Abstract
Objective
To evaluate the effects of replacing time spent in sedentary behavior (SB) with moderate to vigorous physical activity on sleep quality in young adults.
Methods
Multicenter cross-sectional study, carried out with students enrolled in undergraduate courses at universities in Brazil. Sleep quality was assessed using a question of the World Health Organization Quality of Life (WHOQOL-brief) and classified as good or poor sleep quality. SB was evaluated by self-reported total sitting time, and the level of leisure-time PA was classified according to the intensity of moderate-intensity physical activity (MPA) and vigorous-intensity physical activity (VPA), which were assessed using a self-reported questionnaire. An isotemporal replacement logistic model was used to evaluate the effects of different SB, MPA, and VPA sessions on sleep quality.
Results
A total of 8,059 study participants were evaluated, the majority had poor sleep quality (64.79%), were physically inactive (48.28%, defined as practicing < 150 min of MPA or < 75 min of VPA per week), and spent ≥ 9 h/day in SB (55.08%). The multivariate model showed an association between non-adherence to wake-based movement guidelines and poor sleep quality, where those with one altered behavior were 43% more likely to have poor sleep quality (OR:1.43;95%CI:1.27 to 1.60), while individuals with two altered behaviors were 97% more likely (OR:1.97;95%CI:1.73 to 2.24). In the isotemporal analysis, replacing MPA and VPA with equivalent time in SB increased the odds of poor sleep at all times assessed, with peaks of 56% for MPA and 68% for VPA.
Conclusion
The results of the present study indicate that replacing SB with the same amount of MPA or VPA may reduce poor sleep quality.
Similar content being viewed by others
Introduction
Sleep quality is a fundamental pillar for health and well-being, especially among young adults, a population increasingly immersed in sedentary behaviors (SB) and challenges maintaining adequate levels of physical activity (PA). Previous studies have consistently associated SB and insufficient PA with a variety of negative health outcomes, including poor sleep quality [1, 2]. This association between SB and PA with sleep quality is complex and multifaceted. One of the central hypotheses is that SB, especially when it involves exposure to LED-lit screens at night, can suppress the production of melatonin, a hormone crucial for sleep regulation [3]. This suppression can lead to an increase in psychophysiological alertness, thus interfering with the body’s natural circadian rhythms and impairing both the induction and maintenance of sleep [4]. Furthermore, regular PA acts as a synchronizer of these biological cycles, enhancing the robustness of the circadian system and promoting the internal alignment of various rhythms [5].
University students exhibit highly sedentary behavior [6], which has been further exacerbated by the pandemic [7]. The shift to remote education during the COVID-19 pandemic, characterized by prolonged screen time and reduced physical activity, further exacerbated these issues. The nature of distance learning often required extended periods of sitting in front of electronic devices, leading to a more sedentary lifestyle. In addition, restrictions on movement and the closure of public spaces such as gyms and parks during lockdown periods intensified the reduction in PA among young people. Consequently, this transition contributed to poorer sleep quality, highlighting the significant challenges that the pandemic brought to the health and well-being of students [8, 9].
However, the complex interaction between these behaviors and sleep quality has yet to be fully elucidated, particularly about the isotemporal substitution of SB for PA [10]. Recent research has applied isotemporal substitution models to evaluate the hypothetical effects of reallocating time spent on one activity to another, highlighting the importance of considering the balance of behaviors over 24 h [11]. These studies suggest that the interrelationship between these behaviors is a critical factor that interacts to influence mental and physical health.
Despite the growing body of evidence, there remains a gap in knowledge about how the isotemporal substitution of SB for PA affects sleep quality in young adults. Previous studies have focused on specific populations, such as the elderly and adults, but there is a need to explore this association in young adults, a group that is at a critical stage of development and establishment of healthy life patterns [12]. Therefore, this study, conducted during the COVID-19 pandemic, aimed to investigate the association between SB, PA, and sleep quality in young adults. Using isotemporal analysis, we examined the effects of replacing the time devoted to SB with moderate to vigorous PA on sleep quality.
Methods
Design and data collection
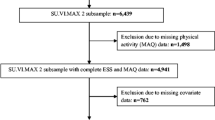
This research presents a cross-sectional study conducted in Brazil, assessing data collected from the survey titled “Symptoms of Anxiety and Depression Disorders in University Students in Minas Gerais: Multicenter Study” (PADu study). The study involved undergraduate students from eight Brazilian Federal Universities of Higher Education, including Universidade Federal de Ouro Preto (UFOP), Universidade Federal de Minas Gerais (UFMG), Universidade Federal de Juiz de Fora (UFJF), Universidade Federal de São João del-Rei (UFSJ), Universidade Federal de Lavras (UFLA), Universidade Federal de Alfenas (UNIFAL-MG), Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM), and Universidade Federal de Uberlândia (UFU).
Students who were over 18 years old and enrolled in undergraduate courses at these institutions were invited to participate in completing a self-administered online questionnaire through Google Forms. As the study was carried out online, participants did not have the option of choosing a printed version of the questionnaire; instead, they could access it via mobile devices such as smartphones, tablets or computers. The survey contained questions about their sociodemographic profile and health conditions. Data collection took place during the COVID-19 pandemic, from October 2021 to February 2022. The inclusion criteria were students aged 18 or over, of both sexes. Exclusion criteria included participants who didn’t complete the entire questionnaire and postgraduate students. For further details about the project’s methodology, refer to the literature [13].
Outcome variable: sleep quality
Sleep quality was assessed through self-reported subjective sleep quality using item 16 of the WHOQOL-bref questionnaire [14], which asks: ‘How satisfied are you with your sleep?’ and has been validated for use in Brazil [15]. The questionnaire was applied exclusively online, with participants completing it using mobile devices, tablets or computers. This item is part of a facet addressing the influence of sleep and rest, as well as related problems, on the individual’s quality of life. It focuses on whether or not sleep is disturbed, regardless of the cause, whether it is related to the individual or the environment. The entire scale was completed, but for this study, only this specific question will be utilized.
When analyzing the data, we adopted a dichotomous approach to classifying sleep quality. The answers included “Very dissatisfied”, “Dissatisfied”, “Neither satisfied nor dissatisfied”, “Satisfied” and “Very satisfied”. When analyzing the data, we adopted a dichotomous approach to classifying sleep quality. The answers ‘Satisfied’ and ‘Very satisfied’ were classified as ‘good sleep’, while ‘Very dissatisfied’, ‘Dissatisfied’, and ‘Neither satisfied nor dissatisfied’ (neutral) were classified as poor sleep’ [16]. We included the “neutral” response in the “poor sleep” category, based on evidence from the literature which suggests that a neutral assessment can hide mild but clinically relevant dissatisfaction. This methodological decision aims for a more conservative and rigorous interpretation of the data, in line with studies that demonstrate the importance of an accurate assessment of sleep quality and its health implications [17, 18].
Physical activity in leisure time
PA during leisure time was evaluated based on the VIGITEL questionnaire, a surveillance system of risk and protective factors for chronic diseases conducted annually by the Brazilian Ministry of Health through telephone surveys. This questionnaire has been validated and shown to be reliable, with substantial agreement for active (k = 0.70) and inactive (k = 0.64) individuals during leisure time and moderate agreement for TV viewing (k = 0.56). In comparison to the Global Physical Activity Questionnaire (GPAQ), a reference method for measuring PA, VIGITEL also demonstrated moderate to high sensitivity and specificity across different domains [19].
Initially, respondents were asked whether they were currently engaged in any type of PA. Those who responded affirmatively were then asked to specify the types of activities they practiced, with the option to select more than one modality. For each activity, they reported the frequency of practice and the duration in minutes.
The intensity of PA was assessed according to the Compendium of Physical Activity Codes and MET Intensities. Activities were classified as moderate physical activity (MPA) or vigorous physical activity (VPA) based on their MET values: moderate activities range from 3 to 5.9 METs, and vigorous activities are greater or equal to 6 METs. For example, running (Code: 12020, 7.5 METs), treadmill running (Code: 12020, 7.5 METs), aerobics (Code: 02000, 7.3 METs), soccer/futsal (Code: 15610, 7.0 METs), basketball (Code: 15055, 7.5 METs), bicycling (Code: 01014, 7.0 METs), wrestling (Code: 02061, 5.0 METs) and tennis (Code: 15675, 6.8 METs) are considered VPA. MPA includes walking (Code: 17160, 3.5 METs), treadmill walking (Code: 17358, 4.8 METs), weight training (Code: 02061, 5.0 METs), water aerobics (Code: 02120, 5.3 METs), general gymnastics (Code: 15300, 3.8 METs), swimming (Code: 18240, 5.8 METs), martial arts (Code: 15425, 5.3 METs), volleyball/foot volleyball (Code: 15710, 4.0 METs), and dancing (Code: 03010, 5.0 METs) [20].
Subsequently, PA was classified according to the guidelines established by the World Health Organization (WHO). Individuals were considered physically inactive if they either did not engage in any PA or did not meet the recommended thresholds of less than 150 min per week of MPA or less than 75 min per week of VPA. Those who met or exceeded these recommendations were classified as active [21].
Sedentary behavior (SB)
SB was measured by the total time spent sitting during weekdays and weekends. The measurement was carried out using the following question: ‘Currently, how much time on average do you spend sitting per day? (Include time used for cell phone, TV, computer, tablet, books, car and bus)’. Based on the answers, a weighted average of the time in SB was calculated using the formula.
Weighted average SB = [(Total sitting timing during the week × 5) + (Total sitting timing during the weekend × 2)] / 7.
Total sitting time was then classified considered a cut-off point of ≥ 9 h, based on a meta-regression analysis of more than 1 million individuals, suggesting that Individuals with a total sitting time per day greater than or equal to 9 h have a higher risk of all-cause mortality [22].
Exposure variables: wake-based movement behaviors
The 24-hour biological clock that controls the sleep-wake cycle is also linked to PA, SB, and sleep. Therefore, the 24-hour cycle can be divided into two categories of behavior that make up the two ends of the circadian cycle: “wake-based” and “sleep-based” movement behavior [23]. In this study, we assessed adherence to wake-based movement guidelines, as measured by time spent in SB and PA. These behaviors were measured continuously and then classified into distinct categories. Subsequently, a composite variable, “Adherence to Movement Guidelines” was created, stratifying participants into three groups: “Complete adherence” for those with less than nine hours of SB and no physical inactivity (PI), “Partial adherence” for more than nine hours of SB or PI and “Non-adherence” for more than nine hours of SB and PI. This classification facilitated an assessment of how these behaviors affect health individually and collectively.
Covariates
The questionnaire included variables for possible confounding controls in the analysis. The variables evaluated were sex (female or male), age (according to the median, in the categories 18–22 years, or > 22 years), current family income (≤ 2 minimum wages; 2 to 5.9; 6 to 10; and > 10 minimum wages), education level of head of household (no education or incomplete primary education; complete primary education or incomplete secondary education; complete secondary education or incomplete higher education; complete higher education), area of knowledge of the course (life sciences, exact sciences, and human and social and applied sciences), and self-reported skin color. The self-reported skin color was based on the categories proposed by the Brazilian Institute of Geography and Statistics (IBGE). The categories were: white, brown, black, yellow, and Indigenous, categories used in demographic and public health research to reflect the ethnic-racial diversity of the Brazilian population [24].
The body mass index (BMI) was calculated by self-reported weight (kg) and height (m²) and classified as not excess weight (BMI < 25 kg/m2) and excess weight (BMI > 25 kg/m2) [25]. Furthermore, the Depression, Anxiety, and Stress Scale (DASS-21) was used to evaluate symptoms of anxiety and depression, classified with a depression symptom score ≥ 19 (DASS-D-21 > 19) and anxiety as a score ≥ 10 (DASS-A-21 > 10) [26, 27].
Statistical analysis
To describe the study sample, statistical analyses were performed in Stata® software, version 15.0. They were described as relative frequencies or mean and 95% confidence interval (CI).
To verify the association between wake-based movement behaviors and sleep quality, unadjusted and adjusted logistic regressions were performed. A theoretical causality model based on a directed acyclic graph (DAG) was developed according to the exposure variables (wake-based movement behaviors), outcome (sleep quality), and confounding variables, using the online software Dagitty, version 3.2 (Fig. 1). To avoid unnecessary adjustment, spurious associations, and estimation errors, the backdoor criterion was used to select a minimum set of confounding variables to fit the analyses. Therefore, all models were adjusted by the following minimum set of variables from the backdoor criterion: sex, age, education level of father/mother or guardian, household income, area of knowledge, and body mass index [28].
Directed acyclic graph (DAG) of the association between wake-based movement behaviors and sleep quality during the COVID-19 pandemic
Legend: The variable in green and with the “►” symbol inside the rectangle was the exposure variable; those in blue and with the letter “I” inside the rectangle were the response variables; variables in blue are the antecedents of the outcome variable; and those in red are antecedents of the outcome and exposure variables
To verify the hypothetical effects of reallocating time spent on SB, MPA, and VPA on sleep quality, the isotemporal substitution approach [29] was used. This statistical approach makes it possible to estimate the redistribution of time between different activities while keeping the total time available constant. In this study, isotemporal substitution models were used to examine the effect of substituting intervals of 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 90, and 120 min per day of SB, MPA and VPA on sleep quality. The analyses were carried out by estimating the odds ratio (OR) with the respective CI of 95% using binary logistic regression. For analysis, a total time variable was first calculated, adding up the minutes dedicated to each activity (total time = SB + MPA + VPA). Subsequently, all the time variables were normalized by the corresponding fractions, facilitating the analysis of the proposed isotemporal substitutions. In the model, we included all the behavioral variables, except the one being replaced (SB, MPA, or VPA), as well as the total time variable. The covariates of interest were also incorporated into the model. The inclusion of total time restricts the model so that the regression estimate for each behavior included reflects the expected average change in the levels of the outcome when there is an increase in the fractions of time defined for an activity, with a concomitant and equivalent decrease in the time spent on sedentary behavior.
The parameters for evaluating the model were assessed (Prob F < 0.001 and goodness-of-fit test), indicating that the variables in the model were appropriate for the analysis. In addition, multicollinearity was tested by variance inflation factors (VIF < 10), with the ‘subsetByVIF’ command in Stata.
Results
A total of 8,059 study participants were evaluated. The majority were female, self-declared white, with a family income of less than four minimum wages and aged between 18 and 22. The age of the participants ranged from 18 to 71 years, with a mean of 23.90 years (± 6.33), and a median of 22.00 (IQR: 5.00). More than half self-reported symptoms of anxiety (59.39%) and 62.66% symptoms of depression (Table 2). In terms of sleep quality, 64.79% of individuals reported poor sleep quality (Fig. 2). Of the wake-based movement behaviors, the average SB was 9.43 h/day (95%CI: 9.34 to 9.50), and 55.08% had a high level of SB. While the average MPA time was 25.43 min/day (95%CI: 24.67 to 26.18), VPA was 7.55 min/day (95%CI: 7.13 to 7.97), and 48.28% were physically inactive. Furthermore, when assessing adherence to movement guidelines, only 26.60% of individuals had complete adherence, meeting physical activity and SB recommendations (Fig. 3).
Distribution of movement behaviors of young adults PADu-multicentric (2021–2022)
Legend: Distribution of movement behaviors of young adults, showing (A) the histogram of time spent in sedentary behavior (hours/day), (B) the histogram of time spent in moderate physical activity (minutes/day), (C) the histogram of time spent in vigorous physical activity (minutes/day), (D) bar graph showing the prevalence of individuals with physical inactivity and high sedentary behavior, and (E) the combination of adherence to movement behavior guidelines, complete adherence (i.e. individuals with less than 9 h of SB per day and no PI), partial adherence (SB elevated or PI) and non-adherence (SB elevated and PI)
The association between wake-based movement behaviors and sleep quality showed that individuals with 9 h or more of SB and who were physically inactive were 42% and 52% more likely to have poor sleep quality, respectively (OR:1.42; 95%CI:1.29 to 1.57 and OR:1.52; 95%CI:1.38 to 1.67). When analyzing adherence to wake-based movement guidelines, individuals with partial adherence were 43% more likely to have poor sleep quality (OR: 1.43; 95%CI: 1.27 to 1.60), while individuals with non-adherence were 97% more likely (OR: 1.97; 95%CI: 1.73 to 2.24) compared to individuals who fully adhered to the guidelines (less than 9 h of SB per day and physically active) (Table 2).
The results obtained from the isotemporal substitution models indicate that increasing the time dedicated to MPA and VPA and reducing the time spent in SB, decreases the odds of poor sleep quality. A significant dose-response gradient was observed, where greater time substitutions resulted in a greater protective effect (p < 0.05). Specifically, the protective effect ranged from 2 to 66.7% for substitutions of 5 to 120 min, respectively (OR:0.98;95%CI:0.97 to 0.99 and OR:0.60;95%CI:0.50 to 0.70). Conversely, replacing 30 min of MPA or VPA with SB resulted in a 14% and 12% increase in the likelihood of poor sleep quality, respectively (OR:1.14;95%CI:1.09 to 1.19 and OR:1.12;95%CI:1.04 to 1.21). When comparing MPA and VPA, it was noted that both showed similar protective effects about SB, with a marginal advantage for MPA in improving sleep quality. The variations can be seen in Table 3, with the most significant change observed for 120 min of substitution.
Discussion
To our knowledge, this is the first study to assess isotemporal change in wake-based movement behaviors and their influence on sleep quality in young adults. Our findings suggest that SB and PI are significantly associated with poor sleep quality, and replacing the time devoted to SB with MPA or VPA may have a significant interface with sleep quality.
The relationship between PA and sleep quality is well documented in the literature, highlighting it as an essential factor for well-being. However, research into SB and its interaction with PA is still relatively new. The association of isolated SB is a risk factor for insomnia and sleep disorders, as demonstrated by Yang et al. (2017) in a systematic review of 16 studies. However, they found no association with overall poor sleep quality and did not consider the interface of PA [30]. In this respect, during the pandemic, a recent study found that high levels of SB were associated with poorer sleep quality, while PA attenuated these adverse effects [31]. However, the population of young adults, especially university students, who tend to have high levels of SB and physical inactivity, has not been extensively evaluated. Furthermore, the potential changes in health outcomes due to the replacement of these wake-based movement behaviors still need further exploration.
Movement behavior modification represents a significant challenge for health research. Understanding the daily variations in movement behavior is a complex task, requiring careful analysis of the interrelationship and substitution between different activities throughout the day. The isotemporal substitution methodology offers a sophisticated statistical approach to investigating the potential effects of reallocating time spent on one activity to another. Recent studies employing this methodology have revealed remarkable findings. For example, systematic review studies have found consistent data on other health outcomes, in which replacing SB with MVPA was associated with reductions in mortality risk, reduced body mass index, body fat percentage and waist circumference, cardiometabolic biomarkers [32] and improved cognitive function [33]. Suggesting that even small changes in daily activity patterns can have profound health consequences [32].
Sleep-related studies that have explored the isotemporal substitution of wake-based movement behaviors (SB and PA) are still scarce. However, existing research provides valuable insights. A study of Japanese older adults found that each 60 min unit of SB or LPA replaced with MVPA was favorably associated with rest by sleep among women (β = 0.16, 95% CI 0.07 to 0.28, p < 0.001; β = 0.18, 95% CI 0.07 to 0.32, p < 0.05, respectively) [2]. In addition, some studies suggest that replacing SB with MVPA can result in significant mental health benefits, including reduced depressive symptoms, increased self-esteem and resilience [34, 35], which may be indirectly related to improved sleep quality [34]. Furthermore, our findings are aligned with the World Health Organization (WHO) guidelines for 2020, which emphasize that replacing sedentary time with physical activity of any intensity, including light intensity, provides health benefits1. This recommendation highlights the importance of even minimal increases in physical activity to counteract the negative health impacts associated with sedentary behavior [36]. The WHO guidelines emphasize that any movement is better than none, reinforcing the idea that small changes in daily habits can lead to significant improvements in health, including sleep quality.
The relationship between SB, PA, and sleep quality is supported by various physiological and behavioral hypotheses. A sedentary lifestyle is associated with an imbalance in circadian rhythms, partly due to reduced exposure to sunlight, which is an essential synchronizer of these rhythms and fundamental in regulating the sleep-wake cycle [21]. Artificial light, especially that emitted by electronic devices, can suppress melatonin production, impairing sleep onset and maintenance [37]. In contrast, PA increases exposure to natural light and promotes circadian regulation, as well as inducing the physical fatigue necessary for sleep pressure, where adenosine plays a central role by accumulating in the brain and signaling the need for rest [38]. Exercise also helps to reduce stress and anxiety, factors that improve sleep quality [39]. In addition, sedentary behavior can lead to musculoskeletal pain, such as lower back pain, which is known to interfere with sleep, while regular PA can help relieve this pain and promote more comfortable and restful sleep [40]. Other hypotheses include the thermoregulatory effect of exercise, which through the elevation and subsequent reduction of body temperature, may facilitate the onset of sleep [41]. The release of endorphins during PA may increase alertness during the day, improving sleep latency at night [42]. Improved respiratory capacity and a reduction in excess adiposity, both effects of regular PA, can contribute to the prevention of sleep disorders and promote better quality sleep [43, 44].
Additionally, PA has positive effects on mental health, which may contribute to reducing sleep disorders related to conditions such as depression and anxiety [42]. Studies have shown that symptoms of depression, anxiety, and stress are prevalent among college students and have a significant negative association on their quality of life, which in turn affects sleep quality. For instance, one study evaluated the association between quality of life and the presence of symptoms of depression, anxiety, and stress in health area students and found that higher symptom severity was associated with lower quality of life scores [45]. Another study compared the quality of life and mental health of healthcare students before and during the COVID-19 pandemic, revealing a deterioration in both aspects during the pandemic, with significant increases in symptoms of depression, anxiety, and stress [46]. All these hypotheses reinforce the importance of an active lifestyle in promoting healthy, quality sleep.
Therefore, this study makes a significant contribution to understanding the effects of wake-based movement behaviors on sleep quality in young adults. General recommendations based on our findings suggest the importance of intervention strategies that promote the reduction of SB and encourage regular PA as an effective means to improve sleep quality. Such strategies are particularly pertinent in remote teaching contexts, where students may be more susceptible to sedentary lifestyle patterns. However, when reflecting on the methodology employed, it is important to recognize both the strengths and limitations of the study.
As limitations, the cross-sectional nature of the research prevents the inference of causal relationships, and the use of self-administered questionnaires may introduce self-report bias. Additionally, the use of a single question from the WHOQOL scale to assess sleep quality limits the depth of understanding regarding sleep patterns. The lack of objective measures of SB and PA may affect the accuracy of the data collected. Furthermore, the period of data collection, during the COVID-19 pandemic, may have influenced the behaviors and perceptions of the participants, as lockdowns and remote learning significantly altered daily routines. It is also important to note the timeframe reference of the questions (e.g., currently, or over the last few months), which may affect the estimates and their interpretation. The non-probability sample, which invited all students to participate, may have introduced response bias, particularly if those with previous experience with mental disorders were more likely to participate. This factor should be carefully considered when interpreting the results.
The strengths of this study include the use of isotemporal analysis to evaluate the hypothetical effects of time reallocation between different wake-based movement behaviors and sleep quality, an approach that offers a more complete view of the health benefits of these activities. In addition to using the counterfactual approach, the DAG was used to guide analysis plans and decisions about possible confounders. The substantial sample size, which includes almost 10,000 students, and the multicenter approach, covering eight higher education institutions in different regions of Brazil, lend robustness and representativeness to the data collected. These characteristics of the study strengthen the reliability of the results and their applicability in a wider context.
Conclusion
Most of the individuals in the study experienced poor sleep quality, which was significantly associated with movement behavior patterns. Replacing sedentary behavior (SB) with an equivalent duration of MVPA was found to reduce the likelihood of poor sleep quality. The longer the duration of MVPA substituted for SB, the greater the observed benefits. It is crucial to note that improving sleep quality not only enhances overall well-being but also promotes various aspects of health.
Data availability
No datasets were generated or analysed during the current study.
References
Duncan MJ, Murphy L, Oftedal S, Fenwick MJ, Vincent GE, Fenton S. The associations between physical activity, sedentary behaviour, and sleep with mortality and incident cardiovascular disease, cancer, diabetes and mental health in adults: a systematic review and meta-analysis of prospective cohort studies. J Activity Sedentary Sleep Behav. 2023;2(1):19. https://doi.org/10.1186/s44167-023-00026-4
Koohsari MJ, Yasunaga A, McCormack GR, et al. Sedentary behaviour and sleep quality. Sci Rep. 2023;13(1):1180. https://doi.org/10.1038/s41598-023-27882-z
Maurya C, Muhammad T, Maurya P, Dhillon P. The association of smartphone screen time with sleep problems among adolescents and young adults: cross-sectional findings from India. BMC Public Health. 2022;22(1):1686. https://doi.org/10.1186/s12889-022-14076-x
Han X, Zhou E, Liu D. Electronic media use and sleep quality: updated systematic review and meta-analysis. J Med Internet Res. 2024;26:e48356. https://doi.org/10.2196/48356
Weinert D, Gubin D. The impact of physical activity on the Circadian system: benefits for health, performance and wellbeing. Appl Sci. 2022;12(18):9220. https://doi.org/10.3390/app12189220
Castro O, Bennie J, Vergeer I, Bosselut G, Biddle SJH. How sedentary are university students? A systematic review and Meta-analysis. Prev Sci. 2020;21(3):332–43. https://doi.org/10.1007/s11121-020-01093-8
Dziewior J, Carr LJ, Pierce GL, Whitaker K. College students report less physical activity and more sedentary behavior during the COVID-19 pandemic. J Am Coll Health Published Online July. 2022;26:1–9. https://doi.org/10.1080/07448481.2022.2100708
Farrahi V, Rostami M. Machine learning in physical activity, sedentary, and sleep behavior research. J Activity Sedentary Sleep Behav. 2024;3(1):5. https://doi.org/10.1186/s44167-024-00045-9
Musa S, Elyamani R, Dergaa I. COVID-19 and screen-based sedentary behaviour: systematic review of digital screen time and metabolic syndrome in adolescents. Jaafar Z, ed. PLoS One. 2022;17(3):e0265560. https://doi.org/10.1371/journal.pone.0265560
Rai A, Aldabbas M, Veqar Z. Effect of physical activity on sleep problems in sedentary adults: a scoping systematic review. Sleep Biol Rhythms. 2024;22(1):13–31. https://doi.org/10.1007/s41105-023-00494-w
Sampasa-Kanyinga H, Colman I, Goldfield GS, et al. Combinations of physical activity, sedentary time, and sleep duration and their associations with depressive symptoms and other mental health problems in children and adolescents: a systematic review. Int J Behav Nutr Phys Activity. 2020;17(1):72. https://doi.org/10.1186/s12966-020-00976-x
Healy KL, Morris AR, Liu AC. Circadian synchrony: sleep, nutrition, and physical activity. Front Netw Physiol. 2021;1. https://doi.org/10.3389/fnetp.2021.732243
Barbosa BCR, de Paula W, Ferreira AD, et al. Anxiety and depression symptoms in university students from public institutions of higher education in Brazil during the covid-19 pandemic: a multicenter study. SciELO Preprints. 2023;25(version 1). https://preprints.scielo.org/index.php/scielo/preprint/view/6080.
THE WHOQOL GROUP. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28(3):551–8. https://doi.org/10.1017/S0033291798006667
da Rocha NS, de Almeida Fleck MP, Rocha NS da, de Fleck MPA. Validity of the Brazilian version of WHOQOL-BREF in depressed patients using Rasch modelling. Rev Saude Publica. 2009;43(1):147–153. https://doi.org/10.1590/S0034-89102009000100019
Barros MB, de Lima A, Ceolim MG, Zancanella MF, de Cardoso E. Quality of sleep, health and well-being in a population-based study. Rev Saude Publica. 2019;53:82. https://doi.org/10.11606/s1518-8787.2019053001067
Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11(06):591–2. https://doi.org/10.5664/jcsm.4758
Scott RQ, Rodríguez AJ. Improving quality of sleep in healthy adults. Curr Pulmonol Rep. 2023;12(2):46–55. https://doi.org/10.1007/s13665-023-00304-1
Moreira AD, Claro RM, Felisbino-Mendes MS, Velasquez-Melendez G. Validade E reprodutibilidade de inquérito telefônico de atividade física no Brasil. Revista Brasileira De Epidemiologia. 2017;20(1):136–46. https://doi.org/10.1590/1980-5497201700010012
Herrmann SD, Willis EA, Ainsworth BE, et al. 2024 adult compendium of physical activities: a third update of the energy costs of human activities. J Sport Health Sci. 2024;13(1):6–12. https://doi.org/10.1016/j.jshs.2023.10.010
WHO WHO. WHO guidelines on physical activity and sedentary behaviour. 2020. https://iris.who.int/bitstream/handle/10665/336656/9789240015128-eng.pdf?sequence=1
Ku PW, Steptoe A, Liao Y, Hsueh MC, Chen LJ. A cut-off of daily sedentary time and all-cause mortality in adults: a meta-regression analysis involving more than 1 million participants. BMC Med. 2018;16(1):74. https://doi.org/10.1186/s12916-018-1062-2
Falck RS, Davis JC, Li L, Stamatakis E, Liu-Ambrose T. Preventing the ‘24-hour Babel’: the need for a consensus on a consistent terminology scheme for physical activity, sedentary behaviour and sleep. Br J Sports Med. 2022;56(7):367–8. https://doi.org/10.1136/bjsports-2021-104487
Telles E, Paschel T. Who is Black, White, or mixed race? How skin Color, Status, and Nation shape racial classification in Latin America. Am J Sociol. 2014;120(3):864–907. https://doi.org/10.1086/679252
WHO. Physical status: the use and interpretation of anthropometry. Technical Report Series no 854. Published online 1995.
Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the Beck depression and anxiety inventories. Behav Res Ther. 1995;33(3):335–43. https://doi.org/10.1016/0005-7967(94)00075-U
Patias ND, Machado WDL, Bandeira DR, Dell’Aglio DD. Depression anxiety and stress scale (DASS-21) - short form: Adaptação E Validação para Adolescentes Brasileiros. Psico-USF. 2016;21(3):459–69. https://doi.org/10.1590/1413-82712016210302
Cortes TR, Faerstein E, Struchiner CJ. Use of causal diagrams in epidemiology: application to a situation with confounding. Cad Saude Publica. 2016;32(8):e00103115. https://doi.org/10.1590/0102-311X00103115
Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–27. https://doi.org/10.1093/aje/kwp163
Yang Y, Shin JC, Li D, An R. Sedentary behavior and sleep problems: a systematic review and meta-analysis. Int J Behav Med. 2017;24(4):481–92. https://doi.org/10.1007/s12529-016-9609-0
de Menezes-Júnior LAA, de Moura SS, Miranda AG, de Souza Andrade AC, Machado-Coelho GLL, Meireles AL. Sedentary behavior is associated with poor sleep quality during the COVID-19 pandemic, and physical activity mitigates its adverse effects. BMC Public Health. 2023;23(1):1–13. https://doi.org/10.1186/S12889-023-16041-8/TABLES/4
Grgic J, Dumuid D, Bengoechea EG, et al. Health outcomes associated with reallocations of time between sleep, sedentary behaviour, and physical activity: a systematic scoping review of isotemporal substitution studies. Int J Behav Nutr Phys Activity. 2018;15(1):69. https://doi.org/10.1186/s12966-018-0691-3
Ehlers DK, Fanning J, Salerno EA, et al. Replacing sedentary time with physical activity or sleep: effects on cancer-related cognitive impairment in breast cancer survivors. BMC Cancer. 2018;18(1):685. https://doi.org/10.1186/s12885-018-4603-3
Meneguci JJ, Galvão LL, Tribess S, et al. Isotemporal substitution analysis of time between sleep, sedentary behavior, and physical activity on depressive symptoms in older adults: a cross-sectional study. Sao Paulo Med J. 2024;142(4):e2023144. https://doi.org/10.1590/1516-3180.2023.0144.r2.04122023
Clemente FM, Chen C, Brown DMY, Kwan MYW. Movement behaviors and mental wellbeing: a cross-sectional isotemporal substitution analysis of Canadian adolescents. Front Behav Neurosci. 2021;15. https://doi.org/10.3389/fnbeh.2021.736587
Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. https://doi.org/10.1136/bjsports-2020-102955
Kozaki T, Kubokawa A, Taketomi R, Hatae K. Effects of day-time exposure to different light intensities on light-induced melatonin suppression at night. J Physiol Anthropol. 2015;34(1):1–5. https://doi.org/10.1186/s40101-015-0067-1
Shen B, Ma C, Wu G, Liu H, Chen L, Yang G. Effects of exercise on circadian rhythms in humans. Front Pharmacol. 2023;14. https://doi.org/10.3389/fphar.2023.1282357
Singh B, Olds T, Curtis R, et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: an overview of systematic reviews. Br J Sports Med. 2023;57(18):1203–9. https://doi.org/10.1136/bjsports-2022-106195
Dzakpasu FQS, Carver A, Brakenridge CJ, et al. Musculoskeletal pain and sedentary behaviour in occupational and non-occupational settings: a systematic review with meta-analysis. Int J Behav Nutr Phys Activity. 2021;18(1):159. https://doi.org/10.1186/s12966-021-01191-y
Harding EC, Franks NP, Wisden W. Sleep and thermoregulation. Curr Opin Physiol. 2020;15:7–13. https://doi.org/10.1016/j.cophys.2019.11.008
Mahindru A, Patil P, Agrawal V. Role of physical activity on mental health and well-being: a review. Cureus Published Online January. 2023;7. https://doi.org/10.7759/cureus.33475
Barskaya G. Your lungs and exercise. Breathe. 2016;12(1):97–100. https://doi.org/10.1183/20734735.ELF121
de Menezes Júnior LAA, Lourenção LG, Andrade AC, de Carraro S, Machado-Coelho JCC, Meireles GLL. Determinants of poor sleep quality in adults during the coronavirus disease pandemic: COVID-inconfidentes, a population-based study. Sao Paulo Med J Published Online Oct. 2022;28. https://doi.org/10.1590/1516-3180.2022.0139.r1.19082022
de Freitas PHB, Meireles AL, Abreu MNS, Barbosa BCR, de Paula W, Cardoso CS. Assessment of the quality of life and mental health of healthcare students during the COVID-19 pandemic. Rev Bras Enferm. 2023;76(suppl 1). https://doi.org/10.1590/0034-7167-2023-0068
de Freitas PHB, Meireles AL, Ribeiro IK da, Abreu S, Paula MNS, de Cardoso W. Symptoms of depression, anxiety and stress in health students and impact on quality of life. Rev Lat Am Enfermagem. 2023;31. https://doi.org/10.1590/1518-8345.6315.3885
Acknowledgements
The authors acknowledge the support of the Federal University of Ouro Preto (UFOP) and the Group for Research and Education in Nutrition and Collective Health (GPENSC) for their support and encouragement, and, the members of the Project on Anxiety and Depression in University Students (PADu).
Funding
The research project “Symptoms of anxiety disorder and depression among university students in Minas Gerais: prevalence and associated factors” has funding approved in Public Notice 001/2018 - DEMANDA UNIVERSAL FAPEMIG, under PROCESS number N. CDS - APQ-01089- 18, with granting term FAPEMIG/DOF nº. 8288757/2019. The National Council for Scientific and Technological Development (CNPq/Brazil), FAPEMIG/Brazil, and the Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil) provided scholarships for undergraduate and graduate students. Cardoso CS received a scholarship from CNPq [Code 302709/2023-1].
Author information
Authors and Affiliations
Contributions
BCRBB and WP: participated in the preparation and writing of the project, coordinated and conducted data collection, writing-review, and editing the manuscript; ADF, CSC, EDF, FCV, LGF, LNN and LSS participated in the project design, coordination of data collection at university, and critical review of the manuscript; ALM: conception and general coordination of the project, study design, statistical analysis, and critical revision of the manuscript. LAAMJ participated in the data analysis and interpretation, article writing, and review. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical approval
All procedures were carried out according to the Brazilian guidelines and standards for research involving human beings of the Declaration of Helsinki and were approved by the Research Ethics Committees of all the IFES. UFOP: 43027421.3.1001.5150; UFMG: 43027421.3.2004.5149; UFJF: 43027421.3.2003.5147; UFSJ: 43027421.3.2002.5545; UFLA: 43027421.3.2006.5148; UNIFAL-MG: 43027421.3.2008.5142; UFU: 43027421.3.2001.5152 and UFVJM: 43027421.3.2009.5108. Informed consent was obtained from all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Menezes-Júnior, L.A.A.d., Barbosa, B.C.R., de Paula, W. et al. Isotemporal substitution analysis of time between sedentary behavior, and physical activity on sleep quality in younger adults: a multicenter study. BMC Public Health 24, 2460 (2024). https://doi.org/10.1186/s12889-024-19995-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19995-5