Abstract
Background
Interstitial lung diseases (ILD) include a wide range of diseases impacting lung parenchyma and leading to fibrosis and architectural distortion. Chronic cough and dyspnea are common symptoms which affect the quality of life (QoL) in ILD patients. The mechanisms of cough in ILD patients are still unknown. The aim of this study was to prospectively investigate histological, radiological, and physiological determinants of cough-related QoL in ILD patients who underwent transbronchial lung cryobiopsy (TBLC).
Methods
All patients (n = 111) filled in The Leicester Cough Questionnaire (LCQ) and The St George’s Respiratory Questionnaire (SGRQ). They underwent lung function tests, forced vital capacity (FVC), forced vital expiratory volume in 1 s (FEV1), diffusion capacity to carbon monoxide (DLCO), high-resolution computed tomography (HRCT), and blood samples before diagnostic TBLC. Two experienced radiologists assessed the extents of following HRCT patterns: ground-glass opacities (GGO), honeycombing, reticulation, traction bronchiectasis, and emphysema. Histology of TBLC were re-analyzed by two experienced pulmonary pathologists and the presence of fibroblast foci, fibrosis, giant cells, granulomas, and honeycombing were recorded.
Results
In the median multivariate regression analysis, BMI (-0.19; 95% CI -0.37- -0.014; p 0.035), GGO (-0.38; 95% CI -0.61- -0.15; p 0.001), granulomas (-3.21; 95% CI -6.12- -0.30; p 0.031), and current smoking (2.49; 95% CI 0.12–4.86; p 0.040) showed independent associations with LCQ total score. BMI (1.3; 95% CI 0.20–2.42; p 0.021) and DLCO (-0.51; 95% CI -0.85 - -0.16; p 0.004) showed independent association with SGRQ total score.
Conclusion
Determinants of cough-related QoL in ILD patients are multifactorial including physiological, radiological and histological parameters.
Similar content being viewed by others
Background
Interstitial lung diseases (ILD) include a wide range of diseases impacting lung parenchyma and leading to fibrosis and architectural distortion [1, 2]. Chronic cough and dyspnea are common symptoms which affect the quality of life (QoL) in ILD patients [3]. Chronic cough in ILD has been most extensively studied in IPF, pulmonary sarcoidosis and connective tissue disease associated interstitial lung disease (CTD-ILD) patients. Cough prevalence has varied in IPF between 68 and 88% [4,5,6], in pulmonary sarcoidosis between 51 and 64% [7, 8] and in other ILD patients between 66 and 71% [9, 10]. Chronic symptoms such as cough, dyspnea and fatigue together with the clinical course of disease progression such as lung function decline, comorbidities, and hospitalizations decrease the QoL of ILD patients [11,12,13]. The level of impairment in the cough-related quality of life (C-QoL) has been shown to predict respiratory hospitalization, death, and time to lung transplantation in ILD [14].
It has been shown that chronic cough in ILD patients correlate with low spirometry values [9]. In IPF, cough has been assumed to be increased by architectural distortion of the airways resulting from fibrosis [15], as well as by cough reflex sensitivity [16], and overexpression of neurotrophins [17]. Mucin 5B (MUC5B) polymorphism has been shown to correlate with chronic cough in IPF patients [18]. However, the mechanisms of cough among ILD patients are still obscure and need further studies, as suggested in the previous review articles [14, 19, 20]. The aim of this study is to investigate histological, radiological and physiological determinants of C-QoL in ILD patients who have underwent transbronchial lung cryobiopsy (TBLC) for diagnostic purposes.
Study design and methods
The patients of this study were prospectively recruited from Kuopio University Hospital (KUH) and Tampere University Hospital (TAUH) pulmonology clinics between January 2015 and December 2021. Inclusion criteria included a referral to a tertiary university hospital (KUH or TAUH) for a suspected ILD and TBLC was suggested by multidisciplinary discussions (MDD) from consecutive patients to confirm the diagnosis. The exclusion criteria of the TBLC included forced vital expiratory volume in 1 s (FEV1) < 50%, total lung capacity < 50%, diffusion capacity to carbon monoxide (DLCO) < 50%, mean pulmonary artery pressure > 55 mmHg in echocardiogram (ECHO), body mass index (BMI) > 30 kg/m² if the mean pulmonary artery pressure in ECHO was > 55 mmHg. The rest of the exclusion criteria are described in more detail in an earlier publication [21].
All patients (n = 111) underwent forced vital capacity (FVC), FEV1, and DLCO tests, high-resolution computed tomography (HRCT), and blood samples before the TBLC. Leicester Cough Questionnaire (LCQ), and The St George’s Respiratory Questionnaire (SGRQ) were filled in at baseline before the TBLC. A bronchoalveolar lavage (BAL) was performed to all patients at the time of the TBLC. After the analysis of the TBLC, the diagnosis of ILD was set in MDD, as recommended by the current international guidelines at that time [22, 23]. A written informed consent was obtained from all participants. This study protocol was approved by the Research Ethics Committee of the Northern Savo Hospital district (statement 80/2014) and the Tampere University Hospital (R15149). This study was conducted in compliance with the Declaration of Helsinki. The collection of the data is described in detail in the earlier publication [21].
For this study, age was calculated at the time when the questionnaires were filled in. Smoking status was recorded in the first visit. Chronic cough comorbidities were recorded in the first appointment or from the patients records. Gender-Age-Physiology (GAP) index is a widely utilized tool to predict the risk of death in IPF. In the present study, we used a modification of GAP in which the MDD-assessed diagnosis of ILD was added to the traditional variables gender, age, and lung physiological variables [24].The extents of several specific HRCT patterns were assessed separately in three zones of each lung by two experienced radiologists. Ground-glass opacities (GGO), honeycombing, and reticulation were graded from 0 to 4 with a maximum score of 24 as follows: 0 = finding absent, 1 = minor peripheral scattered changes, 2 = uniform peripheral or minor central changes, 3 = substantial peripheral changes that penetrated deeply to the lung parenchyma, 4 = very abundant peripheral and central changes. Traction bronchiectasis, and emphysema were graded from 0 to 3 with a maximum score of 18 as follows: 0 = absent, 1 = single scattered changes, 2 = large single changes or several minor changes, 3 = uniform or substantial changes. The interobserver mean score was calculated as a mean score by the sum of six zones for two radiologists. Histology of the TBLC samples were re-analyzed by two experienced pulmonary pathologists, and the presence of fibroblast foci, fibrosis, giant cells, granulomas, and honeycombing were recorded.
Questionnaires
The LCQ is a validated, reproducible, C-QoL questionnaire. It contains 19 questions of which each is scored from 1 to 7 with one indicating the most severe impairment. The LCQ total score ranges from 3 to 21, representing the sum of the three different domains: physical, psychological, and social impacts of cough [25].
The SGRQ is a validated, reproducible, 50-item QoL questionnaire originally developed to measure the overall QoL in obstructive airway diseases [26] and later validated and utilized also in ILDs [27, 28]. It includes questions about respiratory symptoms frequency and severity, activities that cause or are limited by breathlessness and social functions, and psychological disturbances. SGRQ also includes questions about cough. The total score ranges from 0 to 100, the higher score indicating the lower QoL.
Statistical analysis
The LCQ total score was the primary end point of the study and the SGRQ the secondary end point. These parameters were not normally distributed (Kolmogorov-Smirnov test) and non-parametric tests were used. The data is presented as frequencies with percentages or medians and interquartile ranges. Bivariate associations were investigated utilizing Spearman correlation coefficient or Mann-Whitney-U test when appropriate. The independent variables for the multivariable models were selected on two grounds. First, by previous knowledge about the association between a variable and the end points, and second, by the documented statistically significant bivariate association in the present material. Median multivariate regression analysis was used because parameters were not normally distributed. P < 0.05 was accepted as a level for statistical significance. All data was analyzed using IBM SPSS Statistics version 29.0.1.0.
Results
The characteristics of the patients are presented in Table 1. The study population (n = 111) was male dominant, slightly overweight, and with mildly impaired FVC and DLCO. Most of the patients suffered from IPF (63%), and belonged to ILD-GAP index stages 1–2, while only 15% of patients were in stage 3, and no patients in stage 4 (Table 1). In the bivariate analyses, the LCQ total score showed an inverse association with BMI, histological presence of granulomas, and radiological GGO mean score. The LCQ total score was higher in ever smokers than in never smokers. The SGRQ total score correlated with BMI, DLCO, and radiological GGO mean score (Table 2). Other variables, as revealed in the Table 1, showed no statistically significant associations with the end points. The LCQ total score and the SGRQ total score correlated statistically significantly (Spearman’s correlation coefficient − 0.645, p < 0.001).
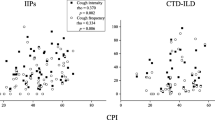
In the median multivariate regression analyses, GGO, BMI, granulomas and current smoking showed independent associations with the LCQ total score (Table 3a) (Figs. 1 and 2, and 3). BMI and DLCO showed independent associations with the SGRQ total score, whereas GGO, and angiotensin converting enzyme (ACE) inhibitor use did not quite reach the statistical significance (Table 3b). The presence of granulomas was seen in eight ILD patients with the following MDD diagnosis: chronic hypersensitivity pneumonitis (CHP) (N = 4), unclassified ILD (N = 2), sarcoidosis (N = 1), and NSIP (N = 1).
Table 3a and 3b. The median multivariate regression analyses about the determinants of the LCQ (3a) and the SGRQ (3b).
In each analysis, the independent variables were age, gender, BMI, current smoking, FVC, DLCO, ground glass opacities, presence of granulomas, presence of comorbidities and the use of ACE inhibitors. The tables present the final steps of the backward-directed analyses indicating the variables showing at least suggestive (p < 0.1) independent associations.
Discussion
In the present study, we prospectively investigated histological, radiological and physiological determinants of C-QoL in ILD patients who underwent TBLC. For the first time, C-QoL was associated with radiological GGO and presence of granulomas. C-QoL was also associated with BMI and current smoking status. The LCQ did not show any relationship with lung functions or BAL total cell count in this study population. Furthermore, the QoL measured by the SGRQ was associated with BMI, and DLCO.
The LCQ total score was associated with BMI in the bivariate and in the median multivariate regression analysis. The impact of BMI was clinically significant since the difference in the median LCQ total score between the fourth quartile (BMI > 31.11) and the second quartile (BMI 26.41–28.1) was over 4 points and the minimal important difference measured by the LCQ is 1.3 points [29]. In our study, overweight in ILD patients increased the risk of having poorer C-QoL and overall QoL. Our results are in line with an earlier study showing that the risk of chronic cough is two to three times higher in obese individuals in general population [30]. Furthermore, in the earlier studies on ILD a suggestive positive association between BMI and cough have been shown [9], as well as, overweight (BMI 25–29.9), and obesity (BMI 30–34.9) have been associated with lower pulmonary function, worse dyspnea and poorer QoL compared to subjects with normal weight [31]. Also, in sarcoidosis patients’ lower QoL, measured by the LCQ, were associated with higher BMI [32].
A recent study suggested a potential association between obesity and ILD revealing that greater amount of pericardial and abdominal visceral adipose tissue were associated with CT measures of early lung injury and lower FVC which may represent a modifiable risk factor for ILD [33]. Kim et al. showed that overweight and obese individuals have lower circulating levels of serum adiponectin and higher circulating levels of proinflammatory adipokines compared with normal weight adults [34]. These mediators are associated with higher attenuation areas and fibrosis in CT scans and with lower lung functions. Individuals with lower circulating adiponectin may be more vulnerable to other mechanisms of repetitive lung injury (e.g., acid reflux, infection, smoking), leading to recurrent injury and abnormal remodelling [34]. GERD in ILD population is a common comorbidity which might be one reason behind the cough. According to the recent study, 51–73% of the ILD patients suffered from GERD [35, 36]. In ILD, higher transdiaphragmatic pressure and low esophagogastric junction pressure and also dissociation between the pressure’s temporal relationship, may explain the high relationship with GERD in ILD [36]. Although, weight loss and lower BMI were associated with mortality in ILD population [37].
For the best of our knowledge, we were able to show that GGO was associated with C-QoL for the first time. We believe that the impact of GGO is clinically significant, since the median LCQ in the fourth quartile was over 4 points lower than that in the first and second quartiles (Fig. 3) perceiving that the minimum clinically important difference of the LCQ total score is 1.3 [29]. Ground-glass refers to an area of increased attenuation that does not completely obscure the underlying structures [38]. The finding is nonspecific, as ground-glass can be caused by any abnormal process that replaces alveolar air by fluid or cells, for example, edema, inflammation, an increase in capillary blood volume, histologic fibrosis, a lepidic pattern of adenocarcinoma, or a combination the above (38). Therefore, we cannot suggest the exact mechanisms linking the GGO with poor C-QoL.
In our study we showed for the first time, that histological presence of granulomas was associated with the impairment of C-QoL. The difference between patients with and without granulomas was over 4 points measured by the LCQ total score median, which made it a clinically significant result (Fig. 2) [29]. There was also bivariate association between the SGRQ and granulomas, probably because the SGRQ included cough questions, although statistical correlation was not seen in the median multivariate regression analysis. Granulomas are unspecific lesions whose appearance do not reveal the etiology or disease unless the inciting agent can be identified within the granuloma [39], and MDD approach plays the key role when diagnosing granulomatous diseases especially in the field of ILD [39]. In a study including 103 patients with HP, 97% reported cough [40]. 52 patients underwent transbronchial lung biopsy or endobronchial lung biopsy and of them, 30 patients had ill-defined granulomas in histological specimen [40]. In sarcoidosis, it has been shown that neurotrophins and their receptors are stained in granulomas and alveolar macrophages [41]. Still, association between cough and neurotrophins remains unproven [42]. In our study histological findings causing architectural distortion (honeycombing or fibrosis) did not correlate with C-QoL. Granulomas in the lung tissue are usually seen in several processes such as sarcoidosis, pulmonary lymphoid lesions as lymphomatoid granulomatosis (LYG), granulomatous lymphocytic interstitial lung disease (GLILD), aspiration, exposure associated diseases as HP, vasculitis and in collagen vascular disorders [43]. In our study population, granulomas were found in sarcoidosis, chronic hypersensitivity pneumonitis, NSIP, and unclassified ILD patients.
Current smokers seemed to have better C-QoL than ever smokers. This finding is probably explained by the “healthy smoker effect” and it has been described in various lung disorders [44] including IPF [45]. In these studies, current smokers had milder disease than former smokers. This effect indicates that patients continue the smoking habits, because they do not get serious symptoms from smoking and their lungs are relatively resistant to it.
This study did not show association with C-QoL and lung function tests or BAL findings. Chronic cough patients with ILD have shown positive association with lung function decline, severity of the lung disease and 6 min walking test in the earlier study with a large study population [9]. However, there is also a large study on ILD-patients in which C-QoL showed no correlation with lung function indices [46]. Another large study with the population of 1447 ILD patients showed correlation between lung functions and C-QoL in baseline but not in 12 months follow up [14]. Also, in pulmonary sarcoidosis lung functions showed no correlation to cough [47]. To our knowledge, association between C-QoL and BAL findings have not been studied earlier.
Our study has several limitations. We used LCQ as a measure of C-QoL in ILD population. LCQ is well validated cough specific quality of life questionnaire, and it is validated in COPD [48], non-cystic fibrosis bronchiectasis [49], and non-tuberculous mycobacterial lung-disease [50], but not in IPF or ILD. However, the LCQ has been used in ILD and IPF patients [14]. Secondly, our study population was quite small, 62% of the population were IPF patients, and the patient material was at early stage of the disease. The mechanisms of the impairment in the C-QoL may be different in more advanced ILDs. Thirdly, there were only eight patients with granulomatous disease which was associated with C-QoL.
Conclusion
Among patients with early stage ILD, the impairment in C-QoL was mainly associated with the presence of granulomas in TBLC samples, GGO in HRCT and high BMI. Impairment of the overall QoL, measured by the SGRQ, was mainly associated with high BMI, age and GGO seen in HRCT. This was the first study to find association between granulomas and C-QoL in ILD population. In the future, these results should be controlled in a larger population, surgical lung biopsies and more advanced disease.
Data availability
Availability of data and materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACE:
-
Angiotensin converting enzyme
- BAL:
-
Bronchoalveolar lavage
- BMI:
-
Body mass index
- CHP:
-
Chronic hypersensitivity pneumonitis
- COP:
-
Cryptogenic organizing pneumonia
- CTD-ILD:
-
Connective tissue disease associated interstitial lung disease
- C-QoL:
-
Cough related quality of life
- DLCO:
-
Diffusion capacity to carbon monoxide
- ECHO:
-
Echocardiogram
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- GERD:
-
Gastro-esophageal reflux disease
- GGO:
-
Ground-glass opacity
- HRCT:
-
High-resolution computed tomography
- ILD:
-
Interstitial lung disease
- ILD-GAP:
-
Index interstitial lung disease Gender-Age -Physiology -Index
- IPF:
-
Idiopathic pulmonary fibrosis
- KUH:
-
Kuopio university Hospital
- LCQ:
-
Leicester cough questionnaire
- NSIP:
-
Nonspecific interstitial pneumonia
- MDD:
-
Multidisciplinary discussion
- QoL:
-
Quality of life
- RB-ILD:
-
Respiratory bronchiolitis interstitial lung disease
- SGRQ:
-
The St George’s Respiratory Questionnaire
- TAUH:
-
Tampere university hospital
- TBLC:
-
Transbronchial lung cryobiopsy
References
American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304.
Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48.
Fujisawa T, Akiyama N, Morita T, Koyauchi T, Matsuda Y, Mori M, et al. Palliative care for interstitial lung disease: a nationwide survey of pulmonary specialists. Respirology. 2023;28(7):659–68.
Saari E, Mononen M, Hasala H, Lätti A, Kaulamo J, Nurmi H, et al. Characteristics of idiopathic pulmonary fibrosis -associated cough. A case-control study. BMC Pulm Med. 2023;23(1):179.
Ryerson CJ, Abbritti M, Ley B, Elicker BM, Jones KD, Collard HR. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology. 2011;16(6):969–75.
Glaspole IN, Chapman SA, Cooper WA, Ellis SJ, Goh NS, Hopkins PM, et al. Health-related quality of life in idiopathic pulmonary fibrosis: data from the Australian IPF Registry. Respirology. 2017;22(5):950–6.
Nardi A, Brillet PY, Letoumelin P, Girard F, Brauner M, Uzunhan Y, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38(6):1368–73.
Kovacova E, Vysehradsky R, Kocan I, Plevkova J, Buday T. Retrospective study of factors potentially influencing occurrence of Cough in Slovak patients with Sarcoidosis. Can Respir J. 2019;2019:3808206.
Lan NSH, Moore I, Lake F. Understanding cough in interstitial lung disease: a cross-sectional study on the adequacy of treatment. InternMedJ. 2020;(Journal Article).
Pan L, Liu Y, Sun R, Fan M, Shi G. Comparison of characteristics of connective tissue disease-associated interstitial lung diseases, undifferentiated connective tissue disease-associated interstitial lung diseases, and idiopathic pulmonary fibrosis in Chinese Han population: a retrospective study. Clin Dev Immunol. 2013;2013:121578.
Rajala K, Lehto JT, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T. Marked deterioration in the quality of life of patients with idiopathic pulmonary fibrosis during the last two years of life. BMC Pulm Med. 2018;18(1):172.
Bloem AEM, Houben-Wilke S, Mostard RLM, Stoot N, Janssen DJA, Franssen FME, et al. Respiratory and non-respiratory symptoms in patients with IPF or sarcoidosis and controls. Heart Lung. 2023;61:136–46.
Kreuter M, Swigris J, Pittrow D, Geier S, Klotsche J, Prasse A, et al. The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time: longitudinal data from the INSIGHTS-IPF registry. Respir Res. 2019;20(1):59.
Lee J, White E, Freiheit E, Scholand MB, Strek ME, Podolanczuk AJ, et al. Cough-specific quality of Life predicts Disease Progression among patients with interstitial lung disease: data from the Pulmonary Fibrosis Foundation Patient Registry. Chest. 2022;162(3):603–13.
Jones RM, Hilldrup S, Hope-Gill BD, Eccles R, Harrison NK. Mechanical induction of cough in idiopathic pulmonary fibrosis. Cough. 2011;7(Journal Article):2–2.
Hope-Gill BD, Hilldrup S, Davies C, Newton RP, Harrison NK. A study of the cough reflex in idiopathic pulmonary fibrosis. AmJRespirCritCare Med. 2003;168(8):995–1002.
Harrison NK. Cough, sarcoidosis and idiopathic pulmonary fibrosis: Raw nerves and bad vibrations. Cough [Internet]. 2013;9(1). https://www.scopus.com/inward/record.uri?eid=2-s2.0-84874523402&doi=10.1186%2f1745-9974-9-9&partnerID=40&md5=8cb1f1176b89ae71e1ed6b0c57590b9c
Scholand MB, Wolff R, Crossno PF, Sundar K, Winegar M, Whipple S, et al. Severity of cough in idiopathic pulmonary fibrosis is associated with MUC5 B genotype. Cough. 2014;10(Journal Article):3–3. eCollection 2014.
Saunders P, Maher TM. Cough in fibrotic lung disease: an unresolved challenge. Respirology. 2017;22(8):1491–2.
Bargagli E, Di Masi M, Perruzza M, Vietri L, Bergantini L, Torricelli E, et al. The pathogenetic mechanisms of cough in idiopathic pulmonary fibrosis. Intern Emerg Med. 2018;14(1):39–43.
Mononen M, Saari E, Hasala H, Kettunen HP, Suoranta S, Nurmi H, et al. Risk factors of clinically significant complications in transbronchial lung cryobiopsy: a prospective multi-center study. Respir Med. 2022;200:106922.
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. AmJRespirCritCare Med. 2011;183(6):788–824.
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(5):e44–68.
Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145(4):723–8.
Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MDL, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339–43.
Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med. 1991;85 Suppl B:25–31; discussion 33–37.
Furukawa T, Taniguchi H, Ando M, Kondoh Y, Kataoka K, Nishiyama O, et al. The St. George’s respiratory questionnaire as a prognostic factor in IPF. Respir Res. 2017;18(1):18.
Suzuki A, Kondoh Y, Swigris JJ, Ando M, Kimura T, Kataoka K et al. Performance of the St George’s respiratory questionnaire in patients with connective tissue disease-associated interstitial lung disease. Respirology. 2018.
Raj AA, Pavord DI, Birring SS. Clinical cough IV:what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol. 2009;187:311–20.
Landt EM, Çolak Y, Nordestgaard BG, Lange P, Dahl M. Risk and impact of chronic cough in obese individuals from the general population. Thorax. 2022;77(3):223–30.
Schaeffer MR, Kumar DS, Assayag D, Fisher JH, Johannson KA, Khalil N, et al. Association of BMI with pulmonary function, functional capacity, symptoms, and quality of life in ILD. Respir Med. 2022;195:106792.
Frye BC, Potasso L, Farin E, Fichtner U, Birring S, Müller-Quernheim J, et al. Abnormal FeV1 and body mass index are associated with impaired cough-related quality of life in sarcoidosis patients. Respir Med. 2021;188:106600.
Anderson MR, Kim JS, Allison M, Giles JT, Hoffman EA, Ding J, et al. Adiposity and Interstitial Lung Abnormalities in Community-Dwelling adults: the MESA Cohort Study. Chest. 2021;160(2):582–94.
Kim JS, Anderson MR, Podolanczuk AJ, Kawut SM, Allison MA, Raghu G, et al. Associations of serum adipokines with subclinical interstitial lung disease among Community-Dwelling adults: the multi-ethnic study of atherosclerosis (MESA). Chest. 2020;157(3):580–9.
Baqir M, Vasirreddy A, Vu AN, Moua T, Chamberlain AM, Frank RD, et al. Idiopathic pulmonary fibrosis and gastroesophageal reflux disease: a population-based, case-control study. Respir Med. 2021;178:106309.
Joshua J, Pathak C, Zifan A, Chen R, Malhotra A, Mittal RK. Selective dysfunction of the crural diaphragm in patients with chronic restrictive and obstructive lung disease. Neurogastroenterol Motil. 2023;e14699.
Comes A, Wong AW, Fisher JH, Morisset J, Johannson KA, Farrand E, et al. Association of BMI and change in Weight with mortality in patients with fibrotic interstitial lung disease. Chest. 2022;161(5):1320–9.
Bankier AA, MacMahon H, Colby T, Gevenois PA, Goo JM, Leung ANC, et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2024;310(2):e232558.
Rosen Y. Pathology of Granulomatous Pulmonary diseases. Arch Pathol Lab Med. 2022;146(2):233–51.
Kumar R, Spalgais S, Ranga V. Hypersensitivity pneumonitis: clinical, radiological and pathological profile of 103 patients from North India. Monaldi Arch Chest Dis. 2020;90(3).
Dagnell C, Grunewald J, Kramar M, Haugom-Olsen H, Elmberger GP, Eklund A, et al. Neurotrophins and neurotrophin receptors in pulmonary sarcoidosis - granulomas as a source of expression. Respir Res. 2010;11(1):156.
Koskela HO, Purokivi MK, Romppanen J. Neurotrophins in chronic cough: association with asthma but not with cough severity. Clin Respir J. 2010;4(1):45–50.
Franquet T, Franks TJ, Galvin JR, Marchiori E, Giménez A, Mazzini S, et al. Non-infectious granulomatous lung disease: imaging findings with pathologic correlation. Korean J Radiol. 2021;22(8):1416–35.
Cerveri I, Cazzoletti L, Corsico AG, Marcon A, Niniano R, Grosso A, et al. The impact of cigarette smoking on asthma: a population-based international cohort study. Int Arch Allergy Immunol. 2012;158(2):175–83.
Antoniou KM, Hansell DM, Rubens MB, Marten K, Desai SR, Siafakas NM, et al. Idiopathic pulmonary fibrosis: outcome in relation to smoking status. Am J Respir Crit Care Med. 2008;177(2):190–4.
Cheng JZ, Wilcox PG, Glaspole I, Corte TJ, Murphy D, Hague CJ, et al. Cough is less common and less severe in systemic sclerosis-associated interstitial lung disease compared to other fibrotic interstitial lung diseases. Respirology. 2017;22(8):1592–7.
Sinha A, Lee KK, Rafferty GF, Yousaf N, Pavord ID, Galloway J, et al. Predictors of objective cough frequency in pulmonary sarcoidosis. Eur Respir J. 2016;47(5):1461–71.
Berkhof FF, Boom LN, ten Hertog NE, Uil SM, Kerstjens HAM, van den Berg JWK. The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes. 2012;10:4.
Murray MP, Turnbull K, MacQuarrie S, Pentland JL, Hill AT. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J. 2009;34(1):125–31.
Takao S, Tabusadani M, Yamane K, Kakuta T, Kuroyama Y, Mori K, et al. Is the Leicester Cough Questionnaire useful for nontuberculous mycobacterial lung disease? Respir Investig. 2021;59(1):120–5.
Acknowledgements
Acknowledgements: The authors wish to thank Tuomas Selander for statistical advice and Leena Tuomisto, Elsa Nylund, Johan Söderström, Aki Vainio, Heikki Pautola, Sumu Lehtola, Markku Pekonen, Päivi Torkko, Anssi Ukkonen, Severi Seppälä, Tiina-Mari Paulow, Minna Tommola, Kirsi Hämäläinen and Satu Nenonen for aiding with the data collection.
Funding
This study was supported by the Foundation of the Finnish Anti-Tuberculosis Association, Väinö and Laina Kivi Foundation, Jalmari and Rauha Ahokas Foundation and The Respiratory Foundation of the Kuopio Region. The funding had no role in the design of the study neither in collection, analysis, or interpretation of data or the writing up of the manuscript.
Author information
Authors and Affiliations
Contributions
E.S. is the guarantor of this paper and takes responsibility for the integrity of the work, from inception to published article. E.S., R.K., M.P., and H.O.K., contributed to study design. E.S., M.M., H.H., H.N., H-P.K., S.S., E.L-B., R.K., and M.P. contributed to data collection. H-P.K., and S.S., contributed to HRCT interpretation. E.L-B., and R.K. contributed to histological interpretation. E.S. and H.O.K. contributed to data analysis. E.S., M.M., H.H., H.N., S.S., R.K., M.P. and H.O.K., contributed to manuscript preparation. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocols were approved by the Research Ethics Committee of the Northern Savo Hospital district (statement 80/2014), and Tampere University Hospital (R15149). The studies were conducted in compliance with the Declaration of Helsinki (as revised in 2013). A written informed consent was obtained.
Consent for publication
Not applicable.
Competing interests
Competing interests: E.S. congress travel costs from Sanofi Finland, Orion Pharma and Astra Zeneca, virtual congress costs from Roche, owns personal stocks from Orion Pharma Ltd, Board member of Finnish allergology- and immunology association outside the submitted work. M.M. personal consulting fee and lecture fee from Boehringer Ingelheim and Astra Zeneca, congress travel and admission cost from Boehringer Ingelheim outside the submitted work. H.H. personal lecture fee from Boehringer Ingelheim outside the submitted work. H.N. personal lecture fees from Boehringer Ingelheim, Duodecim and Nordic Health Group, congress travel costs from Boehringer Ingelheim, and Duodecim outside the submitted work. S.S. owns personal stocks from Merck & Co, CRISPR Therapeutics, 3 M, Organon & Co, Pfizer outside the submitted work. H-P.K. personal lecture fee from Finnish thoracic radiologist society outside the submitted work. R.K. personal consulting fee from Boehringer Ingelheim and MSD and lecture fee from Boehringer Ingelheim, virtual congress travel costs from Novartis, Board member of the Finnish Lung Health Association and the Finnish Medical Foundation outside the submitted work. MP: personal lecture fee from Boehringer Ingelheim and Hengitysyhdistys, advisory board member of Boehringer Ingelheim outside the submitted work. HOK: personal lecture fee from Boehringer Ingelheim, MSD, Chiesi Pharma, owns personal stocks from Orion Pharma Ltd outside the submitted work. E.L-B. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saari, E., Mononen, M., Hasala, H. et al. Determinants of cough-related quality of life in interstitial lung diseases. BMC Pulm Med 24, 427 (2024). https://doi.org/10.1186/s12890-024-03218-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03218-z