Abstract
Background
Metabolic syndrome (MetS) is a risk factor for cardiovascular disease (CVD), and CVD is a major challenge for cancer patients. This study aimed to investigate the prevalence and association of MetS and CVD among adult cancer patients.
Methods
This cross-sectional study included cancer patients aged > 18 years from the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2018. The prevalence of MetS and CVD was calculated using weighted analysis. Multivariable logistic regression was used to assess the association between MetS and CVD.
Results
The study included 2658 adult cancer patients, of whom 1260 exhibited MetS and 636 had CVD. The weighted prevalence of MetS and CVD in cancer patients was 45.44%, and 19.23%, respectively. Multivariable logistic regression showed a 79% increased risk in higher CVD prevalence in cancer patients with MetS, with the OR (95% CI) of 1.79 (1.31, 2.44). Notably, obesity, elevated blood pressure (BP), high glucose, and low high density lipoprotein cholesterol (HDL-C) in the MetS components were significantly associated with higher CVD prevalence after adjusting for covariates. Moreover, the risk of CVD prevalence in cancer patients increased with more MetS components. Notably, MetS was more strongly linked to CVD in patients aged < 65 and women.
Conclusions
Among adult cancer patients, over two-fifths (45.44%) were estimated to have MetS, while about one-fifth (19.23%) were considered to have CVD. Notably, obesity, elevated BP, high glucose, low HDL-C, and higher number of MetS components were found to be significantly associated with higher CVD prevalence among cancer adults. Cancer patients under 65 and women with MetS may be at increased risk of CVD.
Similar content being viewed by others
Introduction
Cancer and cardiovascular disease (CVD) represent prominent contributors to mortality globally, with their burdens continuing to escalate [1,2,3]. As advancements in therapeutic modalities enable extended survival among cancer patients, the cardiac system has been subjected to an intricate interplay between tumor biology and potentially cardiotoxic treatment exposures. Consequently, cancer survivors face an increased susceptibility to developing CVD [4].
The metabolic syndrome (MetS), a group of interconnected risk factors for CVD, which significantly elevate the risk of CVD in adult cancer patients [5]. Notably, MetS is also an important target for secondary prevention to promote cardiovascular health in long-term cancer patients [6]. MetS could be a key factor in predicting CVD risk in long-term cancer survivors due to its strong association with cardiovascular events and mortality [7]. MetS is a condition characterized by a set of metabolic risk factors including abdominal obesity, hyperglycemia, elevated blood pressure (BP), high triglycerides (TG) levels, or low high-density lipoprotein cholesterol (HDL-C) levels, with a minimum of three factors required for diagnosis [8]. These five MetS components are linked to an elevated risk of CVD and diabetes [9, 10]. It is estimated that about 20% to 30% of the world's adult population is affected by MetS [11], while 36.5% American adults have MetS [12].
It is noteworthy that MetS is linked to a higher risk of cancer. Monestime et al. reported that individuals with MetS who are women or older are at a greater risk of developing obesity-related cancers [13]. Another cross-sectional analysis of Israeli adults found that in the presence of MetS, cancer prevalence was higher in those with hyperglycemia and CVD was more likely in men with MetS [14]. These studies suggest that MetS is highly prevalent and interacts with CVD and cancer. Nevertheless, the existing studies have primarily concentrated on the adult general population, leaving a gap in the prevalence and correlation between MetS and CVD in cancer survivors. Therefore, the characteristics and association between MetS and CVD in cancer patients deserve attention.
Cancer therapies have the potential to induce cardiotoxicity [4], likewise, the adverse effects of cancer treatment may contribute to the emergence of MetS [15]. Cancer patients typically have an increased risk of CVD, hospital admissions for CVD, and CVD mortality [16, 17]. Meanwhile, MetS has a significant impact on increasing CVD risk and adversely impacting the prognosis for CVD [9, 10, 18]. MetS may link cancer therapy with long-term CVD risks [5]. However, studies on the prevalence of MetS and CVD and the association between them in cancer patients are limited. Thus, we are committed to addressing this issue.
Methods
Study design and participants
The National Center for Health Statistics (NCHS) conducts the National Health and Nutrition Examination Survey (NHANES), a comprehensive cross-sectional survey, which is administered by the Centers for Disease Control and Prevention (CDC) in the United States. The survey employs a complex sampling methodology, incorporating a multistage probability sampling approach, to generate statistically significant data that better represents the non-institutionalized resident population nationally. The NHANES data are released in a 2-year cycle and encompass a wide range of information, including demographics, physical examinations, health-related questionnaires, laboratory tests, and dietary assessments. The NHANES data were obtained via standardized household interviews person-in-person, followed by physical and health examinations carried out at the Mobile Examination Center (MEC). The investigation was approved by the research ethics review board of NCHS, as outlined in their documentation [19]. Before participating in the study, each participant was required to provide written informed consent. Comprehensive information on NHANES study design, survey methods, and data are provided on the website [20].
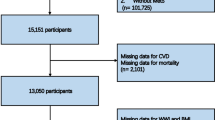
This cross-sectional study aims to analyze the prevalence and association of MetS and CVD among adult cancer patients using data from six cycles of NHANES (2007–2018), a total of 59,842 individuals participated in the study. Cancer participants were established by answering “Yes” to the question “Ever been told by a doctor or other health professional that had cancer or a malignancy of any kind?” in the medical conditions questionnaire (MCQ). Exclusion criteria included participants aged ≤ 18 years, non-cancer patients or non-responders to the MCQ, and those without baseline data on waist circumference, blood pressure (BP), body mass index (BMI), education, smoking, biochemical data. Consequently, a total of 2658 cancer patients were included in the final analysis. The flowchart of participation selection is shown in Fig. 1.
Exposure variables
The exposure variables for this study were MetS and its components, including central obesity, elevated BP, hyperglycemia, high triglycerides (TG) levels, and low high-density lipoprotein cholesterol (HDL-C) levels. According to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATPIII) [8], Mets was defined as a cluster of at least three of the following five components: (1) Obesity (increased waist circumference ≥ 102 cm for men and ≥ 88 cm for women); (2) Elevated BP (systolic BP ≥ 130 and / or diastolic BP ≥ 85 mmHg); (3) High glucose (≥ 100 mg / dl); (4) High TG (≥ 150 mg / dl); (5) Low HDL-C (< 40 mg / dl for men and < 50 mg / dl for women). In addition, we classified the number of MetS components and classified the above five factors into "0–1", "2", "3", and "4–5". Based on the diagnostic criteria of MetS, "0–1" and "2" were categorized as having no MetS.
Waist circumference was measured by connecting the upper lateral edge of the iliac bones on both sides with the horizontal line of the front and back of the abdomen. BP measurements were taken three times consecutively after a five-minute rest in a quiet sitting position. If a BP measurement was incomplete or interrupted, a fourth attempt might be made. The measured mean values were taken to reflect the systolic BP (SBP) and diastolic BP (DBP). Serum glucose, HDL-C and TG samples were shipped to the Collaborative Minnesota Laboratory Service Center for analysis by Beckman coulter UniCel DxC 800 Synchron. Serum glucose concentrations were determined by the glucose oxidase method, while HDL-C and TG concentrations were determined by the timed endpoint method. The serum glucose, cholesterol and TG in this study were venous blood extracted from fasting state. Details of the specific laboratory data and methods are provided on the website [20].
Outcome variable
The study outcome variable is CVD, which is defined by using structured questions in a standardized MCQ. Participants were asked the following questions, "Has your doctor or other health professionals ever told you that you have heart failure, coronary heart disease, angina, heart attack (or myocardial infarction), or stroke?" Participants who responded affirmatively to one or more of them were classified as having CVD.
Covariates
The covariates for this study were demographic characteristics including age, sex, ethnicity, body mass index (BMI, body weight divided by height squared, kg/m2), smoking status, education, and physical activity. Ethnicity was categorized as "Mexican American", "Non-Hispanic Black", "Non-Hispanic White", and "Other". Smoking status was based on two questions "Do you now smoke cigarettes?" and "Have you ever smoked at least 100 cigarettes in life?" to be categorized as "None", "Former" and "Now". Education was categorized as "High school or below" and "College or above". Alcohol consumption was used questionnaire "Ever have 4/5 or more drinks every day?", with a positive answer considered to be "Yes". We classified physical activity as "Inactive" and "Active" according to the physical activity questionnaire, where "Active" included walking or bicycle, both moderate to vigorous or vigorous activity of work and recreation.
Statistical analyses
Since NHANES applied sample weighting, stratification, and cluster variables to address complex survey designs, including oversampling and nonresponse, all statistical analyses in this study were conducted using the complex sampling design of the NHANES database to better represent the target population. The sample for this study encompasses data from six cycles, spanning a total of 12 years of NHANES (2007–2018), with sample weights calculated following NHANES analytic guidelines [21]. Specifically, we weighted the data by the 6-period Full Sample 2 Year MEC Exam Weight (WTMEC2YR), the statistical analysis took into account the weight of 1/6 × WTMEC2YR. Prevalence and 95% confidence intervals (CI) for MetS, CVD, comorbidity or alone, and MetS components classification of cancer patients were calculated using sample weights and converted to predictions for the corresponding numbers in the US population. MetS components classification included specific five components of MetS and number of them, as described in the exposure variables section. Graphs illustrating the prevalence and 95% CI in the overall sample were generated. Furthermore, the prevalence and 95% CI trends across the six NHANES cycles were plotted, with detailed data available in the Supplementary material.
To investigate the association between MetS and CVD among adult cancer participants, baseline characteristics were presented by non-CVD and CVD. Continuous variables including age, waist circumference, SBP, DBP, BMI, serum glucose, TG, and HDL-C were presented as weighted mean with standard error (SE), and comparisons between groups were performed by using Student's t-test (normal distribution) or Mann Whitney U test (skew distribution). We explored the association of MetS and MetS components classification with CVD prevalence in cancer patients by logistic regression analysis. To elucidate the results, we built three models to provide statistical inferences. The Crude model was a univariable analysis. Model I adjusted for age, sex, and ethnicity. Model II additionally adjusted for BMI, education, smoking status, alcohol use, and physical activity in addition to Model I. Given the multicollinearity of BMI and obesity, and BMI is also an important indicator of obesity in MetS [22], we excluded BMI from the multivariable-adjusted Model II to explore the association between obesity in the MetS components and CVD.
In addition, we explored the association between baseline characteristics and CVD in cancer patients by univariable and multivariable logistic regression analysis. For the significant MetS components in multivariable-adjusted Model II, we classified them into quartiles and tested their association with CVD in three models, also performed with trend tests. Further, we performed subgroup analysis of the association of MetS and MetS components classification with CVD prevalence under multivariable-adjusted Model II for age (< 65 and ≥ 65 years) and sex (men and women). Interactions between subgroups were also tested.
Statistical analyses were executed via R software (version 4.2.1), while taking into account sample weights for all analyses conducted. Significance of results was determined when the two-tailed P-value was less than 0.05.
Results
Prevalence of MetS and CVD
A total of 2658 adult caner participants from NHANES 2007–2018 were included in this study (Fig. 1). The 2658 cancer patients represented approximately 6.46% of NHANES 2007–2018 adults, reflecting 19. 97 million US population. The weighted prevalence of MetS in these cancer patients was 45.44%, which represented 9.07 million US population. The weighted prevalence of CVD among these cancer patients was 19.23%, which represented 3.84 million US population. About one tenth (11.89%) cancer patients had MetS comorbid CVD, reflecting 2.37 million US population (Fig. 2A, Supplementary Table S1). The weighted prevalence (95% CI) and projected number in US for MetS without CVD (33.55%, 6.7 million), CVD without MetS (7.34%, 1.46 million), and MetS components classification were also shown in Supplementary Table S1.
We also calculated the weighted prevalence and 95% CI for variables above by each 2-year period for a total of 6 cycles from NHANES 2007–2018 (Supplementary Table S2). Bar graphs (Fig. 2) and line graphs (Fig. 3) were also plotted to visualize the prevalence of variables in Supplementary Table S1-S2.
Baseline characteristics of cancer participants
The weighted mean age of the overall cancer participants was 62.47 (0.38) years. Among them, 636 cancer patients had CVD, and their weighted mean age was 69.29 (0.64) years. Meanwhile, cancer patients without CVD had a weighted mean age of 60.85 (0.44) years. Among them, 1271 (44.28%) were male and 361 suffered from CVD (52.59%). Of the 1260 cancer patients with MetS, 386 (61.83%) had CVD. Among the MetS components, obesity was prevalent in a significant majority (67.53%) of cancer participants, while elevated BP accounted for the second proportion (55.99%). Approximately two-fifths had high glucose (37.41%) and high TG (41.79%). While, followed by low HDL-C (31.01%). Baseline characteristics among cancer participants with and without CVD showed significant differences, except for ethnicity, serum TG, and high TG in MetS components (Table 1).
Association between MetS and CVD among adult cancer participants
We explored the association of baseline characteristics of cancer patients with CVD using univariable and multivariable logistic regression. Multivariable analysis suggested that age, education (high school or below), smoking status (form and now), alcohol consumption, MetS, MetS components (obesity, elevated BP, high glucose, and low HDL-C), and number of MetS components (3, 4–5) were significantly associated with higher CVD prevalence (Table 2).
In three logistic regression models as described in the methods, we observed significant associations between MetS and MetS components classification with CVD in adults with cancer. As results, cancer patients with MetS had a significantly higher CVD prevalence compared to those without MetS. The association between MetS and CVD of the odds ratios (ORs) and 95% confidence intervals (CIs) for the Crude model (univariable), Model I, and Model II (multivariable) were 2.28(1.74,2.98), 2.05(1.56,2.70), and 1.79(1.31,2.44), respectively (Table 2, Supplementary Table S3).
MetS components classification includes five factors in MetS components and No. of MetS components, as described in methods. We found that obesity, elevated BP, high glucose, and low HDL-C had a significant effect on the increased CVD prevalence in cancer patients in all three models. We utilized "0–1" as a reference for the number of MetS components and found that the risk of CVD increased significantly across all three models for individuals with "3" and "4–5" MetS components. The ORs (95% CIs) for "4–5" were higher than those for "3", with values of [3.66(2.62,5.12) VS 2.11(1.46,3.04)], [3.10(2.19,4.37) VS 1.72(1.17,2.52)], and [2.67(1.82,3.92) VS 1.52(1.02,2.26)] in the three models. However, "2" versus "0–1" in number of MetS components had a significant effect on CVD only in the Crude model, with an OR (95% CI) of 1.55(1.14,2.10) (Supplementary Table S3). In order to visually illustrate the associations of MetS and MetS components classification with CVD, error bar plots for the three models were presented (Fig. 4).
Associations of MetS components (4A) and No. of MetS components (4B) with CVD in cancer participants from NHANES 2007–2018. Crude model, Model I, and Model II were shown in black, red, and green, respectively. Crude model was univariate logistic regression model. Model I adjusted for age, sex, and ethnicity. Model II adjusted for age, sex, ethnicity, BMI, education, smoking status, alcohol consumption, and physical activity
Additionally, subgroup analysis showed that MetS was more significantly linked to higher CVD prevalence (higher OR) in adult cancer patients aged under 65 years (OR = 2.63, 95% CI: 1.50–4.61) and women (OR = 2.29, 95% CI:1.48–3.55). The association between MetS components classification and CVD prevalence in subgroup analysis was also presented (Table 3).
For MetS components and CVD in multivariable adjustment Model II has a significant positive correlation between obesity, high glucose and low HDL-C. Thus, we further explored the quartiles of waist circumference, serum glucose and HDL-C on CVD, and trend tests were also depicted. We found that higher waist circumference and serum glucose were associated with higher CVD prevalence, whereas the opposite association for HDL-C and CVD, and the trend tests for all three models were significant (Supplementary Table S4).
Discussion
The 2019 Global Burden of Disease (GBD) study indicated that cancer ranked second to CVD in terms of global deaths, disability-adjusted life years, and years of life lost [3]. Common cardiovascular risk factors in cancer patients, including genetic factors, aging, smoking, obesity, hyperlipidemia, and diabetes, are linked to the development of cancer [23, 24]. MetS is believed to increase the risk of diabetes and CVD [10, 17]. We hypothesize that there is a high prevalence of MetS and CVD among cancer patients, and these conditions are significantly associated. However, limited research has been conducted on this topic. In addition, there is not enough evidence on MetS components and their number affect CVD risk in cancer patients.
The present study conducted an analysis of adult cancer participants using NHANES data from 2007–2018. Weighted prevalence estimates indicated that 45.44% of participants had MetS, and 19.23% had CVD. Notably, 11.83% of cancer patients with MetS also had co-morbid CVD. The study showed the prevalence of MetS components, and their trends over 2-year cycles. The study found a strong link between MetS and CVD in cancer patients, even after considering other covariates. Additionally, obesity, elevated BP, high glucose, and low HDL-C in MetS components were significantly associated with increased CVD prevalence, while high TG were not significant. Cancer patients with more MetS components have a higher risk of CVD. In subgroup analysis, MetS was significantly and highly linked to increased CVD prevalence in aged < 65 years and women cancer patients.
Cancer therapy itself may increase the risk of CVD and MetS. Mediastinal radiotherapy and certain chemotherapy drugs such as anthracyclines can cause direct harm to the heart, while platinum and bleomycin chemotherapy can lead to vascular endothelial injury [25]. Chemotherapy-induced damage to the endothelium may contribute to the development of MetS due to its role in insulin resistance [26]. On the other hand, Nuver et al. reported that patients with testicular cancer who underwent chemotherapy (26%) and surgery (36%) are at a significantly higher risk of MetS than healthy controls (9%) [27]. Post-treatment conditions such as hormone deficiency, hypomagnesemia, and endothelial dysfunction may correlate with the etiology of MetS in cancer survivors [6]. According to an NHANES study (2003–2006) [28], approximately 34% of the participants met the NCEP: ATPIII criteria for MetS. In contrast, the prevalence of MetS was higher (45.44%) among adult cancer patients in our study. Liu et al. reported on a cohort of 710,170 cancer patients that 18% of them had comorbidities related to CVD [29]. Similarly, our results reported that 19.23% of cancer patients had CVD.
MetS is becoming increasingly prevalent, which may be attributed to the obesity epidemic [30, 31]. Our study reveals that obesity is the main factor in MetS, with over three-fifths of cancer patients being obese during each 2-year period. Obesity is positively correlated with an elevated risk of hypertension, diabetes, dyslipidemia, CVD, certain cancers, and mortality [32, 33]. This is consistent with our finding that the higher the waist circumference of cancer patients, the higher risk of CVD. Obesity in breast cancer survivors is common, which is often linked to pre-treatment obesity and a decline in physical activity post-treatment [34]. Hypertension is a prevalent comorbidity among cancer patients, with 38% of them reported to be affected. Due to the increased cardiovascular and mortality risks associated with hypertension, optimal blood pressure management in cancer patients with hypertension needs to be emphasized [35]. A sedentary lifestyle and reduced physical activity are essential risk factors for MetS [36]. Guidelines recommend that a practical, regular, moderate physical activity program (e.g., moderate intensity exercise for 30 min each day) will decrease the risk of all 5 factors of the MetS [37]. In our study, we found a risk effect of inactive physical activity in a univariable logistic regression and considered adjusting for physical activity in Model II as well.
The MetS is a recognized risk factor and prognostic indicator for CVD [10, 18, 38]. Hu et al. found that individuals with MetS had a higher risk of all-cause and cardiovascular mortality compared to those without MetS, even after adjusting for age, blood cholesterol, and smoking [39]. Additionally, Wambui et al. discovered that MetS in women was associated with breast cancer and overall cancer mortality, with waist circumference, BP and serum glucose being independent predictors of death [40]. Similarly, our results show that MetS exhibited a significantly higher CVD risk in women cancer participants. Additionally, waist circumference and serum glucose were identified as significant influential variables in the MetS components. It is noteworthy that the incidence of MetS exhibited an upward trend with advancing age, with a range of 5% in individuals aged 20–30 years to 50% in those aged 50 years [41, 42]. However, in the subgroup analysis of our study, MetS was significantly higher associated with CVD in adult cancer patients aged < 65. The reason behind this seemingly paradoxical phenomenon might be the declining age at which obesity begins [43], while obesity is considered to be a significant contributor to the onset of MetS [30]. Of note, approximately 32% of US adults are obese [44], while over three-fifths of the cancer patients in our study had obesity (67.53%).
Studies have found that individuals with more MetS components have double the cardiovascular risk compared to those with fewer components, even after accounting for age and other traditional risk factors, indicating that MetS is an independent risk factor for CVD [38, 45]. Similarly, our study confirmed that the number of MetS components in cancer patients was significantly associated with higher CVD prevalence. MetS was linked to higher risk of death from CVD and coronary heart disease in two European studies [18, 46]. The significant associations between MetS and myocardial infarction and stroke were found in a NHANES III study enrolling 10,537 participants [47]. A study in Korea found that staying in an unhealthy metabolic state increased the risk of CVD and all-cause death. However, there was no significant association observed between MetS and cancer morbidity and mortality [48]. This seems to suggest that MetS is linked to a higher risk of CVD and worse outcomes, but its impact on cancer remains inconclusive.
MetS and CVD in cancer patients may be associated with growth hormone deficiency (GHD). Growth hormone plays a pivotal role in protein synthesis, promoting lipolysis, and exerting indirect insulin-like effects by stimulating Insulin-like Growth Factor-1 (IGF-1) production in the liver and local tissues, as well as by stimulating glucose uptake by peripheral tissues [49]. GHD in cancer and brain tumor survivors may be caused by tumor growth, surgery, or radiation affecting the hypothalamic-pituitary region [50]. GHD has been linked to changes in body composition, including a decrease in lean body mass and an increase in adiposity. Of note, decreased lean body mass might lead to insulin resistance and dyslipidemia [5]. Adults with GHD are usually overweight or obese and have increased overall fat mass (mainly abdominal), reduced muscle mass, decreased serum IGF-1 concentrations, and reduced exercise capacity [51]. CVD and elevated BP, similar to the MetS are complications in adults with GHD. Furthermore, abdominal obesity and insulin resistance increase the risk of premature atherosclerosis and CVD [51]. Notably, insulin resistance is another description of the MetS [36]. Hyperinsulinemia, which is the precursor to insulin resistance, disturbs the balance of the insulin-growth hormone-IGF axis, downregulating the ratio of growth hormone to insulin, resulting in lower energy expenditure and higher fat accumulation [52].
Possible mechanisms in the development of MetS include genetics, caloric imbalance, inflammation, pro-thrombotic cytokine, and dysregulation of excessive adipose tissue [22, 36]. Similarly, the biological properties of cancer can exert pro-thrombotic and pro-inflammatory effects by activating platelets and affecting neutrophils [53, 54]. Cancer patients have a higher likelihood of developing MetS due to various factors, including comorbidities such as obesity, microalbuminuria, hypertension, dyslipidemia, and insulin resistance post-chemotherapy. These risk factors and metabolic changes could indirectly affect the long-term development of CVD [55].
Treatment of cancer might result in endothelial dysfunction, leading to MetS and early atherosclerosis [11]. Therefore, preventing or treating endothelial dysfunction may be an important measure to prevent CVD in cancer patients. In addition, a study found a 10-year CVD hospitalization rate of 10.8% in early-stage breast cancer patients, with ischemic heart disease being the most common cause and hypertension, diabetes, and chemotherapy as important risk factors for heart failure hospitalization [56]. Focusing on female breast cancer patients and preventing MetS components like atherosclerosis, hypertension, and diabetes could reduce the risk of CVD-related hospitalization and cancer mortality [57]. Therefore, secondary prevention of MetS for cancer patients is beneficial for improving cancer prognosis [11].
This study's strengths include its use of NHANES data representing the U.S. population, weight analysis for MetS and CVD in cancer patients, and adjustment for demographic and lifestyle factors. The study focused on cancer patients, who are susceptible to CVD. To our knowledge, we are the first to explore the prevalence and association of MetS with CVD in cancer patients using weighted analysis in NHANES 2007–2018. Although there have been a large number of studies on MetS and CVD, few studies have linked them in cancer patients. Furthermore, our study examined the MetS components classification among adults with cancer, with particular emphasis on the significant association between waist circumference, serum glucose, and HDL-C levels with CVD. Our findings indicate that having more MetS components increases the risk of CVD in cancer patients. We also performed subgroup analysis by age and gender to identify susceptible populations with significant association between MetS and CVD.
However, it should be acknowledged that this study also has some limitations. First, although several studies have shown that MetS is a risk factor for CVD, due to the nature of cross-sectional studies and temporal differences in the sequence of disease diagnosis, a causal association cannot be clearly established. Moreover, although we adjusted for most of the relevant confounders, residual or unknown confounders cannot be ruled out. Second, the data regarding cancer and CVD may be subject to self-reporting bias. Third, due to methodological definitions and limited sample size, our study involved all types of cancer and no further analysis was performed on specific types of cancer. Prospective cohort studies are necessary in the future to focus on the prognostic impact of MetS interventions, such as diet, weight loss, and exercise, especially on patients with specific types of cancer. Such studies will underscore the importance of maintaining the metabolic and cardiovascular health in cancer patients.
Conclusions
In summary, over two-fifths (45.44%) of US adults with cancer have MetS and about one-fifth (19.23%) have CVD. Significant factors associated with an increased risk of higher CVD prevalence in cancer patients were identified as obesity, elevated BP, high serum glucose, low HDL-C, and higher number of MetS components. Moreover, aged < 65 years and women cancer patients with MetS are more significantly associated with CVD. According to our study, MetS and CVD are prevalent among adult cancer patients and should not be overlooked. It is essential to address the metabolic abnormalities and manage the increased risk of CVD among adults with cancer.
Availability of data and materials
The data can be reached from the dataset published in NHANES (https://www.cdc.gov/nchs/nhanes/index.htm). The primary data generated or analyzed in this article can be provided upon reasonable request to the corresponding author.
Abbreviations
- ATPIII:
-
Adult Treatment Panel III
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CDC:
-
Centers for Disease Control and Prevention
- CVD:
-
Cardiovascular disease
- GHD:
-
Growth hormone deficiency
- HDL-C:
-
High density lipoprotein cholesterol
- IGF-1:
-
Insulin-like Growth Factor-1
- MCQ:
-
Medical conditions questionnaire
- MEC:
-
Mobile Examination Center
- MetS:
-
Metabolic syndrome
- NCEP:
-
National Cholesterol Education Program
- NHANES:
-
National Health and Nutrition Examination Survey
- NCHS:
-
National Center for health statistics; OR (95%CI): odds ratio (95% confidence intervals)
- TG:
-
Triglyceride
References
Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–171, https://doi.org/10.1016/s0140-6736(14)61682-2.
Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151–1210, https://doi.org/10.1016/s0140-6736(17)32152-9.
Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, Henrikson HJ, Lu D, Pennini A, Xu R, et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420–44. https://doi.org/10.1001/jamaoncol.2021.6987.
Karlstaedt A, Barrett M, Hu R, Gammons ST, Ky B. Cardio-Oncology: Understanding the Intersections Between Cardiac Metabolism and Cancer Biology. JACC Basic Transl Sci. 2021;6:705–18. https://doi.org/10.1016/j.jacbts.2021.05.008.
de Haas EC, Oosting SF, Lefrandt JD, Wolffenbuttel BH, Sleijfer DT, Gietema JA. The metabolic syndrome in cancer survivors. Lancet Oncol. 2010;11:193–203. https://doi.org/10.1016/s1470-2045(09)70287-6.
Nuver J, Smit AJ, Postma A, Sleijfer DT, Gietema JA. The metabolic syndrome in long-term cancer survivors, an important target for secondary preventive measures. Cancer Treat Rev. 2002;28:195–214. https://doi.org/10.1016/s0305-7372(02)00038-5.
Felicetti F, Brignardello E, Nuver J. Cardiometabolic Risk, Part 2: Indirect Cardiotoxicity in Cancer Survivors - The Emerging Role of Metabolic Syndrome. Front Horm Res. 2021;54:130–9. https://doi.org/10.1159/000519414.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. https://doi.org/10.1161/circulationaha.109.192644.
Haffner SM. Relationship of metabolic risk factors and development of cardiovascular disease and diabetes. Obesity (Silver Spring). 2006;14(Suppl 3):121s–7s. https://doi.org/10.1038/oby.2006.291.
Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47:1093–100. https://doi.org/10.1016/j.jacc.2005.11.046.
Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36. https://doi.org/10.1161/atvbaha.107.151092.
An S, Ahn C, Jang J, Lee J, Kang D, Lee JK, Park SK. Comparison of the Prevalence of Cardiometabolic Disorders and Comorbidities in Korea and the United States: Analysis of the National Health and Nutrition Examination Survey. J Korean Med Sci. 2022;37:e149. https://doi.org/10.3346/jkms.2022.37.e149.
Monestime S, Beech B, Kermah D, Norris K. Prevalence and predictors of obesity-related cancers among racial/ethnic groups with metabolic syndrome. PLoS ONE. 2021;16:e0249188. https://doi.org/10.1371/journal.pone.0249188.
Rayyan Assi H, Ziv A, Dankner R. The metabolic syndrome and its components are differentially associated with chronic diseases in a high-risk population of 350 000 adults: A cross-sectional study. Diabetes Metab Res Rev. 2019;35:e3121. https://doi.org/10.1002/dmrr.3121.
Emery J, Butow P, Lai-Kwon J, Nekhlyudov L, Rynderman M, Jefford M. Management of common clinical problems experienced by survivors of cancer. Lancet. 2022;399:1537–50. https://doi.org/10.1016/s0140-6736(22)00242-2.
Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, Zaorsky NG. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–97. https://doi.org/10.1093/eurheartj/ehz766.
Kobo O, Raisi-Estabragh Z, Gevaert S, Rana JS, Van Spall HGC, Roguin A, Petersen SE, Ky B, Mamas MA. Impact of cancer diagnosis on distribution and trends of cardiovascular hospitalizations in the USA between 2004 and 2017. Eur Heart J Qual Care Clin Outcomes. 2022;8:787–97. https://doi.org/10.1093/ehjqcco/qcac045.
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. https://doi.org/10.1001/jama.288.21.2709.
Centers for Disease Control and Prevention. National Center for Health Statistics. NHANES 2007–2018, NCHS Ethics Review Board (ERB) Approval. . Availabe online: https://www.cdc.gov/nchs/nhanes/irba98.htm (accessed on July 22nd, 2023).
Centers for Disease Control and Prevention. National Center for Health Statistics. NHANES 2007–2018, questionnaires, datasets, and related documentation. Availabe online: http://www.cdc.gov/nchs/nhanes.htm (accessed on July 22nd, 2023).
Centers for Disease Control and Prevention. National Center for Health Statistics. NHANES 2007–2018, NHANES Survey Methods and Analytic Guidelines. Availabe online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#estimation-and-weighting-proced (accessed on July 22nd, 2023).
Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. https://doi.org/10.1186/1741-7015-9-48.
Moslehi J, Zhang Q, Moore KJ. Crosstalk Between the Heart and Cancer: Beyond Drug Toxicity. Circulation. 2020;142:684–7. https://doi.org/10.1161/circulationaha.120.048655.
Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet. 2014;383:549–57. https://doi.org/10.1016/s0140-6736(13)62224-2.
Nuver J, Smit AJ, van der Meer J, van den Berg MP, van der Graaf WT, Meinardi MT, Sleijfer DT, Hoekstra HJ, van Gessel AI, van Roon AM, et al. Acute chemotherapy-induced cardiovascular changes in patients with testicular cancer. J Clin Oncol. 2005;23:9130–7. https://doi.org/10.1200/jco.2005.01.4092.
Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904. https://doi.org/10.1161/circulationaha.105.563213.
Nuver J, Smit AJ, Wolffenbuttel BH, Sluiter WJ, Hoekstra HJ, Sleijfer DT, Gietema JA. The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol. 2005;23:3718–25. https://doi.org/10.1200/jco.2005.02.176.
Ervin, R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report 2009, 1–7.
Liu D, Ma Z, Yang J, Zhao M, Ao H, Zheng X, Wen Q, Yang Y, You J, Qiao S, et al. Prevalence and prognosis significance of cardiovascular disease in cancer patients: a population-based study. Aging (Albany NY). 2019;11:7948–60. https://doi.org/10.18632/aging.102301.
Hollman G, Kristenson M. The prevalence of the metabolic syndrome and its risk factors in a middle-aged Swedish population–mainly a function of overweight? Eur J Cardiovasc Nurs. 2008;7:21–6. https://doi.org/10.1016/j.ejcnurse.2007.05.003.
Hillier, T.A.; Fagot-Campagna, A.; Eschwège, E.; Vol, S.; Cailleau, M.; Balkau, B. Weight change and changes in the metabolic syndrome as the French population moves towards overweight: the D.E.S.I.R. cohort. Int J Epidemiol 2006;35:190–196. https://doi.org/10.1093/ije/dyi281.
Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207:928–34. https://doi.org/10.1016/j.jamcollsurg.2008.08.022.
Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–1638. https://doi.org/10.1056/NEJMoa021423.
Herman DR, Ganz PA, Petersen L, Greendale GA. Obesity and cardiovascular risk factors in younger breast cancer survivors: The Cancer and Menopause Study (CAMS). Breast Cancer Res Treat. 2005;93:13–23. https://doi.org/10.1007/s10549-005-2418-9.
Kidoguchi S, Sugano N, Tokudome G, Yokoo T, Yano Y, Hatake K, Nishiyama A. New Concept of Onco-Hypertension and Future Perspectives. Hypertension. 2021;77:16–27. https://doi.org/10.1161/hypertensionaha.120.16044.
Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12. https://doi.org/10.1007/s11906-018-0812-z.
Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 2003;107:3109–16. https://doi.org/10.1161/01.Cir.0000075572.40158.77.
Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–73. https://doi.org/10.1161/circulationaha.104.516948.
Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066–76. https://doi.org/10.1001/archinte.164.10.1066.
Gathirua-Mwangi WG, Song Y, Monahan PO, Champion VL, Zollinger TW. Associations of metabolic syndrome and C-reactive protein with mortality from total cancer, obesity-linked cancers and breast cancer among women in NHANES III. Int J Cancer. 2018;143:535–42. https://doi.org/10.1002/ijc.31344.
Ascaso JF, Romero P, Real JT, Lorente RI, Martínez-Valls J, Carmena R. Abdominal obesity, insulin resistance, and metabolic syndrome in a southern European population. Eur J Intern Med. 2003;14:101–6. https://doi.org/10.1016/s0953-6205(03)00022-0.
Rantala AO, Kauma H, Lilja M, Savolainen MJ, Reunanen A, Kesäniemi YA. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J Intern Med. 1999;245:163–74. https://doi.org/10.1046/j.1365-2796.1999.00429.x.
Kang C, LeRoith D, Gallagher EJ. Diabetes, Obesity, and Breast Cancer. Endocrinology. 2018;159:3801–12. https://doi.org/10.1210/en.2018-00574.
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. https://doi.org/10.1001/jama.295.13.1549.
Scuteri A, Najjar SS, Morrell CH, Lakatta EG. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care. 2005;28:882–7. https://doi.org/10.2337/diacare.28.4.882.
Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9. https://doi.org/10.2337/diacare.24.4.683.
Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–6. https://doi.org/10.1161/01.Cir.0000108926.04022.0c.
Lee, J.; Kwak, S.Y.; Park, D.; Kim, G.E.; Park, C.Y.; Shin, M.J. Prolonged or Transition to Metabolically Unhealthy Status, Regardless of Obesity Status, Is Associated with Higher Risk of Cardiovascular Disease Incidence and Mortality in Koreans. Nutrients 2022;14. https://doi.org/10.3390/nu14081644.
Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML, Stephens PA. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91:1621–34. https://doi.org/10.1210/jc.2005-2227.
Boguszewski MCS, Cardoso-Demartini AA, Boguszewski CL, Chemaitilly W, Higham CE, Johannsson G, Yuen KCJ. Safety of growth hormone (GH) treatment in GH deficient children and adults treated for cancer and non-malignant intracranial tumors-a review of research and clinical practice. Pituitary. 2021;24:810–27. https://doi.org/10.1007/s11102-021-01173-0.
Kopchick JJ, Basu R, Berryman DE, Jorgensen JOL, Johannsson G, Puri V. Covert actions of growth hormone: fibrosis, cardiovascular diseases and cancer. Nat Rev Endocrinol. 2022;18:558–73. https://doi.org/10.1038/s41574-022-00702-6.
Janssen, J. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int J Mol Sci 2021;22. https://doi.org/10.3390/ijms22157797.
Langiu, M.; Palacios-Acedo, A.L.; Crescence, L.; Mege, D.; Dubois, C.; Panicot-Dubois, L. Neutrophils, Cancer and Thrombosis: The New Bermuda Triangle in Cancer Research. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms23031257.
Campello E, Ilich A, Simioni P, Key NS. The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues. Br J Cancer. 2019;121:359–71. https://doi.org/10.1038/s41416-019-0510-x.
Meinardi MT, Gietema JA, van Veldhuisen DJ, van der Graaf WT, de Vries EG, Sleijfer DT. Long-term chemotherapy-related cardiovascular morbidity. Cancer Treat Rev. 2000;26:429–47. https://doi.org/10.1053/ctrv.2000.0175.
Abdel-Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV, Fung K, Anderson GM. The Risk of Heart Failure and Other Cardiovascular Hospitalizations After Early Stage Breast Cancer: A Matched Cohort Study. J Natl Cancer Inst. 2019;111:854–62. https://doi.org/10.1093/jnci/djy218.
Akinyemiju T, Moore JX, Judd S, Lakoski S, Goodman M, Safford MM, Pisu M. Metabolic dysregulation and cancer mortality in a national cohort of blacks and whites. BMC Cancer. 2017;17:856. https://doi.org/10.1186/s12885-017-3807-2.
Acknowledgements
We thank all the efforts made by the healthcare workers in NCHS and CDC for the NHANES database. We are also grateful to HOME-for-Researchers (www.example.com) for its assistance in writing and revising our manuscripts.
Funding
This work was supported by grants 2020ZX09201025 from Internationally Standardized Tumor Immunotherapy and Key Technology Platform Construction for Clinical Trials of Drug-Induced Heart Injury, 201805004 from Jinan Medical Science and Technology Innovation Project, and ZR2021MH019 from Natural Science Foundation of Shandong Province.
Author information
Authors and Affiliations
Contributions
AB.L. and Y.Z. (Yu Zhang) did statistical analyses and draft the manuscript. P.T., TT.M., JL.C. and D.Z. were responsible for data collection, methodology, and curation. Y.Z. (Yan Zheng) and GH.S. concepted, designed, and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The investigation was approved by the NCHS research ethics review board (https://www.cdc.gov/nchs/nhanes/irba98.htm).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, AB., Zhang, Y., Tian, P. et al. Metabolic syndrome and cardiovascular disease among adult cancer patients: results from NHANES 2007–2018. BMC Public Health 24, 2259 (2024). https://doi.org/10.1186/s12889-024-19659-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19659-4