Abstract
Background
Physical activity is a crucial demand on cystic fibrosis treatment management. The highest value of oxygen uptake (VO2peak) is an appropriate tool to evaluate the physical activity in these patients. However, there are several other valuable CPET parameters describing exercise tolerance (Wpeak, VO2VT1, VO2VT2, VO2/HRpeak, etc.), and helping to better understand the effect of specific treatment (VE, VT, VD/VT etc.). Limited data showed ambiguous results of this improvement after CFTR modulator treatment. Elexacaftor/tezacaftor/ivacaftor medication improves pulmonary function and quality of life, whereas its effect on CPET has yet to be sufficiently demonstrated.
Methods
We performed a single group prospective observational study of 10 adolescent patients with cystic fibrosis who completed two CPET measurements between January 2019 and February 2023. During this period, elexacaftor/tezacaftor/ivacaftor treatment was initiated in all of them. The first CPET at the baseline was followed by controlled CPET at least one year after medication commencement. We focused on interpreting the data on their influence by the novel therapy. We hypothesized improvements in cardiorespiratory fitness following treatment. We applied the Wilcoxon signed-rank test. The data were adjusted for age at the time of CPET to eliminate bias of aging in adolescent patients.
Results
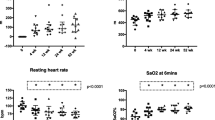
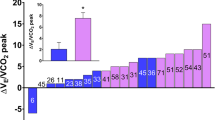
We observed significant improvement in peak workload, VO2 peak, VO2VT1, VO2VT2, VE/VCO2 slope, VE, VT, RQ, VO2/HR peak and RR peak. The mean change in VO2 peak was 5.7 mL/kg/min, or 15.9% of the reference value (SD ± 16.6; p= 0.014). VO2VT1 improved by 15% of the reference value (SD ± 0.1; p= 0.014), VO2VT2 improved by 0.5 (SD ± 0.4; p= 0.01). There were no differences in other parameters.
Conclusion
Exercise tolerance improved after elexacaftor/tezacaftor/ivacaftor treatment initiation. We suggest that the CFTR modulator alone is not enough for recovering physical decondition, but should be supplemented with physical activity and respiratory physiotherapy. Further studies are needed to examine the effect of CFTR modulators and physical therapy on cardiopulmonary exercise tolerance.
Similar content being viewed by others
Background
Cystic fibrosis (CF) is the genetic disorder affecting the lungs worldwide. There is substantial heterogeneity of clinical manifestation in patients with CF. Cystic fibrosis transmembrane conductance regulator (CFTR) mutation results in the development of bronchiectasis, recurrent infectious exacerbations and lung function decline. Thus, patients moreover have increased dead space (VD), which is why the alveolar ventilation is lower compared to healthy subjects. During exercise in CF patients, tidal volume (VT) increases inadequately, the respiratory rate (RR) then increases to heighten minute ventilation (VE) and reach an adequate oxygen uptake (VO2). Response to physical activity is inefficient [1, 2]. Muscle function and muscle mass, the same as cardiac abnormalities, are mostly limitations in mild or moderate CF, patients with severe lung disease, oxygen delivery and non-physiological respiratory mechanics limit exercise capacity [1, 3,4,5,6,7].
Cardiopulmonary exercise testing (CPET) measures aerobic exercise capacity and provides a comprehensive assessment of respiratory, cardiac and musculoskeletal function during exercise and recovery and may be used for prognosis and risk assessment [8]. Not just VO2 peak is a predictor of survival, but also peak workload (W peak), VE/VO2 peak (ventilatory equivalent for oxygen, EQO2) and VE/VCO2 peak (ventilatory equivalent for carbon dioxide, EQCO2) may be significant [8].
Ivacaftor and lumacaftor/ivacaftor combination had no effect on VO2 peak, only sets of case reports in tezacaftor/ivacaftor demonstrated minor improvements [9,10,11,12,13]. The novel triple combination of the modulators of CFTR channel, elexacaftor/tezacaftor/ivacaftor (ETI) provides substantial clinical improvement and prolonged median predicted survival, improves lung functions (ppFEV1 increases by 14.3%); also promotes over-nutrition and overweight, which might affect the physical activity attitude otherwise [14, 15]. The exact quantitative evaluation is still not well known because there is not enough documented evidence of CPET-derived measures of triple combination. The data published so far are not sufficiently convincing. ETI seem to improve VO2 peak, but still lack more detailed data about the physical tolerability mechanism [16]. By performing this trial, we try to fill the gap in the knowledge of additional CPET parameters, than just VO2 peak. These are important tools to evaluate the effect of the new treatment on prognosis and survival.
We hypothesize that patients with CF will improve their physical activity by several adaption mechanisms, e.g. higher myocardial contractility and cardiac output (HR, higher AT which correlate with decreasing values of RQ and VE/VO2), more effective gas exchange (increased VO2 peak and VCO2), lower work of breathing (improved VE due to increased VT, RR and decreased VD/VT), and lower hyperventilation following elexacaftor/tezacaftor/ivacaftor use. Also, the aim of this study is to define other CPET parameters suitable for evaluating the cardiorespiratory demands in cystic fibrosis.
Methods
Subjects
This is a prospective observational, non-randomised study. Inclusion criteria comprised a diagnosis of CF based on current guidelines, EMA-approved genotype for ETI indication and informed consent provided by the patient or their legal representative. All the patients were ETI treatment-naïve. For this study, we analysed data of patients with CF aged 8–19 years at the time of the first testing, who had a full CPET meeting between January 23, 2019, and February 9, 2023. The follow-up measurement was performed at least 12 months after ETI commencement (Kaftrio ®, elexacaftor 100 mg, tezacaftor 50 mg, ivacaftor 75 mg; always used in combination with Kalydeko ®, ivacaftor 150 mg). This was the only intervention made; patients haven’t engaged in standardized exercise training.
Protocol
All the testing was performed during a clinically stable period. We used an exhaustive ramp incremental (10-25W/min) cycling CPET (Ergoline, Ergoselect, Bitz, Germany) protocol. We selected the ramp protocol based on the patient’s physical activity level, body weight and sex. After a 3-min warm-up (10-40W), all the participants completed a test to the point of exhaustion. The protocol was tailored to the individual to yield a fatigue-limited exercise duration of 8–12 min. A five-minute active cool down period followed CPET. Breath-by-breath analysis was provided, and the O2 and CO2 concentrations of exhaled air with ventilatory volume was measured via face mask with connected gas and flow spirometer sensors. The stress test was performed on the ergometer ERGOLINE, and the exhaled gases was analysed by POWER CUBE – Schiller (Switzerland). A ramp protocol was used while VO2, VO2VT1, VO2VT2, VE/VCO2, VE/VO2, VE/VCO2 slope, VE, VT, RQ, VO2/HR, RR parameters were measured every 10 s, and peak values taken as the highest 15 s achieved during the test. Blood pressure and Sp02 were measured during CPET monitoring.
We evaluated anthropometric parameters: height (cm), weight (kg). Our outcome was to evaluate respiratory CPET-derived parameters: maximal workload (W, W/kg, % ref.), RQ max., VO2 peak (L, mL/kg/min, % ref.,), VO2VT1 (L/min, % ref.), VO2VT2, (L/min), VE/VCO2 slope, VE (L/min, % ref.), VT (L, % ref.), VD/VT in rest and in maximal effort, RR (min−1), VO2/HR (ml/beats per minute).
Statistical methods
Numerical parameters are described by mean (standard deviation = SD). Change during two times is tested by paired Wilcoxon signed-rank test. All tests are two-sided on level of significance 5%. Analysis was prepared in the software R (v4.2) (Bell Laboratories, Inc., Windsor, WI, US).
Results
Thirteen patients were eligible, but only 10 patients with CF met the criteria for ETI therapy and were included in the final analysis. The mean age was 14 years, mean ppFEV1 89.4%, 70% of the patients with CF were F508del homozygotes. Baseline patients’ characteristics are presented in Table 1.
We observed significant improvements in VO2 peak, the mean change was 0.8 L (SD ± 0.6; p = 0.002), 15.9% of the reference value (SD ± 16.6; p = 0.014). As well as VO2VT1 improved by 15% of the reference value (SD ± 0.1; p = 0.014) and VO2VT2 improved by 0.5 (SD ± 0.4; p = 0.01). Patients achieved also better VO2VT2, VE/VCO2 slope, VE, VT, RQ, VO2/HRpeak, and RRpeak values. Even VD/VT marker improved. Only RQ remained unchanged.
Complete data are presented in Table 2. The parameters were recalculated based on age and current weight (% ref.), so the impact of aging was eliminated.
During controlled CPET, all the patients indicated the fatigue of lower extremities as their reason for stopping. We did not observe any exercise-induced arrythmia.
Discussion
In this study, we report improvement in most of the parameters, which are valuable predictors of death or lung transplant in CF (VO2 peak, max. effort, peak work rate, VE/VCO2 slope) and parameters valuable to understand the ventilatory efficiency (VO2VT1, VO2VT2, VE, VT, VD/VTVO2/HR peak and RR peak) [8]. An abnormally low exercise capacity and deconditioning in CF results in VO2 peak < 82% predicted and/or peak workload < 93% predicted, VO2VT1 occurring < 50% predicted VO2 peak [17]. Patients in this cohort achieved improvement in two of these parameters, beyond deconditioning. This might suggest improved prognosis in patients with CF treated with ETI for at least one year.
These data provided on triple combination of CFTR modulators therapy are higher than those achieved on double combination (lumacaftor/ivacaftor or tezacaftor/ivacaftor) in Danish patients with CF followed for the same period of use (VO2peak 1.07 mL/min/kg, maximal workload change 14.2W) [18]. Therefore, ETI might be more effective in improving CPET-derived parameters, but improvement is likely multifactorial, and further investigation in a larger patient cohort is necessary.
Older patients with CF aged > 40 years deal with specific comorbidities, while younger patients with CF are healthier than ever due to the variant treatment strategies. Still, physical fitness in CF takes an indisputable position to effect quality of life and prognosis. Medication which reduces the amount of mucus in the respiratory tract and improve pulmonary function could not be the only reason for improved exercise tolerance. Patients eligible into our study were mostly teenagers; the maximal VO2 peak increased significantly in absolute but also in relative values, leaving minimal doubts about the role of ageing bias over the study period [17]. Other CPET variables, VO2VT1 and VO2VT2, demonstrate improved aerobic capacity, physical fitness, and more effective training to the maximal effort. Improvement of VD/VT suggests more effective ventilation and decreased V/Q mismatch, as well as improved lung function in general. This would also hint at statistically significant improvement in VO2/HR.
In this cohort, the mean ppFEV1 is 89.4%; research suggests that in mild lung disease, the respiratory limitation of exercise capacity is rather low [13]. Pulmonary function improvement alone in CFTR modulator users would then be unlikely to change the exercise tolerance. The impact of ETI on the exercise tolerance improvement is hypothesized by several mechanisms [19]. CFTR protein is expressed in myocardial cells, vascular smooth muscle cells, and sarcolemma and sarcoplasm of skeletal muscle cells [20,21,22]. Impaired CFTR function results in local vasoconstriction and affects nitric oxide production [23].Therefore, the CFTR modulators might improve reduced peripheral O2extraction during exercise and utilisation of O2not only by skeletal muscle, but it is also suggested, by reduced VE/ VO2 peak [16]. CFTR protein is hypothesized to be involved in regulating mitochondrial oxidative stress and mitochondrial function in adenosine triphosphate production [24,25].
Decreased systemic inflammation by reducing several interleukins and pro-inflammatory mediators after CFTR modulator use affects cardiorespiratory fitness. Inter alia, chronic inflammation (especially Pseudomonas aeruginosa airway infection) relates to impaired aerobic capacity [26, 27]. Systemic inflammation is demonstrated to lead to muscle atrophy and impaired contractility [28].
To examine body composition change, it is necessary to distinguish the mechanism of VO2 peak change. The pattern of weight gain (whether muscle or fat) due to triple therapy must be studied. This is because increased adiposity has been suggested to contribute to the decreased VO2outcome [19]. Even in this cohort, the patient who gained the most weight (+ 20.4 kg), where BMI increased from 21.86 kg/m2 to 28.38 kg/m2, reported the highest VO2 peak decrease (41.9 mL/kg/min to 35.2 mL/kg/min). Last but not least, it is necessary to consider the change in the mental state of patients on triple therapy and the awareness of new life horizons and possibilities, along with the awareness of the need for more intensive care for overall fitness.
There are several limitations of this study. First, the baseline testing was performed during the COVID-19 pandemic era, so we were not able to recruit more patients in this trial. Even a change in the patient’s physical activity manners during the pandemic era changed to a more sedentary style, which is difficult to quantify. Second, we did not perform a capillary blood gas analysis, and we were therefore not able to assess the V/Q mismatch. Third, the increase in absolute values of certain parameters (e.g. VO2 peak) might partly be attributed to the given adolescent’s growth. However, there were significant improvements also in relative values (% of predicted), therefore aging bias (if any) appears to be limited. Comparative trials with a cohort of same-aged CF patients ineligible for ETI would reveal other perspectives, and the risk of a worsened overall status due to severe CFTR pathogenic variants could bias the results.
Conclusions
We demonstrated improvements in cardiorespiratory fitness in adolescent patients with cystic fibrosis following at least one year of ETI therapy by performing controlled CPET testing. CFTR modulator treatment alone might not be effective in transforming all the mechanisms of exercise intolerance. Understanding the impact of new therapeutical strategies in cystic fibrosis is important for better therapeutical evaluation and survival assessment. Further comparative trials with a larger cohort need to be performed to streamline our results.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AT:
-
Anaerobic threshold
- BMI:
-
Body mass index
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- CF:
-
Cystic fibrosis
- CO2 :
-
Carbon dioxide
- COVID-19:
-
Coronavirus disease of 2019
- CPET:
-
Cardiopulmonary exercise testing
- EQO2 :
-
Ventilatory equivalent for oxygen
- EQCO2 :
-
Ventilatory equivalent for carbon dioxide
- ETI:
-
Elexacaftor/tezacaftor/ivacaftor
- HR:
-
Heart rate
- O2 :
-
Oxygen
- p-value:
-
Probability value
- ppFEV1 :
-
Percent predicted forced expiratory volume in one second
- rpm:
-
Revolution per minute
- RQ:
-
Respiratory quotient
- RR:
-
Respiratory rate
- SD:
-
Standard deviation
- V/Q:
-
Ventilation/perfusion
- VCO2 :
-
Carbon dioxide elimination
- VD :
-
Dead space
- VE :
-
Minute ventilation
- VT :
-
Tidal volume
- VE/VCO2 :
-
Ventilatory equivalent for carbon dioxide
- VE/VO2 :
-
Ventilatory equivalent for oxygen
- VO2 :
-
Oxygen uptake
- VO2VT1 :
-
Oxygen uptake on the first ventilatory threshold
- VO2VT2 :
-
Oxygen uptake on the second ventilatory threshold
- W:
-
Watt
- WR:
-
Work rate
References
Cerny FJ, Pullano TP, Cropp GJ. Cardiorespiratory adaptations to exercise in cystic fibrosis. Am Rev Respir Dis. 1982;126(2):217–20.
Thin AG, Dodd JD, Gallagher CG, Fitzgerald MX, Mcloughlin P. Effect of respiratory rate on airway deadspace ventilation during exercise in cystic fibrosis. Respir Med. 2004;98(11):1063–70.
Regnis JA, Donnelly PM, Robinson M, Alison JA, Bye PT. Ventilatory mechanics at rest and during exercise in patients with cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5):1418–25.
Nixon PA, Joswiak ML, Fricker FJ. A six-minute walk test for assessing exercise tolerance in severely ill children. J Pediatr. 1996;129(3):362–6.
Pastré J, Prévotat A, Tardif C, Langlois C, Duhamel A, Wallaert B. Determinants of exercise capacity in cystic fibrosis patients with mild-to-moderate lung disease. BMC Pulm Med. 2014;14:74.
Pianosi P, Pelech A. Stroke volume during exercise in cystic fibrosis. Am J Respir Crit Care Med. 1996;153(3):1105–9.
Szollosi I, King SJ, Wilson JW, Naughton MT. Tachycardia in adults with cystic fibrosis is associated with normal autonomic function. Intern Med J. 2011;41(6):455–61.
Hebestreit H, Hulzebos EHJ, Schneiderman JE, Karila C, Boas SR, Kriemler S, et al. Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. Am J Respir Crit Care Med. 2019;199(8):987–95.
Edgeworth D, Keating D, Ellis M, Button B, Williams E, Clark D, et al. Improvement in exercise duration, lung function and well-being in G551D-cystic fibrosis patients: a double-blind, placebo-controlled, randomized, cross-over study with ivacaftor treatment. Clin Sci (Lond). 2017;131(15):2037–45.
Wilson J, You X, Ellis M, Urquhart DS, Jha L, Duncan M, et al. VO2max as an exercise tolerance endpoint in people with cystic fibrosis: lessons from a lumacaftor/ivacaftor trial. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2021;20(3):499–505.
Saynor ZL, Barker AR, Oades PJ, Williams CA. The effect of ivacaftor in adolescents with cystic fibrosis (G551D mutation): an exercise physiology perspective. Pediatr Phys Ther Off Publ Sect Pediatr Am Phys Ther Assoc. 2014;26(4):454–61.
Savi D, Schiavetto S, Simmonds NJ, Righelli D, Palange P. Effects of Lumacaftor/Ivacaftor on physical activity and exercise tolerance in three adults with cystic fibrosis. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2019;18(3):420–4.
Ahmed MI, Dayman N, Madge J, Gaillard E. P91 Cardiopulmonary exercise testing in CF adolescents after starting Tezacaftor/Ivacaftor. Thorax. 2021;76(Suppl 1):A136–A136.
Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–19.
Snowball JE, Flight WG, Heath L, Koutoukidis DA. A paradigm shift in cystic fibrosis nutritional care: clinicians’ views on the management of patients with overweight and obesity. J Cyst Fibros. 2023;0(0). https://doi.org/10.1016/j.jcf.2023.03.011.
Causer AJ, Shute JK, Cummings MH, Shepherd AI, Wallbanks SR, Pulsford RM, et al. Elexacaftor-Tezacaftor-Ivacaftor improves exercise capacity in adolescents with cystic fibrosis. Pediatr Pulmonol. 2022;57(11):2652–8.
Hebestreit H, Arets HGM, Aurora P, Boas S, Cerny F, Hulzebos EHJ, et al. Statement on exercise testing in cystic fibrosis. Respir Int Rev Thorac Dis. 2015;90(4):332–51.
Rysgaard UK, Pedersen CL, Jensen JH, Sørensen L, Philipsen LKD, Leo-Hansen C, et al. Change in exercise capacity measured by Cardio-pulmonary Exercise Testing (CPET) in Danish people with cystic fibrosis after initiation of treatment with Lumacaftor/Ivacaftor and Tezacaftor/Ivacaftor. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2022;21(5):844–9.
Caterini JE, Ratjen F, Barker AR, Williams CA, Rendall K, Schneiderman JE, et al. Exercise intolerance in cystic fibrosis-the role of CFTR modulator therapies. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2022;21(2):282–92.
Warth JD, Collier ML, Hart P, Geary Y, Gelband CH, Chapman T, et al. CFTR chloride channels in human and simian heart. Cardiovasc Res. 1996;31(4):615–24.
Robert R, Norez C, Becq F. Disruption of CFTR chloride channel alters mechanical properties and cAMP-dependent Cl- transport of mouse aortic smooth muscle cells. J Physiol. 2005;568(Pt 2):483–95.
Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I. Cystic fibrosis transmembrane conductance regulator in human muscle: Dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol. 2010;67(6):802–8.
Malik FA, Meissner A, Semenkov I, Molinski S, Pasyk S, Ahmadi S, et al. Sphingosine-1-phosphate is a novel regulator of cystic fibrosis transmembrane conductance regulator (CFTR) activity. PLoS One . 2015;10(6):e0130313.
Madácsy T, Pallagi P, Maleth J. Cystic fibrosis of the pancreas: the role of CFTR channel in the regulation of intracellular Ca2+ signaling and mitochondrial function in the exocrine pancreas. Front Physiol. 2018;9:1585.
Velsor LW, Kariya C, Kachadourian R, Day BJ. Mitochondrial oxidative stress in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Respir Cell Mol Biol. 2006;35(5):579–86.
van de Weert-van Leeuwen PB, Slieker MG, Hulzebos HJ, Kruitwagen CLJJ, van der Ent CK, Arets HGM. Chronic infection and inflammation affect exercise capacity in cystic fibrosis. Eur Respir J. 2012;39(4):893–8.
Casey M, Gabillard-Lefort C, McElvaney OF, McElvaney OJ, Carroll T, Heeney RC, et al. Effect of elexacaftor/tezacaftor/ivacaftor on airway and systemic inflammation in cystic fibrosis. Thorax. 2023;78(8):835–9.
Divangahi M, Balghi H, Danialou G, Comtois AS, Demoule A, Ernest S, et al. Lack of CFTR in skeletal muscle predisposes to muscle wasting and diaphragm muscle pump failure in cystic fibrosis mice. PLoS Genet. 2009;5(7):e1000586.
Acknowledgements
We appreciate the willingness of all patients and medical staff to participate in clinical trials and their processing.
Funding
Publication will be supported by the Czech Pulmonological and Phthisiological Society (open access publication free grant).
Author information
Authors and Affiliations
Contributions
N.S.: writing – original draft, methodology, visualization, formal analysis, data curation, L.H.: supervision, methodology, P.H.: writing – original draft, methodology, formal analysis, data curation, L.H.: methodology data curation, M.S.: data curation, formal analysis, K.B.: supervision, writing – review, editing, L.F.: supervision, writing – review, editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approved by University Hospital Brno committee.
Informed consent was obtained from all subject or their legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stastna, N., Hrabovska, L., Homolka, P. et al. The long-term effect of elexacaftor/tezacaftor/ivacaftor on cardiorespiratory fitness in adolescent patients with cystic fibrosis: a pilot observational study. BMC Pulm Med 24, 260 (2024). https://doi.org/10.1186/s12890-024-03069-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03069-8