variables and quartiles) and NAFLD. We constructed unadjusted logistic regression model 1 and model 2 adjusted for confounding factors of gender, race and age. The confounding factor was defined as an interference on both of the independent and dependent variables, but it’s not on their causal relationship chain.
ROC curve and AUC value were used to evaluate the diagnostic efficacy of six indices for NAFLD comprehensively. DeLong test was performed to compare the AUCs between two indices. The underlying non-linear relationship between the six indices and NAFLD state was explored via smooth curve fittings and generalized additive models adjusted for covariates of age, gender and race and weighted by wtsaf4yr. We constructed different generalized additive models with NAFLD (yes/no) as a binary response or CAP and LSM as continuous responses to comprehensively analyze the relationship between NAFLD and indices. Stratified smooth curve fitting was used for subgroup analysis.
Associations between the indices and liver function indicators were assessed by Spearman correlation analysis. P<0.05 was regarded as a significant difference for all the comparisons.
Results
Weighted characteristics of the participants
Weighted characteristics of the 3095 subjects categorized by NAFLD state were described in Table 1. Compared with non-NAFLD, the NAFLD group had a slightly higher percentage of males (P = 0.0578). Meanwhile, individuals in the NAFLD group were generally older than non-NAFLD (P < 0.0001). NAFLD patients presented with significantly worse metabolic parameters and biochemical markers, such as BMI, WC, diabetes proportion, TG, uric acid, HDL-c, ALT, gamma-glutamyl transpeptidase (GGT) and glycated hemoglobin. Moreover, CAP values, LSM values and values of the six indices, namely HOMA-IR, LAP, VAI, TyG, METS-IR and WTI, were remarkably higher in the NAFLD group (all the P < 0.001). Intriguingly, no significant difference was observed for TBil, AST, alkaline phosphatase (ALP), low-density lipoproteins cholesterol (LDL-c) and total Cholesterol between the two groups.
Associations between the six indices and NAFLD
The nonlinear relationship between the six indices and NAFLD was assessed by smooth curve fittings (Fig. 2A1-A6). Overall, all the six indices were positively related to the NAFLD. TyG, METS-IR, WTI showed smooth semi-bell curves indicating a near-linear relationship, while LAP, HOMA-IR and VAI displayed claw-shaped curves with saturation effect and inflection point. Notably, for METS-IR and WTI, the 95% CI band was relatively narrow. For HOMA-IR, VAI and LAP, there were linear correlations with NAFLD in a small numerical range.
Association between indices and NAFLD, CAP, LSM values via smooth curve fittings. The red dotted line represents the smooth curve fit between indices and the prevalence of NAFLD. Blue bands represent the 95% confidence interval from the fit. A1-A6: Associations between six indices and the prevalence of NAFLD; IR indices: A1-A3, ABO indices: A4-A6. B1-B6: Associations between six indices and the CAP values. IR indices: B1-B3, ABO indices: B4-B6.C1-C6: Associations between six indices and the LSM values. IR indices: C1-C3, ABO indices: C4-C6.D1-D6: Association between indices and prevalence of NAFLD stratified by weight grade. IR indices: D1-D3, ABO indices: D4-D6. Dotted lines of various colors represent nonlinear relationship between indices and NAFLD at different weight grades. E1-E6: Associations between six indices and the LSM values stratified by NAFLD. IR indices: E1-E3, ABO indices: E4-E6. Dotted lines of various colors represent nonlinear relationship between indices and median hepatic stiffness in NAFLD or non-NAFLD group.F1-F6: Associations between six indices and the LSM values stratified by fibrosis stages. IR indices: F1-F3, ABO indices: F4-F6. Dotted lines of various colors represent nonlinear relationship between indices and median hepatic stiffness at different fibrosis stages of NAFLD
CAP and LSM values were reported to increase significantly with increasing steatosis grade and fibrosis stage respectively [20]. Therefore, we further assessed the associations between the six indices and CAP values (Fig. 2B1-B6) as well as LSM values (Fig. 2C1-C6) via smooth curve fittings, which showed a consistent trend and a similar shape to that of NAFLD. Compared with LSM, CAP presented better linear correlation with the six indices, indicating higher predicting value for steatosis than fibrosis. As for subgroup analysis, we explored the effect of obesity degree on the relationship between the six indices and NAFLD via smooth curve fitting stratified by weight grade (Fig. 2D1-D6). Those positive correlations still survived in all the groups. We found fusiform curves fitting the relationship between VAI, TyG, METS-IR, WTI and NAFLD, which indicates that within a certain range of values, the heavier an individual is, the higher probability he may have to suffer from NAFLD. On the other hand, the predictive values of LAP and HOMA-IR for NAFLD were rarely influenced by weight grade. In addition, smooth curve fitting stratified by NAFLD showed nonlinear relationship between the six indices and LSM, which varied significantly from NAFLD to non-NAFLD (Fig. 2E1-E6). In the NAFLD group, the wave-like correlation curves were very common, except for METS-IR (Fig. 2E3). On the contrary, in the non-NAFLD group, the correlation curves generally showed a linear trend, except for HOMA-IR (Fig. 2E1). Since the vertical coordinates of Fig. 2E did not contain the extreme values of LSM, we further explored the relationship between the six indices and median hepatic stiffness at different stages of fibrosis (Fig. 2F1-F6), which showed that, at the F4 stage, there was no correlation between LSM and the six indices. In general, when categorized by the shape of the curves (Fig. 2), HOMA-IR, LAP, and VAI should be classified into one group, while TyG, WTI, and METS-IR can be classified into the other.
Table 2 displays the results of the multivariate logistic regression analysis between the six indices and the probability of NAFLD. Regardless of the confounding factors adjustment, all the indices presented significant positive associations with NAFLD. Since different variables have varying extremes, it is challenging to evaluate their predictive potential for NAFLD by odds ratio (OR) value, especially for LAP which has a wide range of values from 65.7 to 119438, resulting in the corresponding OR value very close to 1. Therefore, we converted continuous indices into categorical variables depending on quartiles and reassessed the associations via logistic regression. We found that the statistical results after conversion were more balanced and comparable. In general, the risk of NAFLD increased as categorical indices increased, compared with Q1. The prevalence of NAFLD is significantly higher in the third and fourth quartiles (Q3 and Q4) of all the six indices (P < 0.05 for all). LAP showed higher ORs at each quartile (Q2, Q3, Q4) compared with the other five indices. In addition, confounders posed little effect on the OR values of LAP and its quartiles. On the contrary, VAI showed the lowest predictive capacity for NAFLD.
A correlation map constructed by the Spearman correlation coefficient was used to visualize the correlation between NAFLD severity indicators and the six indices (Fig. 3). We found that all the indices were positively correlated with liver enzymes except for AST. However, the correlation intensity measured by the correlation coefficient varied among different indices and parameters. Particularly in the NAFLD group, the intensity of correlation with ALT was generally stronger for IR indices than ABO indices, which was not observed in the non-NAFLD group. The positive correlation between the six indices and LSM values was stronger and more significant in the NAFLD group compared with the non-NAFLD group. On the other hand, a remarkably positive relationship between the six indices and CAP values was observed in both groups.
Joint assessment of the six indices in diagnosing NAFLD
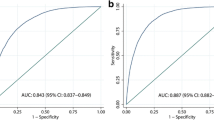
The AUC of the ROC curve is generally regarded as the main evidence for the accuracy of risk assessment instruments [21]. Thus, we used the ROC curve to evaluate the capability of six metabolic indices to predict the risk of NAFLD (all the P < 0.0001). As shown in Fig. 4, the ROC curves of all the six indices for screening NAFLD were located above the diagonal nondiscrimination line. Compared with ALT, all the six indices presented significantly better diagnostic efficiency for NAFLD. The AUCs ranged from 0.728 to 0.799 with the minimum derived from VAI and the maximum derived from LAP. The cutoff values and the corresponding sensitivities, specificities, and Youden indices of the six indices were summarized in Table 3. Interestingly, TyG had two different optimal cutoff values (8.555 and 8.605) which shared the same Youden index of 0.381.
In subgroup analysis, the AUCs of each index between different genders showed insignificant differences (all P > 0.05, Table 4). A pairwise comparison of AUCs of indices was performed to verify whether the difference between each pair of indices was significant. As shown in Table 5, significant differences were observed except for three pairs (HOMA-IR vs LAP, WTI vs METS-IR, TyG vs METS-IR). The similar AUCs (difference between AUCs (DBA): -0.002, P > 0.86) of LAP (AUC = 0.8) and HOMA-IR (AUC = 0.798) indicated their equivalent diagnostic accuracy for NAFLD. Figure 5 intuitively displays the hierarchical relationship of the six indices based on the AUC values, with LAP and HOMA-IR at the top level followed by WTI and METS-IR while VAI at the bottom.
Rank chart of six indices based on AUC values. Abdominal obesity indices are in yellow blocks and insulin resistance indicators are in green blocks. The higher the position, the greater the AUC value. The arrow points from the index with a relatively small AUC to the index with a larger AUC. The dotted line indicates P > 0.05 for DBA of the linked two indices and the solid line represents P < 0.05
Discussion
As the most common chronic metabolic liver disease, NAFLD has become a major threat to human health, with the disease burden growing rapidly from 1990 to 2019 all around the world [22]. Components of MetS have a well-established bidirectional association with NAFLD [1, 23]. IR and central abdominal obesity are closely tied to the progression of NAFLD [7, 9, 24]. Ectopic fat accumulation in liver has been considered as one of the mechanisms for the development of IR [25]. Most previous studies focused on the relationship between NAFLD and single metabolic index or congeneric indices while rare studies compared different metabolic indices for their potential to predict NAFLD. TyG, HOMA-IR and METS-IR are indicators for IR while WTI, VAI and LAP are ABO indices. Quite a few studies suggested associations between these indices and MetS [26,27,28,29], but research on their predictive role in NAFLD remains limited. Since the contribution of MetS to NAFLD has been confirmed by numerous studies, we carried out this research to verify and compare the associations between the six selected indices and NAFLD.
Among the six indices, LAP and HOMA-IR performed best in predicting NAFLD while VAI was the worst, based on logistic regression and ROC analysis. The difference in AUC values between HOMA-IR and LAP for screening NAFLD was insignificant (P for DBA > 0.05). The AUC of LAP (0.8) was slightly lower than that reported by Jinzhou Zhu et al. [30], likely due to difference in races of participants and diagnostic criteria for NAFLD. Despite the controversy, most studies support a significant correlation between LAP and NAFLD [31]. Yaling Li et al. found LAP to be the best predictor for MetS compared with VAI, Tyg and WTI using the NHANES database [29]. And metabolic abnormalities are well-known to be closely linked to NAFLD [19] which may account for the excellent predictive power of LAP for NAFLD. As reported by Bedogni, G. et al., LAP was able to identify individuals with ultrasonographic liver steatosis though without comparing it with other indices [32]. A cross-sectional case–control study [33] comparing the diagnostic performance of VAI, LAP, fatty liver index and hepatic steatosis index for NAFLD indicated that VAI had the lowest diagnostic value, concurring with ourfindings. However, this study focused exclusively on postmenopausal women and the scale was relatively small.
Men are more likely to suffer from NAFLD than women although the statistical significance was not remarkable. This gender-specific influence on metabolic-related fatty liver disease was also witnessed by Lucilla Crudele et al [34].
With regard to Spearman correlation analysis, although most correlation coefficients were less than 0.3, the large study sample was sufficient to judge the correlation [35]. In the NAFLD group, the correlation with ALT, namely the most sensitive liver enzyme, was stronger for IR indices than ABO indices, indicating that IR indicators were probably more sensitive in identifying acute hepatic injury in NAFLD patients, although large-scale prospective studies are required to confirm this conclusion. This may be due to that IR contributes to the progression of NAFLD from simple steatosis to NASH [23]. Rising ALT levels were also reported to be sensitive to reflecting IR and worsening lipid metabolism [36]. Maura PURCELL et al. observed that a higher proportion of obese youth presented elevated ALT levels compared with those overweight [37]. This research also revealed that IR and ABO were risk factors for elevated ALT levels, which is consistent with our findings, although they didn’t compare the correlation intensity. Some experts pointed out that total bilirubin was the best indicator for acute hepatic injury [38]. Thus, we analyzed this item and found that the 95% confidence interval for the average total bilirubin was within the normal range, with no significant difference in total bilirubin levels between the two groups. For other aspects, including smooth curve fitting, logistic regression, or ROC analysis, there was no significant trend from IR indices to ABO indices. This may be due to the tight connection between ABO and IR [39]. IR is considered as impaired insulin action in adipose tissue and is strongly linked to intra-abdominal fat accumulation [40]. ABO indices, such as LAP and VAI, were proven to be effective predictors of IR [41].
Based on the correlation heat map and smooth curve fitting, a significantly positive relationship was observed between indices and CAP values in both groups, indicating that all the indices were sensitive to steatosis grades. Previous studies indicated the severity of steatosis and fibrosis was positively related to IR and central obesity which coincides with our conclusion [9, 42]. A retrospective study [43], conducted on biopsy-confirmed NAFLD, revealed that LAP (r = 0.266, P = 0.001) and VAI (r = 0.189, P = 0.022,) were positively correlated with the grade of steatosis, consistent with our findings. With regard to LSM values, the positive relationship was significant only in the NAFLD group, indicating that these indices can reflect the severity of fibrosis only in NAFLD. Besides, a lack of linearity is evident in the nonlinear relationship curve between LSM and indices in NAFLD, except for METS-IR. But at each stage of fibrosis, except for F4, the relationship curve is near-linear. Previous studies observed significant differences between LAP, quartile-stratified HOMA-IR and TyG-related indices across the NAFLD and liver fibrosis via logistic regression [43, 44]. However, based on smooth curve fitting, we found that LAP, HOMA-IR and TyG didn’t have a linear relationship with fibrosis severity in NAFLD patients, which indicates those indices may not be proper indicators for monitoring fibrosis progression of NAFLD. Thus, it is necessary to combine various analytical approaches to assess the correlation.
Although females had a significantly higher risk for NAFLD and central obesity than males [45], few articles concerns the differences in the predictive value of ABO indicators for NAFLD between sexes. Based on our study, all the selected IR indicators and ABO indices were rarely influenced by sex for predicting NAFLD (P for DBA > 0.05). LAP and TyG were reported to be sex-specific indices for screening MetS [46]. Moreover, the interactions between the two indices and gender were significant for NAFLD [47]. However, we found that gender poses little effect on the diagnostic accuracy of LAP and TyG for NAFLD.
Several limitations exist in our study. The information obtained via questionnaires may be biased due to subjective factors, and some indicators lack quantitative standards. This is a cross-sectional study requiring large-scale prospective studies to validate our findings. In addition, the rich information conveyed by the figures and tables may not fully be mentioned in the text.
Conclusions
In conclusion, all the selected indices, namely LAP, HOMA-IR, TyG, VAI, METS-IR and WTI, presented a positive relationship with NAFLD prevalence, steatosis and fibrosis severity. Among the six indices, LAP and HOMA-IR demonstrated the best diagnostic performance for NAFLD. Compared to ABO indices, IR indicators may be more sensitive to acute hepatic injury in NAFLD.
Availability of data and materials
All the datasets used in the present study can be available on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- IR:
-
Insulin resistance
- ABO:
-
Abdominal obesity
- NHANES:
-
National Health and Nutrition Examination Survey
- LAP:
-
Lipid accumulation product
- VAI:
-
Visceral obesity index
- WTI:
-
Waist circumference-triglyceride index
- TyG:
-
Triglyceride glucose index
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- METS-IR:
-
Metabolic score for insulin resistance
- AUC:
-
Area under the curve
- ROC:
-
Receiver-operating characteristic
- ALT:
-
Alanine aminotransferase
- T2DM:
-
Type 2 diabetes mellitus
- NAFL:
-
Nonalcoholic fatty liver
- NASH:
-
Nonalcoholic steatohepatitis
- MetS:
-
Metabolic syndrome
- MEC:
-
Mobile examination center
- HCV:
-
Hepatitis C virus
- HBV:
-
Hepatitis B virus
- BMI:
-
Body mass index
- FPG:
-
Fasting plasma glucose
- HDL-c:
-
High-density lipoprotein cholesterol
- WC:
-
Waist circumference
- TG:
-
Triglyceride
- CAP:
-
Controlled attenuation parameter
- LSM:
-
Liver stiffness measurement
- SE:
-
Standard error
- CI:
-
Confidence interval
- GGT:
-
Gamma-glutamyl transpeptidase
- ALP:
-
Alkaline phosphatase
- LDL-c:
-
Low-density lipoproteins cholesterol
- OR:
-
Odds ratio
- DBA:
-
Difference between AUCs
References
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the american association for the study of liver diseases. Hepatology. 2018;67(1):328–57.
Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019;70(3):531–44.
Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65(8):1017–25.
Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70(4):1119–33.
Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut. 2022;71(4):778–88.
Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(11):1224–9 9 e1-2.
Fujii H, Kawada N, Japan Study Group Of Nafld J-N. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int J Mol Sci. 2020;21(11):3863.
Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404.
Eguchi Y, Eguchi T, Mizuta T, Ide Y, Yasutake T, Iwakiri R, et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol. 2006;41(5):462–9.
Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203.
American Diabetes A. 2. classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–27.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–57.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26.
Lee AM, Gurka MJ, DeBoer MD. A metabolic syndrome severity score to estimate risk in adolescents and adults: current evidence and future potential. Expert Rev Cardiovasc Ther. 2016;14(4):411–3.
Turemen EE, Cetinarslan B, Sahin T, Canturk Z, Tarkun I. Endothelial dysfunction and low grade chronic inflammation in subclinical hypothyroidism due to autoimmune thyroiditis. Endocr J. 2011;58(5):349–54.
Liu PJ, Lou HP, Zhu YN. Screening for metabolic syndrome using an integrated continuous index consisting of waist circumference and triglyceride: a preliminary cross-sectional study. Diabetes Metab Syndr Obes. 2020;13:2899–907.
Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–835.
Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–30.
Szmukler G, Everitt B, Leese M. Risk assessment and receiver operating characteristic curves. Psychol Med. 2012;42(5):895–8.
Paik JM, Henry L, Younossi Y, Ong J, Alqahtani S, Younossi ZM. The burden of nonalcoholic fatty liver disease (NAFLD) is rapidly growing in every region of the world from 1990 to 2019. Hepatol Commun. 2023;7(10):e0251.
Malaguarnera M, Di Rosa M, Nicoletti F, Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med (Berl). 2009;87(7):679–95.
Milic S, Lulic D, Stimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20(28):9330–7.
Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131–41.
Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32(3):596–604.
Chiu TH, Huang YC, Chiu H, Wu PY, Chiou HC, Huang JC, et al. Comparison of various obesity-related indices for identification of metabolic syndrome: a population-based study from Taiwan Biobank. Diagnostics (Basel). 2020;10(12):1081.
Endukuru CK, Gaur GS, Dhanalakshmi Y, Sahoo J, Vairappan B. Cut-off values and clinical efficacy of body roundness index and other novel anthropometric indices in identifying metabolic syndrome and its components among Southern-Indian adults. Diabetol Int. 2022;13(1):188–200.
Li Y, Zheng R, Li S, Cai R, Ni F, Zheng H, et al. Association between four anthropometric indexes and metabolic syndrome in US adults. Front Endocrinol (Lausanne). 2022;13:889785.
Zhu J, He M, Zhang Y, Li T, Liu Y, Xu Z, et al. Validation of simple indexes for nonalcoholic fatty liver disease in western China: a retrospective cross-sectional study. Endocr J. 2018;65(3):373–81.
Ebrahimi M, Seyedi SA, Nabipoorashrafi SA, Rabizadeh S, Sarzaeim M, Yadegar A, et al. Lipid accumulation product (LAP) index for the diagnosis of nonalcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Lipids Health Dis. 2023;22(1):41.
Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010;10:98.
Vassilatou E, Lafoyianni S, Vassiliadi DA, Ioannidis D, Paschou SA, Mizamtsidi M, et al. Visceral adiposity index for the diagnosis of nonalcoholic fatty liver disease in premenopausal women with and without polycystic ovary syndrome. Maturitas. 2018;116:1–7.
Crudele L, De Matteis C, Novielli F, Di Buduo E, Petruzzelli S, De Giorgi A, et al. Fatty Liver Index (FLI) is the best score to predict MASLD with 50% lower cut-off value in women than in men. Biol Sex Differ. 2024;15(1):43.
Sedgwick P. Spearman’s rank correlation coefficient. BMJ. 2014;349:g7327.
Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91(11):4287–94.
Purcell M, Flores YN, Zhang ZF, Denova-Gutierrez E, Salmeron J. Prevalence and predictors of alanine aminotransferase elevation among normal weight, overweight and obese youth in Mexico. J Dig Dis. 2013;14(9):491–9.
Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46(12):2050–68.
Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, et al. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) family study. Diabetes. 2003;52(10):2490–6.
Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–4.
Wei R, Gao Z, Xu H, Jiang C, Li X, Liu Y, et al. Body fat indices as effective predictors of insulin resistance in obstructive sleep apnea: evidence from a cross-sectional and longitudinal study : BFI as predictors of IR in OSA. Obes Surg. 2021;31(5):2219–30.
Eguchi Y, Mizuta T, Sumida Y, Ishibashi E, Kitajima Y, Isoda H, et al. The pathological role of visceral fat accumulation in steatosis, inflammation, and progression of nonalcoholic fatty liver disease. J Gastroenterol. 2011;46(Suppl 1):70–8.
Li R, Liu J, Han P, Shi R, Zhao L, Li J. Associations between abdominal obesity indices and pathological features of non-alcoholic fatty liver disease: Chinese visceral adiposity index. J Gastroenterol Hepatol. 2023;38(8):1316–24.
Xue Y, Xu J, Li M, Gao Y. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: triglyceride glucose index-related parameters. Front Endocrinol (Lausanne). 2022;13:951689.
Ayonrinde OT, Olynyk JK, Beilin LJ, Mori TA, Pennell CE, de Klerk N, et al. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology. 2011;53(3):800–9.
Anto EO, Frimpong J, Boadu WIO, Korsah EE, Tamakloe V, Ansah E, et al. Cardiometabolic syndrome among general adult population in Ghana: The role of lipid accumulation product, waist circumference-triglyceride index, and triglyceride-glucose index as surrogate indicators. Health Sci Rep. 2023;6(7):e1419.
Lin IT, Lee MY, Wang CW, Wu DW, Chen SC. Gender differences in the relationships among metabolic syndrome and various obesity-related indices with nonalcoholic fatty liver disease in a Taiwanese population. Int J Environ Res Public Health. 2021;18(3):857.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
SJJ analyzed the data and drafted this manuscript. JHC assisted SJJ in cleaning the data. ZZQ polished the language. QGQ supervised the study.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethics review board of the National Center for Health Statistics approved all the NHANES protocols and written informed consents were obtained from participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, J., Chen, J., Zhang, Z. et al. Multi-dimensional comparison of abdominal obesity indices and insulin resistance indicators for assessing NAFLD. BMC Public Health 24, 2161 (2024). https://doi.org/10.1186/s12889-024-19657-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19657-6