Abstract
Background
In recent times, a number of new indices for measuring visceral obesity have been developed. This research sought to investigate the relationship between four visceral obesity indices and prediabetes and diabetes.
Methods
Conducted in 2011 as a cross-sectional analysis in Dalian, China, this study utilized logistic regression models to explore the relationships between four visceral obesity indices and prediabetes and diabetes. It also assessed the dose-response relationships using restricted cubic splines (RCS), performed subgroup analyses, and conducted interaction tests. The predictive values of four visceral obesity indices were evaluated using receiver operating characteristic (ROC) curves.
Results
The study enrolled 10,090 participants, with prediabetes and diabetes prevalence at 80.53%. Multifactorial logistic regression revealed positive relationships between the four visceral obesity indices and prediabetes and diabetes. The results of the RCS analysis revealed a linear relationship between the body roundness index (BRI), cardiometabolic index (CMI) and prediabetes and diabetes. Conversely, a non-linear relationship was observed between Chinese visceral adiposity index (CVAI), lipid accumulation product (LAP) and prediabetes and diabetes. Subgroup analyses demonstrated stronger relationships of CMI, CVAI, and LAP with prediabetes and diabetes among females. ROC curves suggested that LAP could be an effective predictor of these conditions.
Conclusion
This research confirmed that four visceral obesity indices are linked with a higher risk of prediabetes and diabetes in middle-aged and elderly individuals in Dalian. Importantly, LAP could be an effective predictor of prediabetes and diabetes. Effective weight management significantly reduces the risk of both prediabetes and diabetes.
Similar content being viewed by others
Introduction
Diabetes, a condition characterized by elevated blood glucose levels resulting from either malfunctioning pancreatic beta cells or resistance to insulin, has become increasingly prevalent in recent years. It is now one of the leading metabolic disorders globally, significantly impacting the quality of life of millions. According to the International Diabetes Federation, the worldwide diabetic population among adults was estimated at 537 million in 2021, accounting for about 10% of the global adult population. This number is projected to rise to 700 million by 2045. The incidence of diabetes is notably higher in individuals over 40, with a greater prevalence observed in men compared to women [1]. China hosts the largest diabetic population globally, a figure that continues to rise annually—approximately 140 million in 2021, projected to exceed 174 million by 2045 [2]. Moreover, diabetes in China exhibits an aging trend; the proportion of those over 60 is steadily increasing. A 2017 survey indicated that the prevalence rate approached or exceeded 20%. Moreover, many individuals have higher-than-normal blood glucose levels that fall short of diabetes diagnosis criteria, a condition known as prediabetes [3]. Both prediabetes and diabetes are closely linked to insulin resistance [4] and can lead to various complications, including renal and cardiovascular diseases, and cognitive impairment in hypertensive prediabetic frail older adults [5,6,7,8,9,10]. Additionally, diabetes and prediabetes represent a substantial personal and socioeconomic burden [11]. The direct and indirect healthcare costs associated with diabetes are considerable, encompassing treatment expenses as well as economic losses due to disability and premature mortality. Despite these challenges, most individuals with prediabetes are not diagnosed [12], and unmanaged prediabetes not only increases the risk of frailty in older adults with high blood pressure, it also heightens the potential for developing diabetes [13, 14]. Consequently, interventions targeting prediabetes and diabetes to prevent complications are crucial. Such measures are anticipated to enhance the early diagnosis and treatment of diabetes, optimizing healthcare outcomes.
Obesity rates are increasing, influenced by increasing life pressures and changes in dietary habits. According to a survey, over half of Chinese adults are now overweight or obese [15, 16]. The accumulation of fat, particularly visceral fat, is closely linked to insulin resistance. However, the benchmark for assessing insulin resistance—the high insulin-normal glucose clamp technique—is impractical for large sample populations because of its expensive price, invasive nature, and lengthy duration [17]. Therefore, we aimed to assess insulin resistance through visceral obesity indices to explore their relationship with prediabetes and diabetes. Recently, in addition to traditional obesity metrics, several novel indices specifically addressing visceral obesity have emerged, such as body roundness index (BRI) [18], cardiometabolic index (CMI) [19], Chinese visceral adiposity index (CVAI) [20], and lipid accumulation product (LAP) [21]. Currently, Shi et al. found that the atherogenic index of plasma is positively correlated with both prediabetes and diabetes. A cross-sectional study found a non-linear positive correlation between BRI and prediabetes and diabetes [22]. To date, no research has explored the relationship between the BRI, CMI, CVAI, and LAP indices and prediabetes and diabetes in the Chinese population [23]. Thus, our cross-sectional study, which targeted the middle-aged and elderly population of Dalian, China, examined the relationship between four novel visceral obesity indices and prediabetes and diabetes, and compared the predictive value of these indices, aiming to enhance the prevention and treatment of these disorders in specific groups.
Materials and methods
Research subject
The REACTION (Risk Evaluation of Cancers in Chinese Diabetic Individuals: A longitudinal study) is a nationwide, population-based prospective cohort study focusing on community-dwelling individuals aged 40 years or older. This extensive study recruited participants from 25 centers across mainland China, involving a total of 259,657 individuals [24,25,26]. Our specific research was conducted at the Dalian center of the REACTION study between August and December 2011. Our study is a single-center cross-sectional investigation. We excluded individuals with incomplete data and those under 40 years of age, ultimately registering 10,090 participants who fulfilled the eligibility requirements. The screening process is depicted in Fig. 1. The study was performed in accordance with the principles of the Declaration of Helsinki and received approval from the Institutional Review Board of Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine (RUIJIN-2011-14). All participants provided written informed consent.
Data collection
Medical and nursing personnel, who were trained beforehand, participated in conducting this survey. A comprehensive survey was used to collect personal details, covering basic demographic traits (age, gender, height, weight, WC); lifestyle factors (smoking and drinking habits); literacy level; history of previous chronic diseases; and family history of diabetes. Hypertension was defined as having a history of high blood pressure or a reading exceeding 140/90mmHg. Participants were instructed to fast for more than 12 h prior to the day of the survey. Subsequently, a certified technician collected venous blood samples to evaluate fasting plasma glucose (FPG), 2-hour OGTT plasma glucose, HbA1c, lipid profiles, and liver and kidney functions. Subject height and weight were recorded using an RGZ-120 weight-measuring device. Before measurements, subjects were asked to remove their clothing and shoes. Blood measurements were taken on the right arm in a calm state, averaging three readings.
Variable definition
Diabetes is characterized by fulfilling any of the criteria below: (1) FPG level of ≥ 7 mmol/L, 2-hour OGTT plasma glucose level of ≥ 11.1 mmol/L, or an HbA1c level of ≥ 6.5%. (2) Self-reported diabetes diagnosis or ongoing glucose-lowering medication use. Prediabetes is characterized by fulfilling any of the criteria below: FPG level between 5.6 mmol/L and 6.9 mmol/L, 2-hour OGTT plasma glucose level between 7.8 mmol/L and 11.1 mmol/L, or an HbA1c level between 5.7% and 6.4% [27].
Obesity index formula: refer to Fig. 2 for the calculation formula.
Statistical analysis
Categorical variables were described using frequency and constitutive ratios (n%), and comparisons between the two groups were performed using the chi-square test. Data not normally distributed were presented as medians (Q25, Q75), and after transforming these values logarithmically, a t-test was utilized for comparisons between the two groups. For handling missing data, a random forest-based multiple imputation technique was utilized. Multifactorial logistic regression was employed to explore the relationship between the four visceral obesity indices and prediabetes and diabetes. Due to the absence of new cut-off points, these associations were examined using the interquartile method. Three models were constructed for analysis: Model 1 was unadjusted. Model 2 was adjusted for age, gender, and family history of diabetes. Model 3 was further adjusted for the history of hypertension, coronary heart disease (CHD), smoking, alcohol consumption, Creatinine, ALT, and AST. The dose-response relationships between four visceral obesity indices and prediabetes and diabetes were analyzed using 3-knots restricted cubic splines (RCS). Subsequently, we performed subgroup analyses to determine if certain covariates (age, gender, CHD, and hypertension) altered the relationship between four visceral obesity indices and prediabetes and diabetes, with interactions deemed significant at P < 0.05. Lastly, receiver operating characteristic (ROC) curves were used to evaluate the ability of these indices to predict prediabetes and diabetes. Statistical analyses were performed using EmpowerStats (version 4.2) and R software (version 4.3.2). For assessing statistical significance, it is crucial to regard P-values below 0.05 as significant, using a two-sided test.
Results
Baseline characteristics
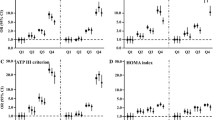
This study included 10,090 participants, who exhibited a combined prevalence of prediabetes and diabetes at 80.53%. As detailed in Table 1, individuals in the prediabetes and diabetes group exhibited higher values for age, systolic blood pressure (SBP), diastolic blood pressure (DBP), BMI, WC, BRI, CMI, CVAI, LAP, Creatinine, LDL, TG, ALT, AST, FPG, and HOMA-IR compared to those in the control group. Conversely, the prediabetes and diabetes group exhibited significantly reduced HDL levels compared to the control group. Furthermore, this group exhibited significantly higher proportions of men, individuals with low education levels, and those with a family history of diabetes, hypertension, and CHD, as well as smokers and alcohol consumers. However, there were no statistically significant differences in marital status between the groups. To further visually represent the differences in four visceral obesity indices between the two groups, violin plots were employed, as illustrated in Fig. 3. Relative to the control group, the prediabetic and diabetic groups exhibited significantly higher values across four visceral obesity indices.
Violin plots comparing four visceral obesity indices between the two groups. The comparison of BRI, CMI, CVAI, and LAP between the prediabetes and diabetes group and the control group. Group 1 represents the prediabetes and diabetes group, whereas Group 2 represents the control group. Abbreviation: BRI, body roundness index; CMI, cardiometabolic index; CVAI, Chinese visceral adiposity index; LAP, lipid accumulation product
Relationship between obesity indices and prediabetes and diabetes
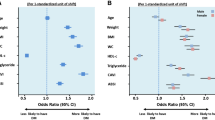
The four visceral obesity indices—BRI, CMI, CVAI, and LAP—were significantly associated with prediabetes and diabetes, regardless of confounder adjustment. As detailed in Table 2, these visceral obesity indices were categorized into quartiles to explore their relationships with prediabetes and diabetes. After adjustments for age, gender, creatinine, AST, ALT, family history of diabetes, hypertension, CHD, smoking, and drinking alcohol, the study revealed significant correlations. Compared with the lowest quartile, the odds ratio (OR) for the highest quartile of BRI was 2.961, 95% CI: (2.521, 3.477); for CMI was 2.899, 95% CI: (2.459, 3.418); for CVAI was 4.451, 95% CI: (3.712, 5.338); for LAP was 3.201, 95% CI: (2.705, 3.787). A progressive increase in the strength of these associations was observed across quartiles, maintaining a significant positive trend (P for trend < 0.001).
RCS curves fitting
Using 3-knots RCS curves fitting, we analyzed the dose-response relationship between four visceral obesity indices and prediabetes and diabetes. Figure 4 indicated that higher visceral obesity indices were linked to higher risks of prediabetes and diabetes. Following adjustments for multiple confounding variables, both BRI and CVAI displayed a linear positive correlation with prediabetes and diabetes. The analysis yielded non-linear p-values of 0.336 and 0.743, with cutoff values set at 5.43 and 139.14, respectively. In contrast, CMI and LAP displayed a non-linear positive correlation with prediabetes and diabetes. The analysis yielded non-linear p-values < 0.05, with cutoff values set at 0.85 and 60.6, respectively. The results from the RCS fit are consistent with those obtained from multivariate regression analysis.
The dose-response relationship between four visceral obesity indices and prediabetes and diabetes. We employed 3-knots RCS to fit the dose-response relationship between four visceral obesity indices and prediabetes and diabetes. We adjusted the model fully for age, gender, family history of diabetes, hypertension, CHD, smoking, alcohol consumption, creatinine, ALT, and AST. The red solid line represents the curve fitting between variables, and the shaded area indicates the 95% CI of the fit. Abbreviation: BRI, body roundness index; CMI, cardiometabolic index; CVAI, Chinese visceral adiposity index; LAP, lipid accumulation product; CHD, coronary heart disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase
Subgroup analyses
We further conducted subgroup analyses based on age, gender, hypertension, and CHD, followed by subsequent multivariable logistic regression analysis. Analysis of Fig. 5 revealed interactions between the CMI, CVAI, and LAP and prediabetes and diabetes within the gender subgroup (P for interaction < 0.05). The links between CMI, CVAI, and LAP and prediabetes and diabetes were stronger in women than in men. No notable differences were found in the remaining subgroups regarding the relationships between the four visceral obesity indices and prediabetes and diabetes.
Subgroup analysis between four visceral obesity indices and prediabetes and diabetes. We adjusted the model fully for age, gender, family history of diabetes, hypertension, CHD, smoking, alcohol consumption, creatinine, ALT, and AST. Abbreviation: BRI, body roundness index; CMI, cardiometabolic index; CVAI, Chinese visceral adiposity index; LAP, lipid accumulation product; CHD, coronary heart disease; OR, odds ratio; ALT, alanine aminotransferase; AST, aspartate minotransferase
ROC curves
As detailed in Fig. 6; Table 3, a baseline model was constructed that included variables such as age, sex, hypertension, CHD, smoking, alcohol consumption, Creatinine, ALT, and AST. The successive addition of the four visceral obesity indices modified the model’s AUC. The baseline AUC was 0.706 (95% CI: 0.693, 0.719). It rose to 0.717 (95% CI: 0.705, 0.730) with the addition of BRI, to 0.721 (95% CI: 0.709, 0.733) with CMI, to 0.724 (95% CI: 0.712, 0.737) with CVAI, and to 0.726 (95% CI: 0.714, 0.738) with LAP. The inclusion of the four visceral obesity indices significantly enhanced the model’s predictive accuracy (P < 0.001), with LAP offering the greatest improvement, followed by CVAI, CMI, and BRI. These findings suggest that LAP could be an effective marker for predicting prediabetes and diabetes.
The AUC curves of four visceral obesity indices to identify prediabetes and diabetes. The AUC curves of four visceral obesity indices to identify prediabetes and diabetes. In the ROC curves, the blue line represents the baseline model, whereas the red line signifies the enhanced model that sequentially incorporates various obesity indices. Abbreviation: BRI, body roundness index; CMI, cardiometabolic index; CVAI, Chinese visceral adiposity index; LAP, lipid accumulation product
Discussion
A study from Korea indicated that CT-based measurements of visceral adipose tissue volume could be valuable in assessing metabolic risk among individuals with prediabetes [28]. CT-based measurement of visceral adiposity offers a more accurate and objective assessment method; however, it has limitations, such as radiation exposure and high cost. Therefore, focusing on the distribution of body fat, we have selected several novel indices to assess visceral obesity more effectively in middle-aged and older adults. Thus, our research investigated the relationship between four visceral obesity indices and prediabetes and diabetes in middle-aged and elderly individuals from Dalian, China. We identified positive correlations between the BRI, CMI, CVAI, and LAP with both prediabetes and diabetes. The positive correlations intensified progressively across quartiles, demonstrating a significant upward trend (P for trend < 0.001). 3-knots RCS analysis indicated that BRI and CVAI were linearly correlated with prediabetes and diabetes, whereas CMI and LAP displayed nonlinear associations. Following further subgroup analysis, it was found that the relationships between CMI, CVAI, and LAP with prediabetes and diabetes were stronger in women. Further, ROC curves indicated that LAP was potentially the most effective predictor of prediabetes and diabetes. This study is the first to examine the potential links between these four visceral obesity indices and prediabetes and diabetes in the Dalian region of China.
Lin and colleagues determined that the BRI is linearly and positively correlated with diabetes, whereas the CVAI and LAP exhibit nonlinear positive correlations, with the CVAI possessing the strongest predictive power for diabetes [29]. A longitudinal study from Korea identified the LAP as a significant indicator for assessing the risk of type 2 diabetes mellitus (T2DM) in middle-aged and elderly non-obese Koreans [30]. Research from a Chinese cohort showed that CVAI, LAP, and visceral adiposity index (VAI) are nonlinearly correlated with T2DM, with LAP displaying stronger predictive capabilities than CVAI [31]. A Taiwanese study reported strong correlations between BRI, LAP, and diabetes, noting that LAP has superior predictive capabilities compared to other indices [32]. In Japan, a longitudinal study revealed that LAP is nonlinearly and positively correlated with newly diagnosed diabetes, possessing the highest predictive strength [33]. A cross-sectional study demonstrated that LAP and VAI are associated with early-onset T2DM, particularly noting that LAP offers the greatest predictive accuracy in males. Another cross-sectional study identified WC, CMI, VAI, and LAP as separately associated with prediabetes, with CMI, VAI, and LAP potentially outperforming BRI and traditional indices in identifying adult prediabetes patients [34]. A case-control study from India indicated that LAP and VAI are closely linked to prediabetes [35]. A cross-sectional study showed that the BRI surpasses traditional obesity indices including BMI and WC in predicting prediabetes [36]. A multicenter study comparing the predictive value of 11 obesity and lipid-related indices for prediabetes in older adults found that the TyG index was the most effective at predicting prediabetes across the whole study group [37]. A longitudinal study from China found that TyG-BMI more accurately predicts diabetes compared to VAI and LAP [38].
The mechanisms underlying our results can be elucidated in the following ways: Firstly, obesity modifies the expression of miRNAs in key metabolic organs [39]. For instance, the overexpression of miR-15b targets the 3′ untranslated region of the insulin receptor, thereby inhibiting its protein expression and disrupting insulin signaling in hepatocytes [40]. Furthermore, miRNAs amplify mTOR signaling pathways, thereby contributing to heightened insulin resistance [41]. Secondly, AM2, an adipokine, has a positive effect on energy metabolism and enhances insulin sensitivity in adipose tissue. In obese individuals, however, decreased expression of receptors and reduced ligand availability may lead to suppressed AM2 signaling, thereby contributing to insulin resistance [42]. Furthermore, adiponectin, an adipocyte-derived protein and key adipokine, demonstrates inverse correlations with body fat mass, especially visceral fat. Low adiponectin levels can impair the inflammatory response and intensify insulin resistance, ultimately leading to diabetes and prediabetes [43, 44]. Thirdly, an increase in visceral fat activates enhanced mitochondrial oxygen consumption via toll-like receptor 4, leading to increased oxidative stress and promoting insulin resistance [45]. Lastly, visceral adipose tissue may augment the expression of CH25H, particularly in the CD206 ATM subgroup within CH25H. This subgroup is associated with insulin resistance and exhibits a pro-inflammatory role [46].
Following subgroup analysis, it was found that the relationships between CMI, CVAI, and LAP with prediabetes and diabetes were stronger in women. Possible explanations might include: Firstly, women typically store adipose tissue subcutaneously, particularly in the buttocks, while men tend to accumulate visceral fat. However, an increase in abdominal fat in women heightens their risk of metabolic diseases, including prediabetes and diabetes, more so than in men [47,48,49]. Secondly, estrogen enhances insulin sensitivity by activating the estrogen receptor α pathway in insulin-responsive tissues. As a result, women generally exhibit higher insulin sensitivity than men. Nonetheless, even minor increases in visceral fat can lead to significant insulin resistance in women, thereby elevating their risk for prediabetes and diabetes [50]. In addition, hormonal fluctuations, particularly the reduction of estrogen during menopause, can decrease metabolic rates and increase visceral fat accumulation in women. These hormonal changes adversely affect glucose and lipid metabolism, consequently raising the risk of diabetes. Lastly, women often have lower levels of the anti-inflammatory mediator adiponectin, whereas men typically exhibit higher levels of pro-inflammatory mediators like leptin and IL-6. This disparity in inflammatory regulation may lead to more pronounced inflammation and oxidative stress when there is an excess of body fat, both of which are crucial in the onset of prediabetes and diabetes [51].
Our research has several advantages: Firstly, our research utilized a considerable number of middle-aged and elderly residents from the Dalian area, China, marking the first exploration of the relationship between four novel visceral obesity indices and prediabetes and diabetes. The uniform regional origin of the samples minimized sample bias and enhanced representativeness. Secondly, we utilized multiple imputation methods to manage missing data, mitigating the impact on the reliability and scientific validity of our results and thereby strengthening our conclusions. Nonetheless, our research possesses several limitations: Firstly, our research did not account for factors such as diet and exercise that significantly influence blood glucose levels, preventing us from fully addressing these potential confounders. Secondly, the research targeted community residents aged 40 and older, limiting its applicability to children, young adults, or populations outside the Dalian region. Additionally, we did not include the use of medications, consumption of specific foods, or beverage intake that could influence blood glucose levels. The likelihood of consuming such foods is particularly higher among obese individuals, which may have led to an overestimation of our findings. Lastly, this cross-sectional study had a less comprehensive design compared to cohort studies. It possessed limited capacity for exploring and testing etiological hypotheses and making extrapolations. Additional cohort studies are required to confirm the association between the four visceral obesity indices and both prediabetes and diabetes.
Conclusion
We observed that both the BRI and the CMI exhibit linear positive correlations with prediabetes and diabetes. In contrast, the CVAI and LAP demonstrate nonlinear positive correlations with these conditions. Furthermore, the relationships between CMI, CVAI, LAP, and prediabetes and diabetes are more pronounced in females. The findings suggest that LAP could be an effective marker for predicting prediabetes and diabetes. This finding supports the implementation of effective interventions and preventive measures in community populations, which can, in turn, help prevent the progression of prediabetes to diabetes, reduce the development of diabetic complications, and enhance health management within these communities.
Availability of data and materials
The data analyzed in this article are not disclosed to the public but are available from the corresponding author on reasonable request.
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Rett K, Gottwald-Hostalek U. Understanding prediabetes: definition, prevalence, burden and treatment options for an emerging disease. Curr Med Res Opin. 2019;35(9):1529–34.
Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–9.
Farhan S, Redfors B, Maehara A, McAndrew T, Ben-Yehuda O, De Bruyne B, Mehran R, Giustino G, Kirtane AJ, Serruys PW, et al. Impact of pre-diabetes on coronary plaque composition and clinical outcome in patients with Acute Coronary syndromes: an analysis from the PROSPECT study. JACC Cardiovasc Imaging. 2019;12(4):733–41.
Welsh C, Welsh P, Celis-Morales CA, Mark PB, Mackay D, Ghouri N, Ho FK, Ferguson LD, Brown R, Lewsey J, et al. Glycated hemoglobin, Prediabetes, and the links to Cardiovascular Disease: Data from UK Biobank. Diabetes Care. 2020;43(2):440–5.
de Jong M, Woodward M, Peters SAE. Diabetes, glycated hemoglobin, and the risk of myocardial infarction in women and men: a prospective cohort study of the UK Biobank. Diabetes Care. 2020;43(9):2050–9.
Kim GS, Oh HH, Kim SH, Kim BO, Byun YS. Association between prediabetes (defined by HbA1(C), fasting plasma glucose, and impaired glucose tolerance) and the development of chronic kidney disease: a 9-year prospective cohort study. BMC Nephrol. 2019;20(1):130.
Wang X, Chen L, Shi K, Lv J, Sun D, Pei P, et al. Diabetes and chronic kidney disease in Chinese adults: a population-based cohort study. BMJ Open Diabetes Res Care. 2024;12(1):e003721.
Mone P, De Gennaro S, Moriello D, Frullone S, D’Amelio R, Ferrante MNV, Marro A, Santulli G. Insulin resistance drives cognitive impairment in hypertensive pre-diabetic frail elders: the CENTENNIAL study. Eur J Prev Cardiol. 2023;30(12):1283–8.
Liu X, Zhang L, Chen W. Trends in economic burden of type 2 diabetes in China: based on longitudinal claim data. Front Public Health. 2023;11:1062903.
Edwards CM, Cusi K. Prediabetes: a Worldwide Epidemic. Endocrinol Metab Clin North Am. 2016;45(4):751–64.
Numata Y, Ohya T, Nitta Y, Yoshinaka Y, Shogakiuchi A, Toyota A. Repetition of Prediabetes Enhances the Risk of Developing Diabetes. J Diab Res. 2019;2019:4916546.
Santulli G, Visco V, Varzideh F, Guerra G, Kansakar U, Gasperi M, Marro A, Wilson S, Ferrante MNV, Pansini A, et al. Prediabetes Increases the Risk of Frailty in Prefrail Older Adults With Hypertension: Beneficial Effects of Metformin. Hypertension (Dallas, Tex: 1979). 2024;81(7):1637–43.
Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–92.
Xi B, Liang Y, He T, Reilly KH, Hu Y, Wang Q, Yan Y, Mi J. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993–2009. Obes Reviews: Official J Int Association Study Obes. 2012;13(3):287–96.
Elahi D. In praise of the hyperglycemic clamp. A method for assessment of beta-cell sensitivity and insulin resistance. Diabetes Care. 1996;19(3):278–86.
Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, Maeda Y, McDougall A, Peterson CM, Ravussin E, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obes (Silver Spring Md). 2013;21(11):2264–71.
Wakabayashi I, Sotoda Y, Hirooka S, Orita H. Association between cardiometabolic index and atherosclerotic progression in patients with peripheral arterial disease. Clin Chim Acta. 2015;446:231–6.
Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, Li Q, Wang D, Hu Y, Pan BS, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214.
Kahn HS. The lipid accumulation product performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26.
Qiu L, Xiao Z, Fan B, Li L, Sun G. Association of body roundness index with diabetes and prediabetes in US adults from NHANES 2007–2018: a cross-sectional study. Lipids Health Dis. 2024;23(1):252.
Shi Y, Wen M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011–2018 population. Cardiovasc Diabetol. 2023;22(1):19.
Ning G. Risk evaluation of cAncers in Chinese diabeTic individuals: a lONgitudinal (REACTION) study. J Diabetes. 2012;4(2):172–3.
Bi Y, Lu J, Wang W, Mu Y, Zhao J, Liu C, Chen L, Shi L, Li Q, Wan Q, et al. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. 2014;6(2):147–57.
Wang T, Lu J, Su Q, Chen Y, Bi Y, Mu Y, Chen L, Hu R, Tang X, Yu X, et al. Ideal Cardiovascular Health Metrics and Major Cardiovascular events in patients with prediabetes and Diabetes. JAMA Cardiol. 2019;4(9):874–83.
5. Prevention or Delay of type 2 diabetes: standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S51–4.
Kim J, Kim K. CT-based measurement of visceral adipose tissue volume as a reliable tool for assessing metabolic risk factors in prediabetes across subtypes. Sci Rep. 2023;13(1):17902.
Lin M, Xie Y, Hong L, Wu S, Deng X, Chen Y, Tan X. Abnormal adiposity indices are associated with an increased risk of diabetes in a non-obese Asian population. Public Health. 2024;226:144–51.
Yang SH, Yoon J, Lee YJ, Park B, Jung DH. Lipid Accumulation Product Index predicts New-Onset type 2 diabetes among non-obese koreans: a 12-Year longitudinal study. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 2022;15:3729–37.
Pan L, Gao Y, Han J, Li L, Wang M, Peng H, Liao J, Wan H, Xiang G, Han Y. Comparison of longitudinal changes in four surrogate insulin resistance indexes for incident T2DM in middle-aged and elderly Chinese. Front Public Health. 2022;10:1046223.
Chung TL, Liu YH, Wu PY, Huang JC, Chen SC. Sex difference in the associations among obesity-related indices with incidence of diabetes mellitus in a large Taiwanese population follow-up study. Front Public Health. 2023;11:1094471.
Liu T, Lu W, Zhao X, Yao T, Song B, Fan H, Gao G, Liu C. Relationship between lipid accumulation product and new-onset diabetes in the Japanese population: a retrospective cohort study. Front Endocrinol. 2023;14:1181941.
Klisic A, Radoman Vujačić I, Kostadinovic J, Patoulias D, Ninic A. Novel anthropometric parameters in the adult population with prediabetes. Eur Rev Med Pharmacol Sci. 2023;27(22):11063–72.
Ramdas Nayak VK, Nayak KR, Vidyasagar S. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabetes Metabolic Syndrome. 2020;14(5):1265–72.
Sánchez M, Sánchez E, Bermúdez-López M, Torres G, Farràs-Sallés C, Pamplona R, Castro-Boqué E, Valdivielso JM, Purroy F, Martínez-Alonso M et al. Clinical usefulness of anthropometric indices to predict the presence of prediabetes. Data from the ILERVAS Cohort. Nutrients. 2021;13(3):1002.
Ramírez-Vélez R, Pérez-Sousa M, González-Ruíz K, Cano-Gutierrez CA, Schmidt-RioValle J, Correa-Rodríguez M, et al. Obesity- and lipid-related parameters in the identification of older adults with a high risk of prediabetes according to the American Diabetes Association: an analysis of the 2015 health, well-being, and aging study. Nutrients. 2019;11(11):2654.
Li X, Sun M, Yang Y, Yao N, Yan S, Wang L, Hu W, Guo R, Wang Y, Li B. Predictive effect of triglyceride glucose-related parameters, obesity indices, and lipid ratios for diabetes in a Chinese Population: a prospective cohort study. Front Endocrinol. 2022;13:862919.
Kim NH, Ahn J, Choi YM, Son HJ, Choi WH, Cho HJ, Yu JH, Seo JA, Jang YJ, Jung CH, et al. Differential circulating and visceral fat microRNA expression of non-obese and obese subjects. Clin Nutr. 2020;39(3):910–6.
Yang WM, Jeong HJ, Park SW, Lee W. Obesity-induced miR-15b is linked causally to the development of insulin resistance through the repression of the insulin receptor in hepatocytes. Mol Nutr Food Res. 2015;59(11):2303–14.
Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging. 2012;4(5):350–8.
Kim J, Lee SK, Kim D, Choe H, Jang YJ, Park HS, et al. Altered expression of Adrenomedullin 2 and its receptor in the adipose tissue of obese patients. J Clin Endocrinol Metab. 2020;105(1):dgz066.
Maeda N, Funahashi T, Matsuzawa Y, Shimomura I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. 2020;292:1–9.
Li X, Zhang D, Vatner DF, Goedeke L, Hirabara SM, Zhang Y, Perry RJ, Shulman GI. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc Natl Acad Sci USA. 2020;117(51):32584–93.
Lin HY, Weng SW, Shen FC, Chang YH, Lian WS, Hsieh CH, Chuang JH, Lin TK, Liou CW, Chang CS, et al. Abrogation of toll-like receptor 4 mitigates obesity-Induced oxidative stress, Proinflammation, and insulin resistance through metabolic reprogramming of Mitochondria in Adipose tissue. Antioxid Redox Signal. 2020;33(2):66–86.
Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c + CD206 + adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–56.
Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biology sex Differences. 2012;3(1):13.
Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, Gourdy P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63(3):453–61.
Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Ben Avraham S, et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care. 2012;35(3):640–7.
Handgraaf S, Riant E, Fabre A, Waget A, Burcelin R, Lière P, Krust A, Chambon P, Arnal JF, Gourdy P. Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas ERαAF-1 is dispensable. Diabetes. 2013;62(12):4098–108.
Ter Horst R, van den Munckhof ICL, Schraa K, Aguirre-Gamboa R, Jaeger M, Smeekens SP, Brand T, Lemmers H, Dijkstra H, Galesloot TE, et al. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler Thromb Vasc Biol. 2020;40(7):1787–800.
Acknowledgements
The authors thank all participants in the study. The authors thank the Guang Ning Team at Ruijin hospital, Shanghai Jiaotong University School of Medicine, for helping establish the data set in the communities.
Funding
The study was supported by grants from the National Key R&D Program of China (2018YFC1311800) and the Dalian Science and Technology Bureau (project 2022RG11).
Author information
Authors and Affiliations
Contributions
Study design: ZN-G and YT-L. Data collection and analysis: HD-J. Drafting and revising the manuscript: YT-L and L-L. All authors have read and agreed to the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This work has been conducted in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The protocol was approved by the Ethical Review Committee of Ruijin Hospital (RUIJIN-2011-14), adhering to the principles outlined in the Declaration of Helsinki. All participants provided written informed consent prior to their involvement.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Jiang, H., Luo, L. et al. Relationship between four visceral obesity indices and prediabetes and diabetes: a cross-sectional study in Dalian, China. BMC Endocr Disord 24, 191 (2024). https://doi.org/10.1186/s12902-024-01718-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01718-x