Abstract
The novel coronavirus disease 2019 (COVID-19) is the latest evidence of an epidemic disease resulting in an extraordinary number of infections and claimed several lives, along with extensive economic and social consequences. In response to the emergency situation, countries introduced different policies to address the situation, with different levels of efficacy. This paper outlines the protocol for developing a model to analyze the burden of COVID-19 in Iran and the effect of policies on the incidence and cumulative death of the disease. The importance of the model lies in the fact that no study, according to the authors’ best knowledge, tried to quantify the impact of the disease on Iran society and the impact of various implemented interventions on disease control. Based on a systematic review of COVID-19 prediction models and expert interviews, we developed a system dynamics model that not only includes an epidemic part but also considers the impact of various policies implemented by the Ministry of Health. The epidemic model estimates the incidence and mortality of COVID-19 in Iran. The model also intends to evaluate the effect of implemented policies on these outcomes. The model reflects the continuum of COVID-19 infection and care in Iran (of which some of its elements are unique) and key activities and decisions in delivering care. The model is calibrated and validated using data published by the Ministry of Health of Iran. Finally, the study aims to provide evidence of the impact of interventions intended to curb COVID-19 in Iran. Insights provided by the model will be necessary for controlling either future waves of the disease or similar future pandemics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The novel coronavirus disease 2019 (COVID-19) is the latest evidence of an epidemic disease resulting in an extraordinary number of infections and claimed several lives, along with extensive economic and social consequences. In addition, it caused different long-term health consequences and a significantly higher burden on health systems. In response, various countries enacted different clinical and non-clinical procedures to curb the spread of disease (e.g., total lockdown and societal health). While such measures are necessary to control the disease, there should be an appropriate balance between them as, along with health benefits, they negatively impact the economy and society. This is only possible when policymakers have access to appropriate information in an in-time manner [1]. On the other hand, the fact that COVID-19 is highly contagious and its evolving nature are among the factors that indicate the pandemic will continue in the future [2]. Furthermore, healthcare policymakers should comprehensively understand the disease, including its transmission pattern, to develop appropriate strategies while having an eye on its future dynamics [3].
In this line, several models are introduced to quantify the effect of COVID-19 on various health outcomes, as well as to determine the risk of future health outcomes [4, 5]. In addition, some models are intended to evaluate the effect of various non-clinical interventions on COVID-19 spread [6,7,8]. The literature includes a wide range of models, including statistical models [9], network-based models [10], simulation models [11], etc. In recent years, simulation-based models have been used as effective solutions, mainly because in rapidly evolving pandemics such as COVID-19, the higher the complexity of statistical and network-based approaches, the lower their applicability, while the need for more data increases [12]. The outputs of such models have been crucial for decision-makers to make evidence-informed decisions intended to curb disease expansion and provide more effective, risk-based treatments based on disease severity [13, 14]. Nevertheless, among various developed models, only a few are of high quality and context-specific, as mentioned by a systematic review [15]. Furthermore, more information about their actual performance should be available. Meanwhile, each country has implemented some specific measures to address the situation, which often are not considered in currently available models, leading to their underperformance.
Therefore, following a susceptible–exposed–infected–recovered epidemic (SEIR) model, this study intended to develop a simulation-based model to comprehend the dynamics of COVID-19 in Iran. In other words, the study investigated the effect of epidemiological, social, economic, and health sector-related variables to estimate the effects of COVID-19 (i.e., infections and mortality) using various scenarios in Iran. The system dynamics (SD) and design of experiment (DOE) approaches are applied to determine the optimal state of variables and their relations in order to match the model with empirical data, leading to a better evaluation of the situation and to forecast the disease’s future dynamics using various scenarios. The model contains dynamics of societal interactions and the effects of various governmental interventions on susceptibility and infectiousness across persons. Hence, the outputs of the model can be used to both investigate and forecast the dynamics of the diseases and to evaluate the effect of various interventions on the defined outcomes. In other words, one of the objectives of this model is to ascertain how governmental interventions changed the course of the pandemic (i.e., number of deaths, infections, hospitalization, etc.). The research team tried to recreate the COVID-19 pandemic as precisely as possible; hence the study has some prominent aspects that are disregarded in previous research. For instance, the model is based on an enhanced SEIR model to realistically simulate the pandemic’s spread and considers susceptible, isolated, infected, and vaccinated cases. In addition, interventions such as lockdowns, social contacts, public awareness, latent time, recovery time, and mortality are included in the model. Noteworthy, the DOE approach is used to develop the model, and integration of this approach is an efficient technique to calibrate the model based on scenarios, which is not widely used in previous studies.
According to the authors’ best knowledge, this is the first study that developed a model based on the Iran context. As a result, it can be argued that the model both tried to address the limitations of other models and to provide a model based on Iran’s context. The model is described in the following, which is structured into five main sections: (a) research background and objectives; (b) literature review, by emphasizing knowledge gaps; (c) methodology; and (d) final model.
Literature review
Since the emergence of COVID-19, several models have been introduced to either predict disease dynamics or estimate its effects, some of which are simulated for Iran. These models used different approaches, including extrapolation, back-calculation, system dynamics, etc. For instance, ShreylL et al. (2020) used an agent-based approach to simulate the COVID-19 pandemic in Australia. They used the ACEMoD model that was previously developed by another researcher. Based on discrete time and stochastic agent-based methods, the research team developed several scenarios [16]. Scott et al. (2020) introduced a model to quantify the effect of governmental interventions on COVID-19 spread in countries with low infection rates. They also used an agent-based approach to simulate the risk of disease transmission networks in families, schools, workplaces, and a wide range of public areas [17]. Richard Stutt O.J et al. (2020) presented a modeling framework to evaluate the effectiveness of using masks and quarantine in the management of the COVID-19 pandemic using a branching process as well as susceptible, infected, and removed model [18]. Hwan Oh et al. (2020) investigated years of lost life due to COVID-19 in countries with high infection rates. They developed a system dynamics model to achieve this goal in 30 countries [19]. Zia et al. (2020) developed a SEIR model to simulate the COVID-19 spread in Oman using epidemiological data [20]. He et al. investigated the effect of quarantine and hospitalization on COVID-19 dynamics using official COVID-19 data from Hubei province (China). They used a particle swarm optimization algorithm to estimate infection and hospitalization rates [21]. Ghaffarzadegan and Rahmandad also developed a simulation-based model to estimate the early spread of COVID-19 in Iran in order to compare actual and confirmed cases of the disease. They combined different sources of data to estimate the magnitude of the outbreak. The findings showed that COVID-19-related deaths are likely 7.1 times more than official counts [22]. Also, Annas et al. (2020) developed a model with vaccination and isolation factors and mentioned the important contribution of vaccination in improving healing and the major contribution of maximum isolation in curbing disease spread [23]. Moreover, Kazempour Dizaji et al. (2022) developed a simulation model for the COVID-19 disease epidemic in Iran using a SIR model using various scenarios. They reported that increased social restrictions and higher health measures translated into a declined reproductive rate. The findings also showed that medications and vaccines had an important role in improving the recovery rate [24]. In the same vein, Chen et al. (2023) investigated the relationship between the COVID-19 spread and governmental interventions using a SEIR model. To estimate the transmission rate, they divided the data into eight different periods with corresponding SEUIR models for each period [25]. Similarly, Liu et al. investigated the effectiveness of measures performed to curb the COVID-19 pandemic (e.g., social distancing and social gatherings) using area-based exposure to infections during travel and quarantine. The model is focused on disease transmission during travel [26].
As mentioned above, a wide range of variables are investigated in the proposed models, as evidenced by the literature review. While variables such as social awareness, public warning, and vaccination are of crucial importance to understanding the dynamics of COVID-19 pandemic, most previous studies have focused on epidemiological variables (e.g., infection period, isolation time, recovery time). Hence, this study attempts to include variables other than epidemiological factors to provide a throughout understanding of the disease dynamics in Iran.
Model description
First, a steering committee (SC) was established, whose responsibility was to ensure that the final model would be a realistic representation of the COVID-19 pandemic and its continuum of care in Iran (i.e. screening to identify newly infected individuals, linkage of newly diagnosed to care, clinical assessment, monitoring of clinical signs, adverse events and drug resistance, and long-term retention of patients in treatment, appropriate data are used for model parameters and calibration, and comprehensive and well-fitted scenarios are developed). The SC is composed of seven well-known experts in public health, health policy, statistics, modeling, and epidemiology.
Data gathering
Based on the compiled model, six groups of information were needed to complete and implement the model:
-
a.
Epidemiological information such as the prevalence of Covid-19 among different groups, the possible proportion of diagnosed people and people who are not aware of their disease; These data were used from the statistics published daily by the Ministry of Health and Medical Education of Iran.
-
b.
Behavioral information: the number of contacts, the number of contacts with infected people, the amount of mask use, the amount of social distancing, the amount of tendency to receive the vaccine.
-
c.
Information about the disease: the probability of transmission per contact with an infected person through breathing, the probability of transmission per contact with an infected person through contact with contaminated surfaces, the probability of disease transmission when using a mask, the rate of disease transmission when receiving a vaccine.
-
d.
Programs and policies related to the control of Covid-19 in Iran and their coverage.
-
e.
Demographic information: population growth rate and the share of different age groups from the total population.
Model development
Since the main question of the current study was ‘actual trend of the COVID-19 pandemic (i.e., incidence and mortality) in Iran’, the first step was to develop a model for the projection of the disease dynamics. Hence, the first step of the study was a systematic review of all published studies on modeling COVID-19, as well as its dynamics in Iran and other middle-income countries. Besides, it was necessary to study unpublished literature. Therefore, a systematic review was performed from the onset of the disease to November 2022. For this purpose, PubMed, EMBASE, Web of Science, Google Scholar, and local databases (i.e., Iranmedex, Magiran, SID, and Irandoc) were searched using the following keywords: prediction models, system dynamics, COVID-19, Coronavirus, SARS-CoV, SARS-CoV-2, healthcare, healthcare system, survival model, medical care. Various combinations of the search terms were used to retrieve related articles from the databases.
The review aimed to identify COVID-19’s dynamics (e.g. high-risk groups, risky behaviors), as well as programs, policies, interventions, organizations, and institutions engaged in policymaking and/or provision of COVID-19-related services in Iran.
The selection criteria referred to COVID-19 transmission, prevention, treatment, the continuum of care, high-risk behaviors, high-risk groups, policymaking or policy analysis, optimization of resources, both globally and in Iran, developing a novel model to predict the incidence or prevalence of COVID-19, reviewing/updating previously developed models, and investigating pros and cons of the current models. In total, 616 studies were deemed to be relevant for full-text review. Then, documents were reviewed to assess their relevance to the objectives. After reviewing titles and abstracts 126 articles were removed due to various reasons such as duplication or being irrelevant. Then, 490 documents were screened. We also searched the references of all eligible studies to find further evidence and asked the experts whether an important study was omitted or not. That resulted in 15 more studies (3 were suggested by experts, and 12 were extracted through searching references). Finally, 137 studies were eligible to be included (Fig. 1).
After reviewing the literature on COVID-19, the next step was to obtain experts’ opinions on COVID-19 dynamics. Then, we interviewed key informants, epidemiologists, researchers, providers engaged in COVID-19 care, public health experts, and system dynamics experts. To select the key informants and experts the following criteria were considered: having experience in modeling infectious diseases; being familiarized with the methodology of the system dynamics; and being engaged in policy-making. Interviewees were selected using the purposive sampling technique. Using a semi-structured approach, interviews intended to explore the dynamics of COVID-19 in the country, including current and future high-risk groups, risky behaviors, the future trend of the disease, and main considerations for modeling the disease.
In total, 15 interviews were conducted. All interviews were transcribed and then analyzed. The mean duration of interviews was about 72 min. The analysis was conducted by a member of the research team. Therefore, a qualitative model of the COVID-19 in Iran was developed. The model was used as a guide to construct various parts of the final model. To better document interviews and facilitate discussions with system experts, the Unified Modelling Language, or UML was used to develop diagrams of system workflows.
Apart from extracting the disease dynamics, the findings of the review and interviews were categorized into two groups: (a) COVID-19-related policies and programs; and (b) COVID-19 dynamics in Iran, which are described in the following.
(A) COVID-19-related policies and program
Table 1 includes interventions and policies that are intended to control COVID-19 spread in Iran since the onset of the Pandemic.
(B) Epidemiological model
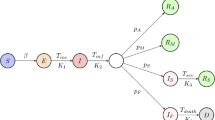
The model is developed following the SD approach, which is widely used to comprehend the nonlinear behavior of complicated systems. SD mostly relies on stocks (represented by a box), flows (symbolized by valves with arrows), auxiliary, and delay components to provide a realistic estimation of the actual situation. To better understand the pandemic dynamics, a deterministic stage-structured SEIR (Susceptible-Exposed-Infected-Removed) model is used in this study; that is, initially, the population is divided into six groups of susceptible (S), exposed (E), infected (I), Hospitalized (H), Isolated (IS), and removed (R). Then, the groups are further divided based on other variables. Also, based on the known information about SARS-CoV-2 transmission, infected states can be either asymptomatic or symptomatic during the simulation period. Each node represents a stock variable containing the number of individuals in the population depending on the state of infection.
The flow rates among the states depend on the interaction of variables and latency under the Iran condition. Noteworthy, it is assumed that each compartment is homogeneous regarding population characteristics. Furthermore, it is assumed that birth and death rates are equal (i.e., no population growth during the simulation period). Also, those in either isolation or hospital compartments are assumed to have zero infection rates. A high-level stock-flow schematic of the model is provided in Fig. 2.
The proposed pandemic model is described in the following. Susceptible might acquire the infection at a given rate in cases where they contact infected patients; then, they enter ‘exposed’. Afterward, they may present symptoms or not (known as symptomatic and asymptomatic). Symptomatic cases enter the compartment of ‘infected’. They may either be isolated, hospitalized, stay at home, or follow their usual behavior. Those in the hospital may require intensive care. The last compartment is ‘removed’ or ‘dead’.
The migration of those in the susceptible compartment to exposure depends on the infection rate (IR), which, as presented in Eq. (1), relies on the infection rate (IRi), contact rate (λ), susceptibility proportion (Sp), and social awareness impact (α).
Noteworthy, the initial infection rate only includes the reproduction number (R0). The infection rate was calculated using Eq. 2.
R0 is the number of times the virus reproduces. IP stands for infection period and refers to an individual’s infectiousness duration. This study considered the R0 and IP as 3.9 and 6, respectively [27]. Noteworthy, as since the onset of the pandemic, the Iranian government has implemented several restrictive interventions, it is assumed that the effect of such interventions (i.e., lockdown (β)) should be considered. In other words, interventions such as lockdowns can significantly reduce contact rates, translating into reduced infection rates. However, there should be a variable to indicate the delay in impact (known as time in this study). Equation 3 intends to estimate the contact rate (λ). It should be noted that the exponential delay of the first order is used to capture its behavior.
The value of tim was estimated using the examination of data published by the Ministry of Health and Medical Education (MoHME), as well as obtaining expert opinions. Equation (4) is used to estimate the behavior of variable β. For this purpose, a similar study performed in the middle-east was used to estimate the behavior of β [27].
The other important variable is ‘proportion susceptibility’, which indicates all cases in the initial population except for the susceptible population. As in Iran, those living in rural areas (with low population density) have a significantly lower rate of infection, this variable was included to consider population density. Equation 5 was used to estimate the proportion susceptibility.
The variable of ‘social awareness’ intends to capture the effect of interventions implemented to control the disease spread, e.g., hand sanitization, social distancing, wearing masks, etc. (Eq. (6)).
Furthermore, it is assumed that personal hygiene and wearing a mask affect the infectivity and are modeled using the v(t) multiplier. Moreover, we defined a time-dependent adjustment factor y(t) for contact rate, Cij. There is a delay function (τ), ranging from one to 20 days, to consider the effect of social awareness interventions, and then the effect starts to decline. The value of τ was obtained from the literature review. The higher the social awareness, the lower the IR. According to evidence, most interventions on social awareness affect the behavior for 50 days, on average [2]. On the other hand, it often takes time to adopt a new behavior (dadopt).
Initially, a proportion of the population is susceptible (S) to the disease (Eq. 5); then, they get exposed to the virus by contacting infected individuals. After exposure, most cases are asymptomatic and become infective only after a latency period (dEA days). Then (after another dAI days), they start to present symptoms and move to the Infectious Symptomatic group. The sum of dEA and dAI represents the average incubation period (dP). The infection rate depends on the contact rate (λ) and the infectivity (p) (Eq. 2). The contact rate for each compartment is the sum of all interactions of all members with other compartments across all contact locations (i.e., home, work, school, and other locations). The study assumes that contacts only occur at home, workplace, school, and all other locations. We assumed that transmission occurs through contact with symptomatic or asymptomatic cases. Also, it is assumed that the relative infectivity of the asymptomatic cases is (1-k) % of the symptomatic cases. Most people in the symptomatic compartment are active in society and transmit the disease, and only a fraction require hospitalization. The rate of admitting patients for inpatient services has been calculated using the percentage of initial hospitalization (IHP) and the stress (σ) and by using an expert’s opinion. In this study, IHP is defined as 0.4; that is, 40 out of every 100 infected cases go to inpatient centers. Afterward, a fraction of hospitalized cases require intensive care, and the rest will recover. A fraction of ICU (Intensive Care Unit) patients recover and the rest die. In order to cover possible deterioration in hospital care due to excessive demand for hospital services and staff burnout, the overflows at hospitals are explicitly modeled. Moreover, based on the availability of beds, patients may either go to the hospital immediately or wait for a while to enter stock H. The same is true for ICU beds. All recovered cases lose their immunity to the disease and enter the susceptible compartment after a period (defined as ‘Waning time (WT)’). Noteworthy, it is assumed that hospitalized patients are in full isolation and do not transmit the disease. The burden on the healthcare system due to extra demand for either outpatient or inpatient services is considered using the variable of ‘stress’. So, the higher the capacity and preparedness of the health system, the lower the value of stress. Hence, when the stress level is zero, the HP is normal, and the higher the stress level, the higher the negative impact on the health system. This variable allows to capture fluctuations in the availability of care to patients. The model also includes the effect of immunization on IR, as well as the vaccine efficacy (ζ). Vaccine efficacy represents the impact of vaccines, and as time passes, the immunity reduces. Several studies show that vaccination could reduce infections, hospitalization, and fatalities [2]. Most Iranians have received the Sinopharm vaccine, so its efficacy is generalized to the whole population (Fig. 3).
The model includes both medical and non-pharmaceutical interventions. Medical interventions include both outpatient and inpatient services, which are included as Hospitalized (H) and Isolated (IS) compartments. Non-pharmaceutical interventions included in the model are quarantining and isolation, which have been widely performed in Iran. These interventions are explicitly captured by stocks A and I. The flow of asymptomatic from A to Q A represents the proportion of patients that are quarantined, which may be due to self-awareness (f1(t)) or contact tracing of positive tested patients (fX(t)). Patients flow from A to LA is defined as patients with a positive test result that are isolated. These patients either recover or develop more severe symptoms. The flow of symptomatic cases from I to QI represents the number of cases chosen to quarantine due to self-awareness (f3(t)). The flow of patients with explicit symptoms from I to LI represents the proportion of isolated cases following a positive result for the COVID-19 test (f4(t)). Noteworthy, all the flows are governed by explicit delays between stages. Other equations are provided in the Parameters section.
The Social Contact Matrix provided by Prem et al. (2017) was used in this study [28]. However, the model was calibrated by applying a correction multiplier of 1.7 to adjust the number of contacts for all locations. Moreover, two pathways are considered to either identify or confirm new patients via testing: (a) when the individual seeks medical help; and (b) via contact tracing and testing symptomatic individuals before presenting severe symptoms. The former is captured by the flow of Disease Progress Rate (DPR) from Infectious Symptomatic (I) to Hospitalized (H) compartments. The latter is captured by the flow of Isolating Symptomatic Rate (LIR) from Infectious Symptomatic (I) to Isolated Symptomatic (LI) compartments.
Feedback loops
Transmission loop
This is the initial loop for the infection that depends on the infection rate. It relies on various factors such as the current infection rate, behavioral transmission rate, susceptible population, and immunity.
Infection loop
It shows the transition from susceptible to exposed and then infected cases, which have a probability of transmitting the virus if not contained, quarantined, or monitored.
Social mobilization loop
It includes all measures intended to minimize the transmission rate (i.e., both pharmaceutical and non-pharmaceutical interventions). This loop covers government mandated practices such as lockdowns, community quarantine, and private institution societal adjustments.
Health system capacity loop
This loop is focused on the first-line measures of COVID-19 patients, including testing and quarantining. It is capped by the effectiveness decline and depreciation due to resource usage. The disease testing and diagnosis loop is provided in Fig. 4.
Simulation
The model presented in this study is developed using the commercial software Vensim ® (Vensim Systems, Inc., Harvard, MA, USA), which may be acquired for free from Ventana Systems, Inc. Vensim PLE is a simulation software that supports continuous simulation by providing a graphical modeling interface for stock-and-flow and causal loop diagrams. The model will be simulated using the Euler integration method with a time-step of 0.0625. concerning the study parameters, values of some parameters are extracted from the literature review (e.g., infection period, recovery time, etc.). Some others are obtained from the MoHME, trials performed in Iran, or online Iranian sources; for instance, hospitalization percentage and days to achieve social awareness. Noteworthy, all data are tailored to Iran’s context based on the expert’s opinions. Also, the results will be provided per day. All units are crosschecked to ensure the consistency of variables. Furthermore, direct structure tests are used to ensure consistency of relations and assumptions, as well as including all necessary variables. Eventually, the outputs of the model can be used to better understand the actual COVID-19 dynamics in Iran and develop appropriate strategies to better control the disease spread.
Parameters
Defined variables and their definition are provided in Table 2.
Noteworthy, the authors are aware that some of the abovementioned parameters are not available for Iran. Hence, data from some countries with similar contexts are generalized and are assumed to hold true for the country.
Data collection
As SARS-Cov-2 has different mutations, each with various characteristics (e.g., infectivity, incubation period, etc.), for each wave of the disease the epidemiological parameters of the dominant mutation were embedded in the model. Data on epidemiological characteristics were obtained from WHO and the Center for Disease Control of the US. Calibration of obtained rates with real data was performed through comparison with official statistics (i.e., confirmed diagnosed cases) and expert opinions. Then, demographic data were obtained from Iran Statistical Center. Data on therapeutic infrastructures were extracted from official reports of the Ministry of Health and Medical Education, as well as the Iran Statistical Center. Data on hospital capacity, including ICU beds, were also obtained from MoHME. Also, data on the extensiveness of various therapeutic measures to control the pandemic were obtained from MoHME, as well as official reports published by medical universities. Meanwhile, data on the infectiveness of various mutations of the SARS-Cov-2 were obtained from the WHO Coronavirus Disease (COVID-19) Dashboard. Meanwhile, the effect of various measures on disease spread was obtained through a literature review. Noteworthy, for all steps, national data were preferred over international data, except for cases data national data were not available; for instance, data on contact matrix, which currently there is no evidence for Iran, and the research team had no other option except to use data of countries with similar context. In such cases, the research team tried to adapt data based on Iran’s context through a steering committee comprised of various experts. Furthermore, the steering committee supervised the data collection process, and in the case of any discrepancy, a third referee (i.e., an expert with sufficient experience in the field) was referred to address the situation. For cases were more than one source of data was available (e.g., daily infection cases), data published by the national institutions were preferred.
Model validation and sensitivity
As SD is a behavior-focused technique, the sensitivity of behavior patterns (e.g., level and time) to changes in parameters should be examined to identify potential leverage points that can enhance the performance of the system. Data on daily incidence, daily mortality of COVID-19, number of recovered cases, and hospitalized cases were extracted from daily reports of the MoHME. The model is calibrated using the data reported by the MoHME of Iran. The research team performed about 140 simulations to tune the model. The validity of the feedback relationship was evaluated by developing a stock-and-flow diagram. Also, testing model structure, consistency, and utility were used to demonstrate the model’s validity. Simple mathematical equations were used in the simulation model to express the relationships between variables, while parameters indicate the weight of these relationships.
Limitations
Like other similar studies, the current study also had limitations. The first, and most important, is not dividing the population based on age groups, which was mainly because of inaccessibility to the required data in Iran. Secondly, the model only contains an epidemic part, and other important risk factors such as super-spreaders and anti-vaccine groups are considered as external variables. A comprehensive model should consider such variables as internal variables which depend on various factors.
Epilogue
The current model has two main implications for the Iranian healthcare system. Firstly, it can be used to predict the future incidence of the COVID-19 and other similar disease in Iran. Secondly, the impact of different interventions on the expansion of the diseases can be evaluated and interventions (both behavioral, diagnostic, and curative). Furthermore, the cost of necessary interventions to reduce the incidence also can be estimated.
Policy implication
The model provided in this article adds to the current body of knowledge regarding the effect of a combination range of policies (pharmaceutical and non-pharmaceutical), as well as behavioral aspects of social interactions, on the incidence and mortality of the COVID-19 pandemic in Iran.
Data availability
Data are available at the request of readers by the research team. For more details please refer to material and method (data gathering) in manuscript.
References
Milne GJ, Xie S, Poklepovich D, O’Halloran D, Yap M, Whyatt D. A modelling analysis of the effectiveness of second wave COVID-19 response strategies in Australia. Sci Rep. 2021;11(1):1–10.
Rehman AU, Mian SH, Usmani YS, Abidi MH, Mohammed MK, editors. Modeling consequences of COVID-19 and assessing its epidemiological parameters: a System Dynamics Approach. Healthcare: Multidisciplinary Digital Publishing Institute; 2023.
Daoust J-F. Elderly people and responses to COVID-19 in 27 countries. PLoS ONE. 2020;15(7):e0235590.
Shreffler J, Petrey J, Huecker M. The impact of COVID-19 on healthcare worker wellness: a scoping review. Western J Emerg Med. 2020;21(5):1059.
Hartnett KP, Kite-Powell A, DeVies J, Coletta MA, Boehmer TK, Adjemian J, et al. Impact of the COVID-19 pandemic on emergency department visits—United States, January 1, 2019–May 30, 2020. Morb Mortal Wkly Rep. 2020;69(23):699.
Mandal M, Jana S, Nandi SK, Khatua A, Adak S, Kar T, Chaos. Solitons Fractals. 2020;136:109889.
Hu H, Kennedy CM, Kevrekidis PG, Zhang H-K. A modified PINN Approach for Identifiable Compartmental models in Epidemiology with Application to COVID-19. Viruses. 2022;14(11):2464.
Alshammari FS, Akyildiz FT, Khan MA, Din A, Sunthrayuth P. A stochastic Mathematical Model for understanding the COVID-19 infection using Real Data. Symmetry. 2022;14(12):2521.
Ahmed I, Modu GU, Yusuf A, Kumam P, Yusuf I. A mathematical model of Coronavirus Disease (COVID-19) containing asymptomatic and symptomatic classes. Results Phys. 2021;21:103776.
Cui Y, Ni S, Shen S. A network-based model to explore the role of testing in the epidemiological control of the COVID-19 pandemic. BMC Infect Dis. 2021;21:1–12.
Li Y, Liu C, Zhao S, Wang Z, Zhao H, Chu S, et al. editors. Simulation Prediction and Control Strategy of COVID-19 Dynamic Contact Network in USA and Various States–Based on Effective Regeneration Number and Improved Discrete SEIQDHR Model. Journal of Physics: Conference Series; 2021: IOP Publishing.
Currie CS, Fowler JW, Kotiadis K, Monks T, Onggo BS, Robertson DA, et al. How simulation modelling can help reduce the impact of COVID-19. J Simul. 2020;14(2):83–97.
Silva PC, Batista PV, Lima HS, Alves MA, Guimarães FG, Silva RC. COVID-ABS: an agent-based model of COVID-19 epidemic to simulate health and economic effects of social distancing interventions. Chaos Solitons Fractals. 2020;139:110088.
Aldekhyl SS, Arabi YM. Simulation role in preparing for COVID-19. Annals Thorac Med. 2020;15(3):134.
Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E et al. Prediction models for diagnosis and prognosis of COVID-19: systematic review and critical appraisal. BMJ. 2020;369.
Chang SL, Harding N, Zachreson C, Cliff OM, Prokopenko M. Modelling transmission and control of the COVID-19 pandemic in Australia. Nat Commun. 2020;11(1):5710.
Scott N, Palmer A, Delport D, Abeysuriya R, Stuart R, Kerr CC, et al. Modelling the impact of reducing control measures on the COVID-19 pandemic in a low transmission setting. Med J Aust. 2020;214(2):79–83.
Stutt RO, Retkute R, Bradley M, Gilligan CA, Colvin J. A modelling framework to assess the likely effectiveness of facemasks in combination with ‘lock-down’in managing the COVID-19 pandemic. Proceedings of the Royal Society A. 2020;476(2238):20200376.
Oh I-H, Ock M, Jang SY, Go D-S, Kim Y-E, Jung Y-S et al. Years of life lost attributable to COVID-19 in high-incidence countries. J Korean Med Sci. 2020;35(32).
Zia K, Farooq U. COVID-19 outbreak in Oman: model-driven impact analysis and challenges. medRxiv. 2020:2020.04. 02.20050666.
He S, Peng Y, Sun K. SEIR modeling of the COVID-19 and its dynamics. Nonlinear Dyn. 2020;101:1667–80.
Ghaffarzadegan N, Rahmandad H. Simulation-based estimation of the early spread of COVID‐19 in Iran: actual versus confirmed cases. Syst Dynamics Rev. 2020;36(1):101–29.
Annas S, Pratama MI, Rifandi M, Sanusi W, Side S. Stability analysis and numerical simulation of SEIR model for pandemic COVID-19 spread in Indonesia. Chaos Solitons Fractals. 2020;139:110072.
Kazempour Dizaji M, Varahram M, Roozbahani R, Abedini A, Zare A, Kiani A, et al. Simulation of COVID-19 disease epidemic in Iran based on SIR model. Health Sci Monit. 2022;1(1):1–9.
Chen Z, Kong G. Hospital admission, facility-based isolation, and social distancing: an SEIR model with constrained medical resources. Prod Oper Manage. 2023;32(5):1397–1414. https://doi.org/10.1111/poms.13702.
Liu Z, Wang XC, Dai J, Li X, Li R. Impacts of COVID-19 pandemic on travel behavior in large cities of China: investigation on the lockdown and reopening phases. J Transp Eng, Part A: Syst. 2022;148(2):05021011.
Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W. Incubation period of COVID-19 caused by unique SARS-CoV-2 strains: a systematic review and meta-analysis. JAMA Netw open. 2022;5(8):e2228008–e.
Prem K, Cook AR, Jit M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput Biol. 2017;13(9):e1005697.
Acknowledgements
The authors would like to thank all those who helped in the development of this article.
Funding
The authors did not receive any financial support to perform this study.
Author information
Authors and Affiliations
Contributions
SG and PM conceived the study. SG conducted data collection and analysis. MM-A and SMH-SH helped with data interpretation and model development. ET and MS conceived the methodology and contributed in model simulation. All authors read and contributed to intellectual development of article.
Corresponding author
Ethics declarations
Ethics approval
The research purpose and methodology, including experimental protocols, were approved by the Internal Research Ethics Committee of the Baqiatallah University of Medical Sciences. Moreover, written informed consent was obtained from all participants before entering the study and after a comprehensive introduction to the study.
Consent for publication
The study did not use any data on single individuals and only macro data are used.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghazanfari, S., Meskarpour-Amiri, M., Hosseini-Shokouh, SM. et al. Designing a model to estimate the burden of COVID-19 in Iran. BMC Public Health 24, 2609 (2024). https://doi.org/10.1186/s12889-024-19920-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19920-w