Abstract
Objective
The relationship between thyrotropin (TSH), free triiodothyronine (FT3), free thyroxine (FT4) and diabetic kidney disease (DKD) is still controversial, and this study analyzed the correlation between TSH, FT3, FT4 and DKD in patients with type 2 diabetes mellitus (T2DM).
Methods
T2DM patients (1216) were divided into five groups based on serum TSH, FT3, and FT4 levels, differences in urinary albumin excretion rate (UACR), estimated glomerular filtration rate (eGFR) were compared. Binary logistic regression verified independent correlations among TSH, FT3, FT4 and UACR, eGFR. TSH and FT3 predictive values for DKD were analyzed using receiver operating characteristic (ROC) curves.
Results
The prevalence of albuminuria with decreased eGFR was higher in T2DM patients with subclinical hypothyroidism and overt hypothyroidism than that in patients with normal thyroid function. TSH positively correlated with UACR (r = 0.133, p < 0.001) and positively correlated with eGFR (r = -0.218, p < 0.001), FT3 negatively correlated with UACR (r = -0.260, p < 0.001) and positively correlated with eGFR (r = 0.324, p < 0.001). With the change from the lower normal level to the increased level of TSH and the change from the higher normal level to the reduced level of FT3, the prevalence of albuminuria gradually increased, the prevalence of decreased eGFR gradually increased in TSH groups and FT3 groups. After adjusting for age, BMI, duration of diabetes, TPOAb, TGAb, smoking, drinking, hypertension, the use of anti-diabetic medications (metformin, sodium–glucose cotransporter 2 inhibitors), HbA1c, CRP, TC, TG, LDL-C, and HDL-C, both TSH and FT3 correlated with increased UACR (TSH: OR 1.253, p = 0.001; FT3: OR 0.166, p < 0.001) and decreased eGFR (TSH: OR 1.245, p < 0.001, FT3: OR 0.579, p < 0.001), but this correlation of TSH with eGFR < 60 mL/min/1.73 m2 was not found in male. The area under the ROC curve (AUC) for FT3 was greater than that for TSH (FT3: 0.64; TSH: 0.61).
Conclusions
Increased TSH and reduced FT3 levels were associated with DKD in T2DM patients, but in a sex-dependent manner. FT3 had a higher predictive value for DKD.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease affecting human health. The prevalence of DM in adults has reached 10.5% with 780 million people currently living with diabetes and is expected to increase to 46% by 2045 [1]. DM increases the risk of chronic complications. Diabetic kidney disease (DKD), one of the most common microvascular complications, has a prevalence rate of up to 25% in DM patients and is the main cause of chronic kidney disease and end-stage kidney disease [2], often imposing a large economic burden on patients and causing high morbidity and mortality.
Thyroid-related hormones are closely related to glucose metabolism, and both overt and subclinical thyroid dysfunction have adverse effects on disease control in DM patients, especially those with T2DM. The prevalence of diabetes in patients with thyroid dysfunction is higher than that in the normal population [3], and the prevalence of hyperthyroidism and hypothyroidism in T2DM patients is higher than that in the normal population [4, 5].
As a chronic complication of DM, the clinical diagnosis of DKD is mainly based on an increase in urinary albumin-to-creatinine ratio (UACR) and a decrease in estimated glomerular filtration rate (eGFR). Studies have found an independent correlation between subclinical hypothyroidism and the incidence of DKD in DM patients, and the serum thyrotropin (TSH) level is an independent risk factor for proteinuria [6, 7]. Serum FT3 levels in DKD patients with normal thyroid function were also significantly lower than those in non-nephrotic patients [6, 8]. However, relatively few studies have addressed the relationship between overall thyroid-related hormones and DKD; several studies found no correlation between the two [9, 10]. Therefore, we conducted a cross-sectional study to explore the correlation between DKD and thyroid-related hormones (TSH, FT3, and FT4) in T2DM patients to provide references for the monitoring and treatment of thyroid dysfunction in diabetes patients.
Subjects, materials, and methods
Subjects
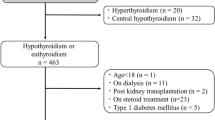
T2DM patients hospitalized in the Department of Endocrinology of the First Hospital of Lanzhou University between 2022 and February 2023 were recruited for this study. This study was approved by the Ethics Committee of First Hospital of Lanzhou University, China.
The inclusion criteria were: (1) age > 18 years and (2) presence of T2DM. The exclusion criteria were: (1) patients with a history of thyroid diseases, such as Hashimoto’s thyroid disease or thyroid surgery; (2) patients currently treated with thyroxine, amiodarone, glucocorticoids, or other drugs that affect thyroid function; and (3) patients with acute severe infections or serious cardiac or pulmonary vascular diseases, or other possible complications that can cause low T3 syndrome.
Clinical characteristics
The participants’ general characteristics were collected, including sex, age, BMI, duration of diabetes, history of smoking or alcohol consumption, history of hypertension, the use of anti-diabetic medications (insulin, metformin (MET), sodium–glucose cotransporter 2 inhibitors (SGLT2i)), HbA1c, c-reactive protein (CRP), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), UACR, eGFR, TSH, FT3, FT4, TT3, TT4, TPOAb, TGAb.
Laboratory measurements
The venous blood of each subject was collected after overnight fasting for 10 h to measure triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and serum creatinine (CR) via a BS-220 automatic biochemical analyzer (Shenzhen Mindray Bio-Medical Electronics Co., Ltd., China). The serum CRP levels were measured using immunoturbidimetric assay, HbA1c was measured by High Performance Liquid Chromatography (Bio-RAD-10 glycated hemoglobin analyzer).
Serum FT3, FT4, TSH, antithyroid peroxidase antibody (TPOAb), and antithyroid globulin antibody (TGAb) levels were measured using chemiluminescence (Roche, Cobas e801, Germany). The within-batch variation was 1.60% for TSH, 1.90% for FT4, and 1.40% for FT3, and the between-batch variation was 1.20% for TSH, 1.40% for FT4, and 1.10% for FT3.
Morning fasting spot urine samples were collected, and the concentration of urinary albumin was measured using a rate-scatter turbidimetry with the creatinine oxidase method. The UACR (mg/g) was calculated, and the eGFR (mL/min/1.73 m2) was calculated using the Chronic Kidney Disease Epidemiology Collaborative (CKD-EPI) formula [11].
Definition of variables
The normal reference range of thyroid-related hormones were: FT3, 2.3–4.8 pg/mL; FT4, 0.62–1.24 ng/dL; TSH 0.56–5.91 mIU/L; TPOAb 0–9 IU/L; and TGAb 0–4 IU/L. FT4 > 1.24 ng/dL with TSH < 0.56 mIU/L was defined as overt hyperthyroidism; FT4 > 1.24 ng/dL with TSH in the normal range was defined as subclinical hyperthyroidism. FT4 < 0.62 ng/dL with TSH > 5.91 mIU/L was defined as overt hypothyroidism; FT4 < 0.62 ng/dL with TSH in the normal range was defined as subclinical hypothyroidism. eGFR ≥ 90 mL/min/1.73 m2 was defined as normal, whereas < 90 mL/min/1.73 m2 was defined as decreased [12, 13]. UACR < 30 mg/g was considered as normal albuminuria, whereas while ≥ 30 mg/g was considered albuminuria [14]. DKD following multiple laboratory measurements performed at intervals of < 6 months that showed a UACR ≥ 30 mg/g, and/or an eGFR < 90 mL/min/1.73 m2.
Grouping of subjects
First, all T2DM patients were divided into normal, increased, and decreased groups according to the respective TSH, FT3, and FT4 levels. Second, the normal level group was divided into a lower-, medium-, and higher-level normal groups according to the respective tertiles of TSH, FT3, and FT4.
For TSH levels, T2DM patients were grouped as follows: reduced level group (TSH < 0.56 mIU/L, group 1), lower normal levels group (TSH = 0.57–1.97 mIU/L, group 2), medium normal levels group (TSH = 1.98–3.23 mIU/L, group 3), higher normal level group (TSH = 3.24–5.91 mIU/L, group 4), and increased level group (TSH > 5.91 mIU/L, group 5).
For FT3 levels, T2DM patients were grouped as follows: reduced level group (FT3 < 2.30 ng/dL, group 1), lower normal levels group (FT3 = 2.30–3.03 ng/dL, group 2), medium levels group (FT3 = 3.04–3.46 ng/dL, group), higher normal levels group (FT3 = 3.47–4.80 ng/dL, group 4), and increased level group (FT3 > 4.80 ng/dL, group 5).
For FT4 levels, T2DM patients were grouped as follows: reduced level group (FT4 < 0.62 ng/dL, group 1), lower normal levels group (FT4 = 0.62–0.87 ng/dL, group 2), medium normal levels group (FT4 = 0.88–0.99 ng/dL, group 3), higher normal levels group (FT4 = 1.00–1.24 ng/dL, group 4), and increased level group (FT4 > 1.24 ng/dL, group 5).
Statistical analysis
All data were analyzed by SPSS (24.0 version) statistical software (IBM Corp., Armonk, NY, USA). The chi-square test was used to compare ratios, the correlation between two continuous variables (non-normally distributed) was analyzed using the Spearman test, the correlation between binary variables and continuous variables (non-normally distributed) was analyzed using the Mann–Whitney test, and the differences in levels of TSH, FT3, and FT4 between the different groups were analyzed using the Kruskal–Wallis test. The Bonferroni correction was used again if the above tests were significant. Binary logistic regression was used to analyze the independent correlations of eGFR and UACR with TSH, FT3, and FT4 levels. Considering the physiological differences in the thyroid gland in different sexes, the relationships between eGFR, UACR, TSH, FT3, and FT4 in the different sexes were analyzed separately. Finally, the diagnostic values of TSH and FT3 for DKD were analyzed using the receiver operating characteristic (ROC) curve and the area under the curve (AUC). All reported p-values were two-sided, and p < 0.05 was considered statistically significant.
Results
Characteristics and comparison of prevalence of the different renal damage in T2DM patients with different thyroid function status groups
After screening, a total of 1216 patients were included in this study, including 780 males and 436 females, and the specific patient information are shown in Table 1.
Among them, T2DM patients were divided into normal thyroid function, subclinical hyperthyroidism, subclinical hypothyroidism, overt hyperthyroidism, and overt hypothyroidism groups, and differences in the prevalence of different degrees of kidney damage were compared. As shown in Table 2, compared with the normal thyroid function group, the prevalence of albuminuria with decreased eGFR was higher in the subclinical hypothyroidism group and overt hypothyroidism group (p < 0.05), whereas no significant difference was found among the subclinical hyperthyroidism and overt hyperthyroidism groups. No significant difference was found in the prevalence of albuminuria with normal eGFR among the normal thyroid function, subclinical hyperthyroidism, subclinical hypothyroidism, overt hyperthyroidism, and overt hypothyroidism groups (all p > 0.05).
Correlations between thyroid-related hormones and UACR and eGFR
UACR correlated positively with TSH (r = 0.133, p < 0.001) and negatively with FT3 (r = -0.260, p < 0.001). eGFR correlated negatively with TSH (r = -0.218, p < 0.001) and positively with FT3 (r = 0.324, p < 0.001); however, neither UACR nor eGFR correlated with FT4 (all p > 0.05). In addition, eGFR correlated negatively with positive TGAb (Z = -0.105, p = 0.001), and did not correlate with TPOAb. Age, sex, BMI, duration of diabetes, drinking, smoking, hypertension, MET, SGLT2i, HbA1c, CRP, HDL-C levels were correlated with eGFR (all p < 0.05). Age, diabetes duration, hypertension, MET, SGLT2i, HbA1c, CRP and TG levels were correlated with UACR (all p < 0.05) (Table 3).
Comparison of the levels of UACR and eGFR among the different thyroid-related hormone level groups
According to the TSH, FT3 and FT4 levels, T2DM patients were divided into five groups: reduced, lower normal, medium normal, higher normal, and increased level groups (groups 1–5, respectively). Differences were then compared between the levels of UACR and eGFR among the five groups, respectively. As shown in Fig. 1, with the change from the lower normal level to the increased level of TSH (group 2–5), UACR gradually increased (all p < 0.05) and eGFR gradually decreased (all p < 0.05). With the change from the higher normal level to the reduced level of FT3 (group 4–1), UACR gradually increased (all p < 0.05) and eGFR gradually decreased (all p < 0.05). No significant differences were present in UACR and eGFR among the different FT4 levels (all p > 0.05).
Comparison of the differences in UACR, eGFR levels among the different TSH, FT3, FT4 levels groups. A. Comparison of UACR levels. B. Comparison of eGFR levels. The groups of TSH, FT3, and FT4 in A and B were as described above, with reduced levels group (group 1), lower normal levels group (group 2), medium normal levels group (group 3), higher normal levels group (group 4), and increased levels group (group 5). *: p < 0.05
Comparison of the prevalence of different degrees of kidney damage in T2DM patients among the different thyroid-related hormones level groups
Similarly, T2DM patients were again divided into five groups (groups 1–5) based on TSH, FT3 and FT4 levels, and the differences compared among these of the prevalence of albuminuria and the prevalence of decreased eGFR.
As shown in Fig. 2, the prevalence of albuminuria gradually increased as TSH levels changed from reduced to increased (groups 1–5, all p < 0.05). The prevalence of decreased eGFR gradually increased with TSH levels changing from the lower normal level to the increased level (groups 2–5, all p < 0.05). The prevalences of albuminuria and decreased eGFR gradually increased as FT3 levels changed from the higher normal level to the reduced level (groups 4–1, all p < 0.05). Moreover, no significant difference was present in the prevalence of albuminuria and decreased eGFR among the groups with different levels of FT4 (p > 0.05).
The prevalence of albuminuria, decreased eGFR among the different TSH, FT3, FT4 levels groups. A. Comparison of the prevalence of albuminuria, B. Comparison of the prevalence of decreased eGFR. The groups of TSH, FT3, and FT4 in A and B were as described above, with reduced levels group (group 1), lower normal levels group (group 2), medium normal levels group (group 3), higher normal levels group (group 4), and increased levels group (group 5). *: p < 0.05
Independent correlation of UACR and eGFR with thyroid-related hormones
To investigate the independent relationship among UACR, eGFR and TSH, FT3, FT4, binary regression analyses were performed with albuminuria and decreased eGFR as dependent variables (normal albuminuria with normal eGFR as the control).
As shown in Table 4, After adjusting for the two thyroid antibodies in model 1, TSH positively correlated with albuminuria and decreased eGFR (UACR, 95% CI: 1.156–1.307, p < 0.001; eGFR, 95% CI: 1.064–1.246, p < 0.001), whereas FT3 negatively correlated with albuminuria and decreased eGFR (UACR, 95% CI: 0.089–0.182, p < 0.001; eGFR, 95% CI: 0.154–0.412, p < 0.001). After adjusting for sex, age, BMI, and duration of diabetes based on model 1, TSH remained positively correlated with albuminuria and decreased eGFR (UACR, 95% CI: 1.156–1.312, p < 0.001; eGFR, 95% CI: 1.168–1.319, p < 0.001) while FT3 remained negatively correlated with albuminuria and decreased eGFR (UACR, 95% CI: 0.109–0.228, p < 0.001; eGFR, 95% CI: 0.411–0.742, p < 0.001) in model 2. After adjusting for smoking, drinking, hypertension, MET, SGLT2i, HbA1c, CRP, TC, TG, LDL-C, and HDL-C in model 2, TSH independently and positively correlated with albuminuria and decreased eGFR (UACR, 95% CI: 1.171–1.341, p < 0.001; eGFR, 95% CI: 1.168–1.328, p < 0.001), and FT3 independently and negatively correlated with albuminuria and decreased eGFR in model 3 (UACR, 95% CI: 0.113–0.245, p < 0.001; eGFR, 95% CI: 0.426–0.787, p < 0.001).
Correlation of thyroid-related hormones with UACR and eGFR among different sexes
Considering the significantly sex differences in eGFR observed in the correlation analysis, and that sensitivity to thyroid disease varied between males and females, we performed regression models for stratified analyses to understand the independent correlations of UACR and eGFR with thyroid hormones among different sexes. Binary regression analyses were performed with albuminuria, eGFR < 90 mL/min/1.73 m2, eGFR < 60 mL/min/1.73 m2 as dependent variables (normal albuminuria with normal eGFR as the control).
As shown in Table 5, after adjusting for age, BMI, duration of diabetes, TPOAb, TGAb, smoking, drinking, hypertension, MET, SGLT2i, HbA1c, CRP, TC, TG, LDL-C, and HDL-C, both albuminuria and eGFR < 90 mL/min/1.73 m2 significantly correlated with FT3 and TSH in male and female patients. eGFR < 60 mL/min/1.73 m2 significantly correlated with FT3 in male patients, but not those with TSH. Conversely, eGFR < 60 mL/min/1.73 m2 significantly correlated with TSH and FT3 levels in female patients.
Diagnostic value of TSH and FT3 in DKD
The predictive values of serum TSH and FT3 levels for DKD were analyzed using ROC curves. As seen in Fig. 3, the AUC of FT3 was the largest at 0.64 (95% CI: 0.61–0.67; p < 0.001), with an optimal cut-off value of 2.955, a sensitivity of 39.36%, and a specificity of 83.74%. The AUC of TSH was 0.61 (95% CI: 0.57–0.64; p < 0.001), with an optimal cut-off 3.075, a sensitivity of 52.48%, and a specificity of 63.65%. In male patients, FT3 had a greater predictive value with an AUC of 0.63 (95% CI: 0.59–0.67, p < 0.001), an optimal cut-off of 2.955, sensitivity of 36.21%, and specificity of 84.99%, TSH had a low predictive value with an AUC of 0.56 (95% CI: 0.52–0.60, p = 0.002), an optimal cut-off of 3.265, sensitivity of 40.63%, and specificity of 70.21%. However, for female patients, both TSH and FT3 had a high predictive value (TSH: AUC 0.66, 95% CI: 0.61–0.71, p < 0.001, optimal cut-off value of 2.72, sensitivity of 74.65%, and specificity of 50.23%; FT3: AUC 0.64, 95% CI: 0.59–0.70, p < 0.001, optimal cut-off of value 2.94, sensitivity of 42.86%, and specificity of 84.93%).
Discussion
In this cross-sectional study, we analyzed the relationship between thyroid-related hormones and DKD and found that the UACR correlated positively with TSH and negatively with FT3, whereas the eGFR correlated negatively with TSH and positively with FT3. This trend was especially apparent in normal or reduced FT levels and in normal or increased TSH levels.
DM and its complications involve complex interactions with the thyroid gland. Hyperinsulinemia and high leptin levels, which are common in T2DM patients, can stimulate the synthesis of TSH [15, 16], whereas excessive or reduced thyroid-related hormones can exacerbate glucose metabolism disorders [17]. The prevalence of thyroid function disorders in T2DM patients is reportedly higher than that in the normal population [18], and DKD, a major complication of DM, is also closely related to thyroid dysfunction. Our study also confirmed that T2DM patients with thyroid dysfunction have a higher prevalence of abnormal renal function than that in the normal population. Our study showed a greater probability of abnormal renal function in T2DM patients with higher TSH levels than in those with lower TSH levels. This is consistent with the results reported by Han et al. This study, which included 61 meta-studies, concluded that the prevalence of subclinical hypothyroidism was higher in T2DM patients, and that subclinical hypothyroidism increased the prevalence of diabetic nephropathy (odds ratio [OR] 1.74, 95% CI: 1.34–2.28) [19]. In addition, similar results were found in clinical studies, where two cross-sections of 414 T2DM patients with no prior history of thyroid disease and 8418 normoglycemic individuals found that higher UACR ratios were positively associated with subclinical hypothyroidism (OR, 3.51, 95% CI: 1.10–10.0), and increased TSH was negatively correlates with eGFR independently of age, body mass index, and glucose [20, 21]. A Japanese longitudinal study that included 7609 health-screened members of the general population showed that those with above-normal TSH levels had a significantly higher risk of developing chronic kidney disease within 3 years than the risk in those with below-normal TSH levels (OR 1.58, 95% CI: 1.02–2.45) [22].
We found that FT3 was associated with abnormal renal function in all sexes. The ROC curve analysis showed that the AUC value of FT3 was greater than that of TSH, i.e., a low FT3 level may be more strongly associated with DKD than a high TSH level. The results of the METAL study also showed that the AUC of FT3 with decreased eGFR, higher UACR, and higher UACR and/or decreased eGFR was much higher than those of any other hormone [23]. While some studies have shown that TSH does not predict decreased renal function in T2DM patients, FT3 or the FT3/FT4 ratio seems to be independently associated with the development of DKD [24,25,26], and a lower FT3 within the normal range still increases the risk of DKD in T2DM [27]. Our study also found that the prevalence of an abnormal UACR and eGFR tended to decrease with reduced FT3 levels, which is consistent with our results. Several studies have found improvements in renal function after thyroxine supplementation [28, 29]; however, a meta-analysis by Meuwese et al. incorporating 16 cohort studies showed that hypothyroidism (especially subclinical hypothyroidism) was not associated with renal function deterioration, and the authors hypothesized that several of the cross-sectional studies may have been affected by renal dysfunction [30]. A Mendelian randomization analysis by Chen et al. showed no causal relationship between genetically elevated TSH levels and eGFR [31]. Thus, we hypothesize that changes in FT3 may be more valuable as a predictor of DKD progression, even if it is within the normal range.
Mechanistically, thyroid hormones and DKD can influence each other. Firstly, diabetes affects thyroid function. Hyperglycemia-induced inflammation can inhibit 5’-deiodinase activity to reduce peripheral T4 to T3 deiodination [32], FT3 decreased, affecting the hypothalamic–pituitary–thyroid axis [33]. In the context of metabolic disorders and with the development of DKD, damage to glomerular structures can cause protein loss, which further reduces FT3, leading to hypothyroidism [34, 35]. And the kidney, as an important organ for iodine metabolism, can experience iodine accumulation, elevated thyroxine, and negative feedback to elevated TSH levels in pathological conditions, which also affects thyroid function [36]. Secondly, thyroid dysfunction can also affect kidney function. Elevated TSH levels can cause increased peripheral vascular resistance, decreased cardiac output and eGFR [37,38,39]. Low FT3 levels can consequently aggravate impaired tubular concentrating and diluting functions [40], reduce renal plasma flow and glomerular transcapillary hydrostatic pressure [41], and deteriorate renal function. As mentioned above, the more severe the development of DKD, the lower the FT3 level will be and the more it will affect the thyroid function, and the thyroid dysfunction in turn will aggravate the development of DKD, and both of these interactions will contribute to the development of the disease on both sides.
We found an interesting phenomenon in the different sex analyses, TSH was significantly associated with decreased eGFR in those with eGFR < 90 mL/min/1.73 m2, while eGFR < 60 mL/min/1.73 m2 significantly correlated with FT3 but not TSH in male patients, whereas these exhibited correlations in all patients, although the AUC for FT3 was higher. Previous studies have also shown that the prevalence of chronic kidney disease and decreased eGFR is higher in female patients than in male patients. There are several possible reasons regarding this difference: firstly, the mean age of female in our included participants was greater than 60 years, higher than that of male, with lower TSH in female compared to male (3. 32 (3.26) vs. 2.48 (2.29)). This is consistent with previous studies that found that the proportion of hypothyroidism in T2DM was 1.7-fold higher in female patients than in male patients, and that the risk was higher in patients older than 60 years [42]. Differences in age and TSH levels may have combined to influence eGFR levels, contributing to the correlation between TSH and lower eGFR in female [43, 44]. Secondly, estrogen affects the hypothalamo-hypophyseal-thyroidal axis, Increased estrogen lead to increased amounts of T3 and TRH receptors in the anterior pituitary, an increased thyroxine-5′-deiodinase activity, and an induction of the conversion of T4 to T3, but an increase in androgens may mildly suppress 5′-deiodinase activity [45, 46]. Differences in thyroid hormone levels caused by this difference in physiologic environment can also affect renal function. In addition, considering that the thyroid antibody positivity rate is generally higher in female patients than in male patients, logistic regression analysis was performed, and neither TPOAb nor TGAb were found to be associated with DKD among the different sexes, which is consistent with a study by Suher et al., suggesting that thyroid autoimmunity is not strongly associated with renal dysfunction in patients with low TSH levels [47]. However, because additional biological or sociocultural factors (e.g., sex hormones) were not collected in our study, which is a limitation of this study. Whether increased TSH levels in female patients exacerbate the increased prevalence of DKD compared with that in male patients requires verification via further research.
Conclusion
T2DM patients with subclinical hypothyroidism had a higher prevalence of DKD than that in patients with normal thyroid function. TSH correlated positively with UACR and negatively with eGFR, with the prevalence of renal abnormalities increasing as TSH increased. FT3 correlated negatively with UACR and positively with eGFR, with the prevalence of renal abnormalities increasing as FT3 decreased. Both albuminuria and eGFR < 90 mL/min/1.73 m2 significantly correlated with FT3 and TSH in male and female patients. In male patients, eGFR < 60 mL/min/1.73 m2 significantly correlated with FT3 in male patients, but not those with TSH. FT3 has a higher predictive value for DKD than that of TSH. Potentially, the early assessment of FT3 can be used to predict or further treat kidney injury in T2DM patients.
Data availability
The data and materials involved in the study are not publicly available due to the containing information that could compromise research participant privacy/consent but are available from the corresponding author on reasonable request.
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109–19.
Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:117–24.
Chaker L, Ligthart S, Korevaar TIM, Hofman A, Franco OH, Peeters RP, et al. Thyroid function and risk of type 2 diabetes: a population-based prospective cohort study. BMC Med. 2016;14:150.
Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes Mellitus: two closely Associated disorders. Endocr Rev. 2019;40:789–824.
Gyawali P, Takanche JS, Shrestha RK, Bhattarai P, Khanal K, Risal P, et al. Pattern of thyroid dysfunction in patients with metabolic syndrome and its relationship with components of metabolic syndrome. Diabetes Metab J. 2015;39:66–73.
Yasuda T, Kaneto H, Kuroda A, Yamamoto T, Takahara M, Naka T, et al. Subclinical hypothyroidism is independently associated with albuminuria in people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94:e75–7.
Shimizu Y, Nabeshima-Kimura Y, Kawashiri S-Y, Noguchi Y, Minami S, Nagata Y, et al. Association between thyroid-stimulating hormone (TSH) and proteinuria in relation to thyroid cyst in a euthyroid general population. J Physiol Anthropol. 2021;40:15.
Zou J, Tian F, Zhang Y, Li Z, Yang C, Chen H, et al. Association between thyroid hormone levels and Diabetic kidney disease in Euthyroid patients with type 2 diabetes. Sci Rep. 2018;8:4728.
Shi W, Song D, Chen D, Duan W, Zhang J. The association of thyroid parameters with markers of chronic kidney disease in euthyroid patients with type 2 diabetes. Endocr J. 2023;70:687–96.
Huang X, Ding L, Peng K, Lin L, Wang T, Zhao Z, et al. Thyroid hormones associate with risk of incident chronic kidney disease and rapid decline in renal function: a prospective investigation. J Transl Med. 2016;14:336.
Palacio-Lacambra M-E, Comas-Reixach I, Blanco-Grau A, Suñé-Negre J-M, Segarra-Medrano A, Montoro-Ronsano J-B. Comparison of the Cockcroft-Gault, MDRD and CKD-EPI equations for estimating ganciclovir clearance. Br J Clin Pharmacol. 2018;84:2120–8.
Gao Z, Zhu Y, Sun X, Zhu H, Jiang W, Sun M, et al. Establishment and validation of the cut-off values of estimated glomerular filtration rate and urinary albumin-to-creatinine ratio for diabetic kidney disease: a multi-center, prospective cohort study. Front Endocrinol (Lausanne). 2022;13:1064665.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 11. Chronic kidney Disease and Risk Management: standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S191–202.
KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney int. 2022;102.
Ortiga-Carvalho TM, Oliveira KJ, Soares BA, Pazos-Moura CC. The role of leptin in the regulation of TSH secretion in the fed state: in vivo and in vitro studies. J Endocrinol. 2002;174:121–5.
Eom YS, Wilson JR, Bernet VJ. Links between thyroid disorders and glucose homeostasis. Diabetes Metab J. 2022;46:239–56.
Duntas LH, Orgiazzi J, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol. 2011;75:1–9.
Ward RJ, Heald AH, Ogunmekan S, Fryer AA, Duff CJ. Should we be screening for thyroid dysfunction in patients with type 2 diabetes mellitus? Br J Gen Pract. 2018;68:94–5.
Han C, He X, Xia X, Li Y, Shi X, Shan Z, et al. Subclinical hypothyroidism and type 2 diabetes: a systematic review and Meta-analysis. PLoS ONE. 2015;10:e0135233.
Furukawa S, Yamamoto S, Todo Y, Maruyama K, Miyake T, Ueda T, et al. Association between subclinical hypothyroidism and diabetic nephropathy in patients with type 2 diabetes mellitus. Endocr J. 2014;61:1011–8.
Sun M-T, Hsiao F-C, Su S-C, Pei D, Hung Y-J. Thyrotropin as an independent factor of renal function and chronic kidney disease in normoglycemic euthyroid adults. Endocr Res. 2012;37:110–6.
Toda A, Hara S, Kato M, Tsuji H, Arase Y. Association of Thyrotropin Concentration with chronic kidney disease in a Japanese General Population Cohort. Nephron. 2019;142:91–7.
Chen Y, Zhang W, Wang N, Wang Y, Wang C, Wan H, et al. Thyroid parameters and kidney disorder in type 2 diabetes: results from the METAL Study. J Diabetes Res. 2020;2020:4798947.
Liu M-C, Li J-L, Wang Y-F, Meng Y, Cai Z, Shen C, et al. Association between thyroid hormones and diabetic kidney disease in Chinese adults. BMC Endocr Disord. 2023;23:56.
Iwakura H, Takagi T, Inaba H, Doi A, Ueda Y, Uraki S, et al. Thyroid function, glycemic control, and diabetic nephropathy in patients with type 2 diabetes over 24 months: prospective observational study. BMC Endocr Disord. 2023;23:146.
Siddiqui K, Nawaz SS, Alfadda AA, Mujammami M. Low circulating free triiodothyronine levels are Associated with the Progression of Diabetic Nephropathy in patients with type 2 diabetes. Int J Gen Med. 2022;15:8659–68.
Zhang Y, Chang Y, Ryu S, Cho J, Lee W-Y, Rhee E-J, et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: the Kangbuk Samsung Health Study. Int J Epidemiol. 2014;43:1624–32.
Liu P, Liu R, Chen X, Chen Y, Wang D, Zhang F, et al. Can levothyroxine treatment reduce urinary albumin excretion rate in patients with early type 2 diabetic nephropathy and subclinical hypothyroidism? A randomized double-blind and placebo-controlled study. Curr Med Res Opin. 2015;31:2233–40.
Chen Y, Wu G, Xu M. The effect of L-thyroxine substitution on oxidative stress in early-stage diabetic nephropathy patients with subclinical hypothyroidism: a randomized double-blind and placebo-controlled study. Int Urol Nephrol. 2018;50.
Meuwese CL, van Diepen M, Cappola AR, Sarnak MJ, Shlipak MG, Bauer DC, et al. Low thyroid function is not associated with an accelerated deterioration in renal function. Nephrol Dial Transpl. 2019;34:650–9.
Chen C, Xia F, Chen Y, Zhang K, Cheng J, Li Q, et al. Association between thyroid-stimulating hormone and renal function: a mendelian randomization study. Kidney Blood Press Res. 2018;43:1121–30.
Ogbonna SU, Ezeani IU. Risk factors of thyroid dysfunction in patients with type 2 diabetes Mellitus. Front Endocrinol (Lausanne). 2019;10:440.
Meuwese CL, Dekkers OM, Stenvinkel P, Dekker FW, Carrero JJ. Nonthyroidal illness and the cardiorenal syndrome. Nat Rev Nephrol. 2013;9:599–609.
Zhang L, Wu Y, Nie Y, Lv W, Li Y, Zhu B, et al. The serum free triiodothyronine to free thyroxine ratio as a potential prognostic biomarker of chronic kidney disease in patients with glomerular crescents: a retrospective study. Front Endocrinol (Lausanne). 2022;13:977355.
Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012;23:22–6.
Iglesias P, Bajo MA, Selgas R, Díez JJ. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord. 2017;18:131–44.
Owen PJD, Rajiv C, Vinereanu D, Mathew T, Fraser AG, Lazarus JH. Subclinical hypothyroidism, arterial stiffness, and myocardial reserve. J Clin Endocrinol Metab. 2006;91:2126–32.
Ripoli A, Pingitore A, Favilli B, Bottoni A, Turchi S, Osman NF, et al. Does subclinical hypothyroidism affect cardiac pump performance? Evidence from a magnetic resonance imaging study. J Am Coll Cardiol. 2005;45:439–45.
Asvold BO, Bjøro T, Vatten LJ. Association of thyroid function with estimated glomerular filtration rate in a population-based study: the HUNT study. Eur J Endocrinol. 2011;164:101–5.
Chen Y-C, Cadnapaphornchai MA, Yang J, Summer SN, Falk S, Li C, et al. Nonosmotic release of vasopressin and renal aquaporins in impaired urinary dilution in hypothyroidism. Am J Physiol Ren Physiol. 2005;289:F672–8.
Vargas F, Moreno JM, Rodríguez-Gómez I, Wangensteen R, Osuna A, Alvarez-Guerra M, et al. Vascular and renal function in experimental thyroid disorders. Eur J Endocrinol. 2006;154:197–212.
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–99.
Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14:151–64.
Brar A, Markell M. Impact of gender and gender disparities in patients with kidney disease. Curr Opin Nephrol Hypertens. 2019;28:178–82.
Zhang L, Yang G, Su Z, Yang J. Correlation between subclinical hypothyroidism and renal function in patients with diabetes mellitus. Nephrol (Carlton). 2017;22:790–5.
Suzuki S, Nishio S-I, Takeda T, Komatsu M. Gender-specific regulation of response to thyroid hormone in aging. Thyroid Res. 2012;5:1.
Suher M, Koc E, Ata N, Ensari C. Relation of thyroid disfunction, thyroid autoantibodies, and renal function. Ren Fail. 2005;27:739–42.
Acknowledgements
None.
Funding
This study was funded by the Lanzhou Science and Technology Bureau [grant number: 2022-3-46]; the National Natural Science Foundation of China [grant number: 82360161].
Author information
Authors and Affiliations
Contributions
Jingfang Liu contributed to the study conception and design. Data collection and analysis were performed by Jie Gao. The first draft and revisions of the manuscript was written by Jie Gao. All authors discussed the results and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved and informed consent from all subjects of our study was waived by the Ethics Committee of First Hospital of Lanzhou University, China. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, J., Liu, J. Correlation of serum thyrotropin and thyroid hormone levels with diabetic kidney disease: a cross-sectional study. BMC Endocr Disord 24, 170 (2024). https://doi.org/10.1186/s12902-024-01699-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01699-x