Abstract
Background
In Sweden, most children with slipped capital femoral epiphysis (SCFE) are operated on with a single smooth pin or a short-threaded screw, allowing further growth of the femoral neck. Using the Swedish Pediatric Orthopaedic Quality registry, SPOQ, we investigated whether angular remodelling occurs adjacent to the proximal femoral epiphysis after fixation of SCFE using implants, allowing continued growth of the femoral neck.
Methods
During 2008–2010 a total national population of 155 children were reported to the SPOQ registry. Following our strict inclusion criteria, radiographs of 51 hips were further assessed. The lateral Head Shaft Angle (HSA), the Nötzli 3-point α-angle, the anatomic α-angle, and the Anterior Offset Ratio (AOR) on the first postoperative radiographs and at follow-up were measured to describe the occurrence of remodelling. Slip severity was categorised as mild, moderate or severe according to postoperative HSA.
Results
Mean and SD values for the change in HSA were 3,7° (5,0°), for 3-point α-angle 6,8° (8,9°), and anatomic α-angle 13,0° (16,3°). The overall increase in AOR was 0,038 (0.069). There were no significant differences between the slip severity groups.
Conclusions
We found limited angular remodelling after in situ fixation with smooth pins or short threaded screws for SCFE. The angular remodelling and the reduction of the CAM deformity was less than previously described after fixation of SCFE with similar implants. Results about the same magnitude with non-growth sparing techniques suggest that factors other than longitudinal growth of the femoral neck are important for angular remodelling.
Similar content being viewed by others
Introduction
Slipped Capital Femoral Epiphysis (SCFE) is Sweden´s most common adolescent hip disease, with an incidence rate of 5 per 10 000 children aged 9–15 [1]. In SCFE, the metaphysis slips in relation to the proximal femoral physis. With increasing slip angles, the anterosuperior head-neck junction will be more convex than the normal concave shape. This prominent shape of the femoral neck metaphysis, called CAM-deformity, increases the risk of symptomatic femuro-acetabular impingement (FAI) [2]. FAI is a mechanical conflict between the head-neck junction and the acetabular rim with hip flexion resulting in subsequent damage to the labrum and the acetabular cartilage [3].
With FAI after SCFE, the cartilage damage is seen early, and the severity seems worse in hips with intermediate slip angles [4].
Surgery with fixation in situ is the most commonly used method to prevent slip progress and minimise the CAM-prominence after SCFE. Generally, a percutaneous screw with threads crossing the physis to achieve epiphysiodesis is used. However, epiphysiodesis might cause abnormal hip anatomy. In addition, with a CAM deformity, the short neck might interfere with the hip’s normal mechanics [5]. Therefore, most children in Sweden with SCFE are operated upon with a single smooth pin or a short-threaded screw that reliably prevents slip progress and allows continued growth of the femoral neck [6,7,8] (Fig. 1).
Remodelling of bone is a complex process to repair and replace old bone and regain a more normal shape of the affected bone [9]. Remodelling is suggested to occur after in situ fixation of SCFE regardless of the surgical method used [10,11,12]. The early phase of SCFE remodelling occurs by osteoclastic resorption of the anterosuperior femoral metaphysis and callus formation at the inferior-posterior angle between the head and neck [13, 14].
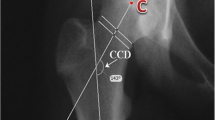
The lateral head shaft angle (HSA), as defined by Southwick [15], is the most common way to measure epiphyseal angulation (Fig. 2). Measures to quantify the CAM deformity include the anterior offset ratio (AOR) [16, 17] and the α-angle (Fig. 2). Although first described by Nötzli [18] on radial magnetic resonance imaging, the α-angle is now commonly measured on lateral radiographs [19, 20], as is the AOR [21]. Two ways for measuring the α-angle are described, the 3-point method (α3p) as defined by Nötzli [18, 22, 23] and the anatomic method (αA) described by Bouma [24]. In a normal hip, α3p will equal the αA.
(A) The lateral head shaft angle (HSA) is the angle between the femoral shaft axis and a line perpendicular to a line connecting the femoral head’s physeal edges. (B) The 3-point α-angle method as defined by Nötzli (α3p). Place a circle over the femoral head’s bony contour and another circle over the femoral neck’s narrowest part. Draw a line connecting the centre of these two circles. Then draw a line from the femoral head’s centre to where the head-neck contour exits the femoral head circle. The α3p is the angle between these two lines. (C) The anatomic α-angle (αA) as described by Bouma. Determine the axis of the femoral neck by placing three circles touching the contour of the femoral neck. Draw a line through the centres of the neck circles to decide the anatomic femoral neck axis. The neck axis is translated to the centre point in the best-fit circle over the femoral head. A second line is drawn from the central point to where the head-neck contour first exits the femoral head circle. The αA is the angle between these two lines. (D) The anterior offset ratio. First, draw a line through the centre of the femoral neck. Then two parallel lines are drawn along the anterior edge of the neck and the femoral head. The distance between the latter (AO) divided by the femoral head diameter (d) gives the anterior offset ratio (AOR), also called the head-neck offset ratio (HNOR)
A mean angular remodelling of 9 degrees using a single smooth pin was recently reported [19]. In addition, other European studies with similar implants suggest that angular remodelling does occur [25, 26].
There is no consensus on whether remodelling will alter the shape of the hip after fixation in situ and thereby prevent FAI symptoms. New treatment algorithms, including safe surgical dislocation with capital realignment, are gaining popularity [27,28,29,30].
The present study aimed to investigate whether angular remodelling, with a subsequent reduction of CAM-deformity, occurs adjacent to the proximal femoral epiphysis after fixation of SCFE using an implant allowing continued growth of the femoral neck. Data from the Swedish Pediatric Orthopaedic Quality (www.spoq.se) registry was used.
Methods
This study is based on the radiographs of all children (155 children, 171 hips) consecutively reported to the Swedish Pediatric Orthopaedic Quality (SPOQ) registry, 2008–2010. The registry compared its database with individual-based data from the Swedish National Board of Health and Welfare, utilising the Swedish National Patient Register. The study population included a total national population for the study period. Thus, the first author assessed the radiographs of all persons who had surgery because of SCFE in Sweden during these three years (MA). Each participant consented to inclusion in the Swedish Pediatric Orthopaedic Quality registry.
The radiographs were routine examinations from all Swedish hospitals that treated children with SCFE during the study period. Postoperative and follow-up radiographs were assessed using the standard picture archiving and communication system (IDS7, Sectra, Linköping, Sweden).
Inclusion criteria were SCFE surgery performed with one smooth hook-pin or one smooth short-threaded screw and frog-leg lateral (Lauenstein) postoperative and follow-up radiographs with at least eight months intervals. For each hip, we used radiograph pairing to identify unacceptable rotational differences between the postoperative and follow-up radiographs. The Southwick head shaft angle (HSA) assessment required no less than 2 cm of the femoral shaft visible [31]. To rule the radiographs as comparable or not, apart from the shaft length, was a judgement based on a combination of the following criteria: we did not accept a variance in the visible amount of the lesser trochanter, the direction of the hook of the Hansson hook pin, or other differences in the implant position.
Thus, radiographs with inconsistent quality or lateral view other than Lauenstein, surgery performed with other methods, primary intentional physeodesis, subsequent early surgery performed (e.g., proximal femoral osteotomy) or development of avascular necrosis were excluded.
Following our strict inclusion criteria, the study material included radiographs of 51 hips. Each hip was given a unique number, thus blinding the observer to age and name. The first author assessed postoperative and follow-up radiographs twice, with a minimum interval of one month. The first measurement findings were unavailable for the observer during the second measurement. Therefore, each hip was presented with the mean of the two measurements. The baseline measurements were done on the first postoperative radiograph. All follow-up radiographs had signs of finished growth at the femoral neck and, importantly, were of the same frog-leg lateral projection as the immediate postoperative control.
The lateral head-shaft angle (HSA) was used as defined by Southwick [15]. The slip severity was classified according to the baseline HSA (mild < 30°, moderate 30–60°, and severe > 60°) [12, 19]. The AOR and lateral offset angle (α-angle) describe the CAM deformity of the head-neck junction. The anatomic method (αA) described by Bouma [24] was compared with the 3-point method (α3p) as defined by Nötzli [18, 22, 23] (Fig. 2).
When comparing slip severity groups according to baseline HSA, the hips excluded from further study were not significantly different from those included for further research. Slip severity at baseline HSA for the excluded group was comparable with the group included in the study: mild (mean HSA 19,0°), moderate (mean HSA 42,0°) and severe (mean HSA 66,0°). Median age at surgery was 12.4 years in the excluded group and 12.5 years in the study group. We believe the study group represents the whole group of patients.
Statistics
Results are presented as mean values with range or standard deviation. We set the significance threshold at 0.05. The Student t-test (independent samples) was used to compare means for postoperative and follow-up variables for gender and age groups. The paired sample t-test was used to compare the means of postoperative and follow-up variables. The Pearson correlation coefficient was used to test the correlation between HSA, the two alpha angle methods, HSA at follow-up, AOR, and age at surgery. ANOVA with post hoc Tukey test was used to compare variables with the groups of different slip severity. Intra-rater reliability (ICC) and 95% confidence interval (CI) are based on an absolute-agreement, 2-way mixed-effects model for single measures. Variation of intraobserver measurements was also presented as Bland-Altman plots. The statistical analysis was performed using SPSS Statistics (version 25; IBM, Armonk, NY).
Results
Matching radiographs of good quality were found in 51 hips (48 children; 3 bilateral). The fixation method used was one smooth hook-pin (n = 28) or one smooth short-threaded screw (n = 23). The type of implant did not significantly affect the measured variables.
We studied radiographs of 18 boys and 30 girls with a median age of 12.5 years at surgery, girls 11.6 years (range; 9.5–14.6) and boys 13.9 years (range; 11.2–14.6) (p < 0,001). The median interval between postoperative and follow-up radiographs was 25.2 months (range; 9.6–68.4). Except for age at surgery, we found no statistical differences between boys and girls regarding HSA, α3A, α3p, or AOR at baseline.
Between the baseline and follow-up radiographs, the mean reduction of HSA was 3.7° (SD 5°), the mean decrease in α3p was 6.8° (SD 8.9°), the mean reduction of αA was 13° (SD 16.3°), and the mean increase of the AOR was 0,038 (SD 0.069). The differences found were statistically significant (p < 0.001). Table 1.
Intra-observer variation for the HSA measurements was assessed using the mean difference, with its 95% limits of agreement [31, 32]. For the postoperative HSA, the intra-observer difference was 0.8°, and for the HSA at follow-up the difference was 0.2° (Figs. 3 and 4). The intra-observer reliability for the HSA was excellent, with an ICC of 0.97 (CI 0.95–0.98).
According to baseline HSA, there were 19 mild (mean HSA 21,1°), 26 moderate (mean HSA 43,7°) and six severe (mean HSA 71,1°) slips. The mean HSA did decrease during growth, but it did not differ significantly between the groups of severity (Fig. 5).
The difference between the three groups of slip severity concerning HSA, αA, and AOR was statistically significant at baseline. However, for the postoperative α3p, the difference between the moderate and severe groups of slip severity was not statistically significant. There were no significant differences between the slip severity groups regarding reducing HSA, α3p, αA, or the increase of AOR (Table 2).
There was a significant inverse correlation between the postoperative HSA and AOR at follow-up (R=-0.69, p < 0.001) as well as a significant correlation between postoperative HSA and both methods to measure the α-angle at follow-up (α3p; R = 0,68 and αA; R = 0,71, p < 0.001), (Fig. 6).
Fourteen children were ten years of age or younger, and 11 children were 14 years of age or older at surgery. The mean follow-up time between the age groups differed significantly. The mean HSA at baseline was higher in the older group (34,6° and 47,3°, respectively) (p = 0,07). At follow-up, the mean HSA was significantly lower in the younger age group (28,9° and 42,5°, respectively) (p = 0,05). The reduction of HSA, α3p, αA, and the increase of AOR was larger for the younger group but not statistically significant. (Table 3; Fig. 7).
Discussion
Compared to other studies of surgery for SCFE with growth-sparing techniques, we found statistically significant (p < 0.001) but only limited angular remodelling. Wensaas and Svenningsen found an average HSA remodelling of 15°, Guzzanti et al. found 13,5°, and Örtegren et al. found 9° compared to our 3,7° [19, 25, 26]. Our strict inclusion criteria, for eligible hips to be assessed, might explain this difference.
Unlike Örtegren et al. [19], we did not find statistically significant differences regarding the reduction of HSA between the different slip severity groups. In the study by Örtegren et al., only three hips were included in the severe group showing a mean HSA reduction of as much as 31,3°, whereas our six severe hips remodelled by a mean of 6,5°.
We don´t believe remodelling is clinically important for the group with a mild slip. The group with a severe slip has a high risk for symptomatic FAI, with a remodelling to a mean of 64,6° HSA. Whether the slight mean HSA decrease of 4° in the moderate group might reduce the risk for symptomatic FAI is unclear but unlikely. Accabled et al. found a significant correlation between slip severity and patient-reported outcome showing that HSA values exceeding 35° gave increased risk for symptomatic FAI [33]. Also, Nectoux et al. and Murgier et al. have pointed out the threshold of slip angles of 30–35° for symptomatic FAI to appear more regularly [34, 35].
The importance of continued longitudinal growth of the femoral neck for angular remodelling and, thereby, a decreased risk for symptomatic FAI is supported by Örtegren et al. [19]. With a growth-sparing technique, the femoral neck growth continues with 3–4 mm/year [7] to a total of 8,9–15,2 mm [8]. We did not measure the longitudinal growth of the femoral neck. However, angular remodelling about the same magnitude with non-growth sparing techniques suggests other factors’ importance [11, 12].
Remodelling depends on the remaining growth potential. Örtegren et al. found a statistically significant difference in HSA reduction between children younger than 11 years of age and children older than 13 [19]. We did the same analysis with the finding of a more minor and statistically not significant difference. Regardless of the age at surgery, we found strong correlations between HSA postoperatively and α3p, αA, AOR, and the HSA at follow-up. These findings support the importance of factors other than femoral neck growth for angular remodelling.
Our study showed a statistically significant difference between the severity groups at baseline regarding αA and AOR but not for the α3p. The better correlation at follow-up between AOR and αA, than for α3p, suggests that αA corresponds better to the anterior head-neck offset, as pointed out in other reports [24, 36].
We found that assessing the morphology of the SCFE hip was simplified using the anatomical alpha angle rather than the 3-point method. The αA correlates better with the AOR as both methods reference the femoral neck axis. For hips with poor head-neck offset, i.e. higher HSA, the alpha angle will increase accordingly. Therefore, HSA and the αA probably better judge the risk of symptomatic FAI connected to SCFE than the α3p does, which is also pointed out by Ucpunar et al. (39).
The gender distribution in our study included a higher percentage of girls than boys compared to the population-based study by Herngren et al. [1]. Therefore, we do not have any reason to believe that the remodelling is different between girls and boys.
Limitations
The exclusion of 72 hips was because of inconsistent quality and rotational error. The pair-wise inspection of the radiographs to ensure the same visibility of the lesser trochanter and identical implant position on the frog-leg views contributed to this. However, we became confident that we ended up with 51 pairs of radiographs with as small a rotational error as possible.
Another limitation is that only the first author made measurements. However, the first and second measurements were blinded, assessed randomly and made twice, at least one month apart. The excellent intraobserver reliability, with an ICC of 0.97 for the HSA, is a factor that makes us believe we have reliable measurements for the analysis [37].
The setup based only on radiographs available in a registry is a limitation. This retrospective study revealed that clinical practice regarding the type of radiographs and projections varied considerably in Sweden from 2008 to 2010. Many radiographs were of inferior quality and the type of projections used varied with time. The majority of exclusions were made because the correct and comparable measurements was not possible to make. Nevertheless, the study material was based on a total national population in Sweden during the study period chosen.
Conclusion
We found limited angular remodelling after in situ fixation with smooth pins or short threaded screws for SCFE. The angular remodelling and the reduction of the CAM deformity were less than previously described after the fixation of SCFE with similar implants.
Results about the same magnitude with non-growth sparing techniques suggest that factors other than longitudinal growth of the femoral neck are important for angular remodelling.
Data Availability
The datasets in this study are available from the corresponding author on reasonable request.
Abbreviations
- SCFE:
-
slipped capital femoral epiphysis
- SPOQ:
-
Swedish Pediatric Orthopaedic Quality registry
- HSA:
-
Head Shaft Angle
- α3p :
-
3-point α-angle
- αA :
-
Anatomic α-angle
- AOR:
-
Anterior Offset Ratio
- SD:
-
Standard deviation
- FAI:
-
Femuro-acetabular impingement
- ICC:
-
Intra-rater reliability
References
Herngren B, Stenmarker M, Vavruch L, Hagglund G. Slipped capital femoral epiphysis: a population-based study. BMC Musculoskelet Disord. 2017;18(1):304.
Pollard TC, Villar RN, Norton MR, Fern ED, Williams MR, Simpson DJ, et al. Femoroacetabular impingement and classification of the cam deformity: the reference interval in normal hips. Acta Orthop. 2010;81(1):134–41.
Leunig M, Casillas MM, Hamlet M, Hersche O, Notzli H, Slongo T, et al. Slipped capital femoral epiphysis: early mechanical damage to the acetabular cartilage by a prominent femoral metaphysis. Acta Orthop Scand. 2000;71(4):370–5.
Ziebarth K, Leunig M, Slongo T, Kim YJ, Ganz R. Slipped capital femoral epiphysis: relevant pathophysiological findings with open Surgery. Clin Orthop Relat Res. 2013;471(7):2156–62.
Leblanc E, Bellemore JM, Cheng T, Little DG, Birke O. Biomechanical considerations in slipped capital femoral epiphysis and insights into prophylactic fixation. J Child Orthop. 2017;11(2):120–7.
Ortegren J, Bjorklund-Sand L, Engbom M, Siversson C, Tiderius CJ. Unthreaded fixation of slipped capital femoral epiphysis leads to continued growth of the femoral Neck. J Pediatr Orthop. 2016;36(5):494–8.
Holmdahl P, Backteman T, Danielsson A, Karrholm J, Riad J. Continued growth after fixation of slipped capital femoral epiphysis. J Child Orthop. 2016;10(6):643–50.
Hagglund G, Bylander B, Hansson LI, Selvik G. Bone growth after fixing slipped femoral epiphyses: brief report. J bone Joint Surg Br Volume. 1988;70(5):845–6.
Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010;11(4):219–27.
Jones JR, Paterson DC, Hillier TM, Foster BK. Remodelling after pinning for slipped capital femoral epiphysis. J bone Joint Surg Br Volume. 1990;72(4):568–73.
Wong-Chung J, Strong ML. Physeal remodeling after internal fixation of slipped capital femoral epiphyses. J Pediatr Orthop. 1991;11(1):2–5.
Bellemans J, Fabry G, Molenaers G, Lammens J, Moens P. Slipped capital femoral epiphysis: a long-term follow-up, with special emphasis on the capacities for remodeling. J Pediatr Orthop B. 1996;5(3):151–7.
Kallio PE, Foster BK, LeQuesne GW, Paterson DC. Remodeling in slipped capital femoral epiphysis: sonographic assessment after pinning. J Pediatr Orthop. 1992;12(4):438–43.
Moreau MJ. Remodelling in slipped capital femoral epiphysis. Can J Surg J canadien de chirurgie. 1987;30(6):440–2.
Southwick WO. Osteotomy through the lesser trochanter for slipped capital femoral epiphysis. J bone Joint Surg Am Volume. 1967;49(5):807–35.
Eijer H, Leunig M, Mahomed M, Ganz R. Cross-table lateral radiographs for screening of anterior femoral head-neck offset in patients with femoro-acetabular impingement. Hip Int. 2001;11(1):37–41.
Peelle MW, Della Rocca GJ, Maloney WJ, Curry MC, Clohisy JC. Acetabular and femoral radiographic abnormalities associated with labral tears. Clin Orthop Relat Res. 2005;441:327–33.
Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J bone Joint Surg Br Volume. 2002;84(4):556–60.
Ortegren J, Bjorklund-Sand L, Engbom M, Tiderius CJ. Continued growth of the femoral Neck leads to Improved Remodeling after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 2018;38(3):170–5.
Beaule PE, Hynes K, Parker G, Kemp KA. Can the alpha angle assessment of cam impingement predict acetabular cartilage delamination? Clin Orthop Relat Res. 2012;470(12):3361–7.
Clohisy JC, Carlisle JC, Beaule PE, Kim YJ, Trousdale RT, Sierra RJ, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J bone Joint Surg Am Volume. 2008;90(Suppl 4):47–66.
Loder RT. Unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2001;21(5):694–9.
Clohisy JC, Nunley RM, Otto RJ, Schoenecker PL. The frog-leg lateral radiograph accurately visualized hip cam impingement abnormalities. Clin Orthop Relat Res. 2007;462:115–21.
Bouma H, Slot NJ, Toogood P, Pollard T, van Kampen P, Hogervorst T. Where is the neck? Alpha angle measurement revisited. Acta Orthop. 2014;85(2):147–51.
Guzzanti V, Falciglia F, Stanitski CL. Slipped capital femoral epiphysis in skeletally immature patients. J bone Joint Surg Br Volume. 2004;86(5):731–6.
Wensaas A, Svenningsen S. [Slipped capital femoral epiphysis treated with a specially designed screw]. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin. ny Raekke. 2005;125(20):2788–90.
Passaplan C, Gautier L, Gautier E. Long-term follow-up of patients undergoing the modified Dunn procedure for slipped capital femoral epiphysis. Bone Jt Open. 2020;1(4):80–7.
Masquijo JJ, Allende V, D’Elia M, Miranda G, Fernandez CA. Treatment of slipped capital femoral epiphysis with the Modified Dunn Procedure: a Multicenter Study. J Pediatr Orthop. 2019;39(2):71–5.
Tannast M, Jost LM, Lerch TD, Schmaranzer F, Ziebarth K, Siebenrock KA. The modified Dunn procedure for slipped capital femoral epiphysis: the Bernese experience. J Child Orthop. 2017;11(2):138–46.
Venkatadass K, Durga Prasad V, Jain D, Al Ahmadi NMM, Rajasekaran S. Is there a role for controlled repositioning and mini-open primary osteoplasty in the management of unstable slipped capital femoral epiphysis? J Hip Preserv Surg. 2022;9(4):211–8.
Lehmann TG, Vetti N, Laborie LB, Engesaeter IO, Engesaeter LB, Rosendahl K. Intra- and inter-observer repeatability of radiographic measurements for previously slipped capital femoral epiphysis at skeletal maturity. Acta Radiol. 2013;54(5):587–91.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
Accadbled F, Murgier J, Delannes B, Cahuzac JP, de Gauzy JS. In situ pinning in slipped capital femoral epiphysis: long-term follow-up studies. J Child Orthop. 2017;11(2):107–9.
Nectoux E, Décaudain J, Accadbled F, Hamel A, Bonin N, Gicquel P. Evolution of slipped capital femoral epiphysis after in situ screw fixation at a mean 11 years’ follow-up: a 222 case series. Orthop Traumatol Surg Research: OTSR. 2015;101(1):51–4.
Murgier J, de Gauzy JS, Jabbour FC, Iniguez XB, Cavaignac E, Pailhé R, et al. Long-term evolution of slipped capital femoral epiphysis treated by in situ fixation: a 26 years follow-up of 11 hips. Orthop Rev (Pavia). 2014;6(2):5335.
Ucpunar H, Mert M, Camurcu Y, Buyuk AF, Cobden A, Sofu H. Validity of the alpha angle measurements on plain radiographs in the evaluation of cam-type femoroacetabular impingement in patients with slipped capital femoral epiphysis. Skeletal Radiol. 2019;48(11):1787–94.
Herngren B, Lindell M, Hagglund G. Good inter- and intraobserver reliability for assessment of the slip angle in 77 hip radiographs of children with a slipped capital femoral epiphysis. Acta Orthop. 2018;89(2):217–21.
Acknowledgements
Bo Rolander, PhD, statistician at the Futurum Academy for Health and Care, Jönköping county council, Sweden.
Funding
Funding was only by institutional grants and government funding for medical training and research in Sweden.
Open access funding provided by Linköping University.
Author information
Authors and Affiliations
Contributions
Study design: MA, BH, HT, and OR; data collection: MA and BH; data analysis: MA, BH, and OR; manuscript preparation: MA, BH, HT, and OR.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Furthermore, the study protocol was reviewed and approved by the Regional Ethical Review Board in Linköping, Sweden (registration number 2014/421 − 31). In addition, ethical approval was also obtained from the Regional Ethical Review Board in Lund, Sweden (registration number 2013/87) to assess radiographs from the Swedish Pediatric Orthopaedic Quality registry. The Regional Ethical Review Boards in Sweden are not affiliated with a university; hence work independently on a governmental assignment. Informed consent was obtained from parent, legal guardians and all individual participants included in the study.
Consent for publication
Consent for publication was obtained from each participant included in the Swedish Pediatric Orthopaedic Quality registry.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Anderson, M., Herngren, B., Tropp, H. et al. Limited angular remodelling after in-situ fixation for slipped capital femoral epiphysis. BMC Musculoskelet Disord 25, 11 (2024). https://doi.org/10.1186/s12891-023-07117-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-07117-y