Abstract
Background
The purpose of this study was to evaluate the osseointegration of zirconia and titanium implants in the rat maxilla in specimens under systemic antiresorptive therapy.
Materials and methods
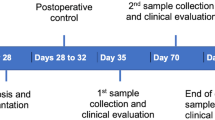
After 4 weeks of systematic medication administration (either zoledronic acid or alendronic acid), 54 rats received one zirconia and one titanium implants that were immediately inserted in the rat maxilla after tooth extraction. Twelve weeks after implant placement, histopathological samples were evaluated for implant osteointegration parameters.
Results
The bone-implant-contact (BIC) ratio revealed no significant inter-group or inter-material differences. The distance between the implant shoulder to the bone level was significantly greater around the titanium implants of the zoledronic acid group compared to the zirconia implants of the control group (p = 0.0005). On average, signs of new bone formation could be detected in all groups, although often without statistical differences. Signs of bone necrosis were only detected around the zirconia implants of the control group (p < 0.05).
Conclusions
At the 3-month follow-up, no implant material was demonstrably better than the others in terms of osseointegration metrics under systemic antiresorptive therapy. Further studies are necessary to determine whether there are differences in the osseointegration behavior of the different materials.
Similar content being viewed by others
Introduction
Antiresorptive medications, such as bisphosphonates and monoclonal antibodies, interfere with physiological bone metabolism and are intended to inhibit pathological bone resorption [1]. However, bisphosphonates also prevent the renewal of injured bone and have a half-life of approximately 11 years in bone because of their irreversible binding to bone [2]. In 2008, 200 million doses of bisphosphonates were prescribed in Germany [3]. Since the incidence of bisphosphonates increases with the aging of society, these numbers are likely to increase in the coming years [3]. Furthermore, bisphosphonate accumulates in bone over 100 times faster than when administered orally and leads to a higher bone mineralization [4]. The incidence of tumor patients on systemic antiresorptive medication (such as in cases of multiple myeloma, prostate carcinoma, bronchial carcinoma, and breast carcinoma) in Germany alone is around 200,000 new cases per year and rising [5].
If these drugs are used, surgical interventions such as implantation represent a risk for jaw necrosis [6, 7]. Dental implants can be used, but they come with a greatly increased risk of complications due to oral antiresorptive therapy [8]. Patients with low oral doses will have an increased risk of jaw necrosis during implantation; for patients with high doses, for example in the case of systematic medication, no dental implantation is recommended at all according to a recent systematic review [3]. Extensive operations, such as jaw bone augmentations, can represent a contraindication for the risk group mentioned. According to this guideline, patients with a high-risk profile should not be treated with implants, which is why no sufficiently fixed restoration is possible in these cases, and patients have to live with the risk of prosthetic pressure points.
Osseointegration is a special form of bony healing in which vital bone cells and bioserological components bond with the implant surface [9]. Provided that no relevant disturbing influences occur, this connection is permanent. Titanium proved to be a biologically suitable material on which chemical bonds can form with the surrounding tissues, which are also sufficiently stable biomechanically [10]. Among other things, it has been proven based on the first osseointegrated implants can remain in function for over 40 years.
In dental implantology, titanium is considered the gold standard according to numerous long-term studies. The emergence of the all-ceramic, high-performance material zirconium dioxide has raised hopes for a suitable all-ceramic material. Initial preclinical and clinical studies look promising. According to the findings, zirconia ceramic is biocompatible, and the ceramic implant surface is well accepted by the bone tissue. Furthermore, authors concluded that one-piece zirconia implants can be used as an alternative for titanium implants [11,12,13,14]. Both titanium and zirconia implant materials showed good osseointegration behavior in a preclinical study and also presented high success and survival rates under physiological condition in patients, even with immeditate implant treatment [15,16,17,18]. This inevitably leads to the question of what implant material may have a lower risk of complication under systemic antiresorptive therapy due to the individual material properties. The primary aim of this study was to evaluate the histopathological osseointegration using the bone-implant-contact (BIC) ratio by comparing immediate zirconia and titanium implants in the rat maxilla. Furthermore, peri-implant bone levels and bone behaviors were investigated.
Material and methods
Experimental protocol
This animal study enrolled 54 adult male Sprague–Dawley rats, each weighing 250 g and aged 7 weeks (Janvier Labs, Le Genest-Saint-Isle, France). Two experimental groups and one control group, each with 18 animals, were randomly assigned to the following treatments: zoledronic acid (Group 1), alendronic acid (Group 2), and no medicine (Group 3). Systemic antiresorptive medicine was started 4 weeks prior to implantation and continued for 4 months. Before administration, the medicines were diluted in physiologic phosphate-buffered saline. Once a week, rats in Group 1 were given 0.04 mg/kg of body weight of zoledronic acid (Mylan dura GmbH, Darmstadt, Germany) intravenously in the tail vein [19]. The rats in Group 2 were given 0.2 mg/kg of body weight of alendronic acid (alendronate sodium trihydrate, Sigma Aldrich GmbH, Munich, Germany) subcutaneously five times a week [20].
One examiner performed the surgery, and another examiner assessed the histopathological samples using blinded data evaluation. This study was carried out in accordance with the guidelines of the European Parliament and of the Council on the protection of animals used for scientific purposes, Animal Research: Reporting of In Vivo Experiments (ARRIVE) and Directive 2010/63/EU. The study protocol received ethical approved from the appropriate local authority (Landesamt für Natur und Verbraucherschutz, Recklinghausen, Germany; Ref. 2018A314). The rats were given free access to food and water, with only soft moistened food provided following implantation until the end of the study.
Implant placement
Surgery was conducted after 4 weeks of medication administration to both test groups. The Straumann Company (Institute Straumann AG, Basel, Switzerland) custom made a total of 54 microrough titanium or zirconia implants with a polished shoulder (4-mm length and 2-mm diameter, Fig. 1) using the same method as commercially available implants. This surface was generated with a macro roughness by rough sandblasting. Subsequently, a micro-roughness is generated by acid etching. The titanium surface was optimized by sandblasting and the zirconia surface by zirconia particle blasting. Mean surface roughness values of titanium were 1.23 versus zirconia 0.59 (micrometer).
The rodents were injected with an intraperitoneal sedative mixed injection comprised of 90 mg/kg of body weight of ketamine (Medistar GmbH, Ascheberg, Germany) and 0.2 mg/kg of body weight of medetomidine hydrochloride (Domitor, Bayer Austria, Vienna, Austria). A split-mouth design was used. After extraction of the primary molar of the upper jaw on each side, one zirconia and one titanium implants were immediately inserted. The individual implant material was distributed on the maxillary side by randomization. Implantation was performed according to the manufacturer’s guidelines with a transgingival healing procedure. Toward the end of surgery, 0.8 mg/kg of body weight of the antitoxin atipamezole hydrochloride (Orion Pharma, Espoo, Finland) was injected subcutaneously. During the 3 initial postoperative days, the animals received 4 mg/kg of carprofen (Rimadyl, Zoetis GmbH, Berlin, Germany) subcutaneously once per day as indicated by a score sheet. The animals were sacrificed at the end of the study under deep isoflurane anesthesia by cervical dislocation. We then studied the histopathological results of all implants that were incorporated.
Histopathological sample evaluation
At 12 weeks after implant placement, the animals were killed by cervical dislocation under deep isoflurane anesthesia (4%). The tibia samples were stored in 4% formalin (neutrally buffered with methanol; Otto Fischar GmbH & Co. KG, Saarbrücken, Germany) for 48 h. The samples were dehydrated using an ascending ethanol gradient (50–100%) prior to embedding in methyl methacrylate resin (Technovit 9100, Heraeus Kulzer GmbH, Frankfurt, Germany). Coronal sections of the embedded undecalcified specimens were obtained at a thickness of about 200 µm using the EXAKT cutting unit (EXAKT Technologies Inc., Oklahoma City, Oklahoma, USA), and then they were thinned and polished manually to a final thickness of about 50–70 µm [21]. Final specimens were stained with toluidine blue according to manufacturer’s protocol and were analyzed using digital microscopy. One slide was obtained for each implant in the coronal section through the implant center.
The tissue structures were analyzed by digital microscopy by one specialized pathologist. The set of parameters evaluated in the histomorphometric analysis included a quantitative evaluation of the peri-implant bone. The bone-to-implant ratio (μm) was calculated by measuring the complete circumference of the implant and then recording the area that had histologic bone contact. Using this, the implant contact through bone in relation to the entire surface (BIC ratio) was determined. The distance between the implant shoulder and the peri-implant bone level (mm) was measured on both sides. Additionally, semiquantitative analyses were performed for new bone formation, bone resorption, necrosis, signs of inflammation, and connective tissue proliferation based on a previously published score (0 = normal, 1 = minimal count, 2 = progressing count, and 3 severe amount) [22]. All parameters were examined under 40–600 times magnification with the OLYMPUS digital microscope DSX-1000 (Olympus, Hamburg, Germany) and the integrated morphometric stream desktop software [22].
Statistical analyses
Analyses were performed using the Prism 8 software (GraphPad, La Jolla, CA, USA) running on Apple OS X. Variables were analyzed using the Kolmogorov–Smirnov normality test. Kruskal–Wallis and Dunn’s multiple comparison tests with adjustment were used to identify differences between parameters. A p value of less than 0.05 was considered statistically significant.
Post-hoc power analysis was performed with the G-Power software (Heinrich-Heine-Universität, Düsseldorf, Germany) using the post hoc ANOVA test by means of groups to determine the power of 100% (primary study aim: BIC) based on the total sample size of 52 with an effect size of 8.7 and an alpha of 0.05.
Results
This study investigated histological results in an animal model at one time point at month follow-up. At the end of the test series, the following implants were osseointegrated and used for histopathological evaluation: 15 titanium and 13 zirconia implants in Group 1 (zoledronic acid), 9 titanium and 10 zirconia implants in Group 2 (alendronic acid), and 4 titanium and 11 zirconia implants in Group 3 (control group). At 3 months after implant insertion, the BIC ratio revealed no significant inter-group or inter-material differences (Fig. 2A; p > 0.05). Group 1 showed the highest BIC mean values for both materials. However, the titanium implants of Group 2 and zirconia implants of Group 3 showed the lowest BIC ratios (Table 1).
A The implant contact through bone in relation to the entire surface of the implant (BIC ratio; %) was determined. B The distance between the implant shoulder and the peri-implant bone level (mm) was measured on both sides of the implant. C New bone formation, D bone resorption, E bone necrosis, F signs of inflammation, and G connective tissue proliferation were evaluated according to a previously published score (0 = normal, 1 = minimal count, 2 = progressing count, and 3 severe amount)
The distance of the bone to the implant shoulder presented a significantly value around titanium implants of the zoledronic acid group compared to zirconia implants of the control group (p = 0.0005). The remaining group and material comparisons showed no statistical differences (Fig. 2B). A pronounced bone formation according to score was recorded around zirconia implants of the control group compared to titanium implants of Group 2 (Fig. 2C; p = 0.04). On average, signs of new bone formation could be detected in all groups, although often without statistical differences.
In addition to the new bone formation, bone resorption around the different implants was also present but did not show any significant differences between groups (Fig. 2D). Signs of bone necrosis around the zirconia implants were detected in the control group (Fig. 2E). The score was significantly higher than in the titanium of Group 1 (p = 0.0134), titanium of Group 2 (p = 0.0413), zirconia of Group 2 (p = 0.0161), and zirconia of Group 3 (p = 0.0246).
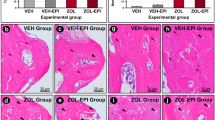
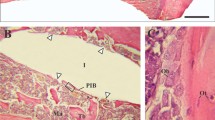
Peri-implant signs of infection were significantly higher around the zirconia implants of the control group compared to the zirconia implants of Group 1 (p = 0.0026) and Group 2 (p = 0.0035, Fig. 2F). The histomorphometrical analysis revealed no differences regarding connective tissue proliferation (Fig. 2G). Figures 3 and 4 show histological preparations of the zirconia and titanium implants of one rat of the zoledronic acid group. Not only the control groups but also the test groups showed a large BIC area. Squamous epithelium of the gingiva in the area of the implant shoulder and the adjacent osteoid and new bone formation were detected in these examples. No evidence of inflammatory reaction or necrosis was noted in this example of the test group.
A A zirconia implant in the zoledronic acid test group. B Squamous epithelium of the gingiva is clearly visible (#). C, D Implant tip revealed a large BIC area. The adjacent osteoid and new bone formation was detected (+). E Focally, new bone formation was present (+). No evidence of inflammatory reaction or necrosis was found. F Beside the neck of the implant, circumscribed connective tissue formation was visible (*)
A Titanium implant in the zoledronic acid test group. B Squamous epithelium of the gingiva (#) and connective tissue (*) adjacent to the implant neck. C, D Osteoid and bone formation (+) with focal BIC can be seen laterally and in the area of the implant tip. E No evidence of inflammatory response or necrosis. F Squamous epithelium of the gingiva (#) and connective tissue adjacent to the implant neck
Discussion
This study aimed to evaluate histopathological implant osseointegration comparing immediate zirconia versus titanium implants in the rat maxilla under systemic antiresorptive therapy. An animal model using rats for the study of dental surgical interventions in relation to wound healing and Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) was previously described in the literature [23]. Additionally, the rat maxilla’s mesial root socket of the first molar is a good area for dental implant research [24]. At the end of our study, 62 implants were found to be integrated.
Studies have shown that surgical placement of dental implants in humans, regardless of the onset timing of bisphosphonates, is a risk factor for the development of osteonecrosis [25,26,27]. According to a literature review, the use of antiresorptive drugs increases the risk of osteonecrosis in patients with implants that are subjected to functional loading [28]. In our study, small signs of bone necrosis were detected only around zirconia implants in rats treated with alendronate. Signs of peri-implant infection could be detected in most of the groups, but the differences were not significant. A possible explanation for the low occurrence of necrosis zones is due to the fact that only implants that were still integrated after three months were examined. In the case of BRONJ, the implant may have been lost beforehand.
It has been shown that differences in implant design features influenced the osseointegration pathway [29]. Interactions between proteins, cells and tissues, but also implant surfaces can affect the implant integration. Surface treatments can differ outcomes and the surfaces applied in this study are not the same between zirconia and titanium implants [30]. However, it could be shown in a preclinical study, that both materials osseointegrated equally, so without significant difference [31]. Hou et al. [32] evaluated the osseointegration of titanium implants in rats with systemic zoledronate administration and found that bone-to-implant contact was negatively influenced by the drugs. Similarly, Dikicier et al. [33] demonstrated that there is an unfavorable implant osseointegration regarding the BIC value after administering systemic zoledronic acid in rats. In contrast, our results regarding the BIC ratio showed that there were no significant differences between the test groups and the control group.
Viera-Negron et al. [34] concluded that the osseointegration of implants in the rat maxilla was improved by the systemic administration of alendronic acid. In another study, systemic bisphosphonate delivery enhanced implant osseointegration in 15 animals with induced osteoporotic conditions [6]. Furthermore, in animal models, systemic injection of zoledronate improved osseointegration of orthopedic implants [20, 35]. However, because of the inherent variations between preclinical and clinical populations, the results regarding a meta-analysis should be regarded with caution [36]. The potential risk of bisphosphonate-related BRONJ in patients undergoing dental implant therapy cannot be disregarded.
The final implant survival of this study could be due to various causes. In this animal experiment, occlusal overload could only be excluded to a limited extent, despite soft food. Furthermore, the hygiene of the implants was only conditionally given, since food residues from the soft food could have accumulated around the implants. In addition, the tooth extraction and simultaneous immediate implantation could have led to an overstressing of the bone bed. Nevertheless, according to the inclusion criteria, only integrated implants at the end of the study were enrolled in this assessment.
The aim of this investigation was to analyze a high-risk group for dental implantation due to a systemic antiresorptive medication in the rat model using a high application rate. We applied antiresorptive medication per body weight comparable with that of humans. We recognize that pathophysiology can vary between rodents and humans [37,38,39,40,41,42,43]. Intravenous administration, as applied in this study, is associated with a higher risk than oral administration (i.v.—> oral application) [44]. While no single animal model can entirely replicate human bone and joint formation and repair, different species can help with implant development [45]. It should be noted that there are differences in bone behavior between rats and humans. Bone growth in rats lasts significantly longer than it does in humans following sexual maturity [45]. At places like the jaw bones, rats have better bone repair potential than humans. Further limitations were that only histological results with the the “BIC” osseointegration value at one time point were measured. It also should be noted that each specific implant surface individually influences the osseointegration behavior.
Conclusions
To find out if the various materials behave differently during osseointegration, more preclinical and clinical research is required. Regarding osseointegration parameters under systemic antiresorptive therapy, no implant material was clearly superior to the others at the 3-month follow-up.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Zhang Q, Yu W, Lee S, Xu Q, Naji A, Le AD. Bisphosphonate induces osteonecrosis of the jaw in diabetic mice via NLRP3/caspase-1-dependent IL-1beta mechanism. J Bone Miner Res. 2015;30:2300–12.
Marx RE. A decade of bisphosphonate bone complications: what it has taught us about bone physiology. Int J Oral Maxillofac Implants. 2014;29(2):e247-258.
Walter C, Sagheb K, Bitzer J, Rahimi-Nedjat R, Taylor KJ. Analysis of reasons for osteonecrosis of the jaws. Clin Oral Investig. 2014;18(9):2221–6.
Cole LE, Vargo-Gogola T, Roeder RK. Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Adv Drug Deliv Rev. 2016;99(Pt A):12–27.
Then C, Horauf N, Otto S, Pautke C, von Tresckow E, Rohnisch T, Baumann P, Schmidmaier R, Bumeder I, Oduncu FS. Incidence and risk factors of bisphosphonate-related osteonecrosis of the jaw in multiple myeloma patients having undergone autologous stem cell transplantation. Onkologie. 2012;35(11):658–64.
Vohra F, Al-Rifaiy MQ, Almas K, Javed F. Efficacy of systemic bisphosphonate delivery on osseointegration of implants under osteoporotic conditions: lessons from animal studies. Arch Oral Biol. 2014;59(9):912–20.
Deutsche Gesellschaft für Zahn- M-uKeV, Düsseldorf, Germany: Zahnimplantate bei medikamentöser Behandlung mit Knochenantiresorptiva (inkl. Bisphosphonate). 2016. https://www.dgzmk.de/zahnimplantate-bei-medikamentoeser-behandlung-mit-knochenantiresorptiva-inkl.-bisphosphonate-s3-.
Javed F, Almas K. Osseointegration of dental implants in patients undergoing bisphosphonate treatment: a literature review. J Periodontol. 2010;81(4):479–84.
Branemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstruct Surg Suppl. 1977;16:1–132.
Lekholm U, Zarb G. Patient selection and preparation. In: Branemark P, editor. Tissue integrated prosthesis: osseointegration in clinical dentistry. Chicago: Quintessence Publishing Co Inc; 1985. p. 199–209.
Ichikawa Y, Akagawa Y, Nikai H, Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo. J Prosthet Dent. 1992;68(2):322–6.
Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: a histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005;7(Suppl 1):S13-20.
Kohal RJ, Wolkewitz M, Hinze M, Han JS, Bachle M, Butz F. Biomechanical and histological behavior of zirconia implants: an experiment in the rat. Clin Oral Implant Res. 2009;20(4):333–9.
Thiem DGE, Stephan D, Kniha K, Kohal RJ, Röhling S, Spies CB, Stimmelmayr M, Grötz KA. German S3 guideline on the use of dental ceramic implants. Int J Implant Dent. 2022;8(1):43.
Spies BC, Balmer M, Patzelt SB, Vach K, Kohal RJ. Clinical and patient-reported outcomes of a zirconia oral implant: three-year results of a prospective cohort investigation. J Dent Res. 2015;94(10):1385–91.
Gahlert M, Rohling S, Wieland M, Sprecher CM, Kniha H, Milz S. Osseointegration of zirconia and titanium dental implants: a histological and histomorphometrical study in the maxilla of pigs. Clin Oral Implant Res. 2009;20(11):1247–53.
Kniha K, Kniha H, Mohlhenrich SC, Milz S, Holzle F, Modabber A. Papilla and alveolar crest levels in immediate versus delayed single-tooth zirconia implants. Int J Oral Maxillofac Surg. 2017;46(8):1039–44.
Kniha K, Schlegel KA, Kniha H, Modabber A, Holzle F, Kniha K. Evaluation of peri-implant bone levels and soft tissue dimensions around zirconia implants-a three-year follow-up study. Int J Oral Maxillofac Surg. 2018;47(4):492–8.
Abtahi J, Agholme F, Sandberg O, Aspenberg P. Effect of local vs. systemic bisphosphonate delivery on dental implant fixation in a model of osteonecrosis of the jaw. J Dent Res. 2013;92(3):279–83.
Bernhardsson M, Sandberg O, Aspenberg P. Anti-RANKL treatment improves screw fixation in cancellous bone in rats. Injury. 2015;46(6):990–5.
Kamal M, Andersson L, Tolba R, Al-Asfour A, Bartella AK, Gremse F, Rosenhain S, Hölzle F, Kessler P, Lethaus B. Bone regeneration using composite non-demineralized xenogenic dentin with beta-tricalcium phosphate in experimental alveolar cleft repair in a rabbit model. J Transl Med. 2017;15(1):263.
Kniha K, Buhl EM, Hermanns-Sachweh B, Al-Sibai F, Bock A, Peters F, Hölzle F, Modabber A. Implant removal using thermal necrosis-an in vitro pilot study. Clin Oral Investig. 2021;25(1):265–73.
Barba-Recreo P, de Del Castillo Pardo de Vera JL, García-Arranz M, Yébenes L, Burgueño M. Zoledronic acid—related osteonecrosis of the jaws. Experimental model with dental extractions in rats. J Cranio-Maxillo-Facial Surg Off Publ Eur Assoc Cranio-Maxillo-Facial Surg. 2014;42(6):744–50.
Du Z, Lee RS, Hamlet S, Doan N, Ivanovski S, Xiao Y. Evaluation of the first maxillary molar post-extraction socket as a model for dental implant osseointegration research. Clin Oral Implant Res. 2016;27(12):1469–78.
Giovannacci I, Meleti M, Manfredi M, Mortellaro C, Greco Lucchina A, Bonanini M, Vescovi P. Medication-related osteonecrosis of the jaw around dental implants: implant surgery-triggered or implant presence-triggered osteonecrosis? J Craniofac Surg. 2016;27(3):697–701.
Kuroshima S, Sasaki M, Sawase T. Medication-related osteonecrosis of the jaw: a literature review. J Oral Biosci. 2019;61(2):99–104.
Kuroshima S, Sasaki M, Murata H, Sawase T. Medication-related osteonecrosis of the jaw-like lesions in rodents: a comprehensive systematic review and meta-analysis. Gerodontology. 2019;36(4):313–24.
Escobedo MF, Cobo JL, Junquera S, Milla J, Olay S, Junquera LM. Medication-related osteonecrosis of the jaw. Implant presence-triggered osteonecrosis: case series and literature review. J Stomatol Oral Maxillofac Surg. 2020;121(1):40–8.
Bergamo ET, de Oliveira PG, Jimbo R, Neiva R, Gil LF, Tovar N, Witek L, Bonfante EA, Coelho PG. The influence of implant design features on bone healing pathways: an experimental study in sheep. Int J Periodontics Restor Dent. 2022.
Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23(7):844–54.
Gahlert M, Rohling S, Wieland M, Eichhorn S, Kuchenhoff H, Kniha H. A comparison study of the osseointegration of zirconia and titanium dental implants. A biomechanical evaluation in the maxilla of pigs. Clin Implant Dent Relat Res. 2010;12(4):297–305.
Hou M, Lee RSB, Du Z, Hamlet SM, Vaquette C, Ivanovski S. The influence of high-dose systemic zoledronate administration on osseointegration of implants with different surface topography. J Periodontal Res. 2019;54(6):633–43.
Dikicier E, Karaçaylı Ü, Dikicier S, Günaydın Y. Effect of systemic administered zoledronic acid on osseointegration of a titanium implant in ovariectomized rats. J Cranio-Maxillo-Facial Surg Off Publ Eur Assoc Cranio-Maxillo-Facial Surg. 2014;42(7):1106–11.
Viera-Negron YE, Ruan WH, Winger JN, Hou X, Sharawy MM, Borke JL. Effect of ovariectomy and alendronate on implant osseointegration in rat maxillary bone. J Oral Implantol. 2008;34(2):76–82.
Kim JH, Park YB, Li Z, Shim JS, Moon HS, Jung HS, Chung MK. Effect of alendronate on healing of extraction sockets and healing around implants. Oral Dis. 2011;17(7):705–11.
He Y, Bao W, Wu XD, Huang W, Chen H, Li Z. Effects of systemic or local administration of zoledronate on implant osseointegration: a preclinical meta-analysis. Biomed Res Int. 2019;2019:9541485.
Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 2010;16(7):674–85.
Abtahi J, Agholme F, Sandberg O, Aspenberg P. Bisphosphonate-induced osteonecrosis of the jaw in a rat model arises first after the bone has become exposed. No primary necrosis in unexposed bone. J Oral Pathol Med. 2012;41(6):494–9.
Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitriou C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580–7.
Walter C, Al-Nawas B, Grotz KA, Thomas C, Thuroff JW, Zinser V, Gamm H, Beck J, Wagner W. Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur Urol. 2008;54(5):1066–72.
Giro G, Sakakura CE, Gonçalves D, Pereira RMR, Marcantonio E Jr, Orrico SRP. Effect of 17β-Estradiol and Alendronate on the removal torque of osseointegrated titanium implants in ovariectomized rats. J Periodontol. 2007;78(7):1316–21.
Duarte PM, de Vasconcelos Gurgel BC, Sallum AW, Filho GR, Sallum EA, Nociti FH Jr. Alendronate therapy may be effective in the prevention of bone loss around titanium implants inserted in estrogen-deficient rats. J Periodontol. 2005;76(1):107–14.
Choi H, Lee JH, Lee JH, Kim JH. Genetic association between VEGF polymorphisms and BRONJ in the Korean population. Oral Dis. 2015;21(7):866–71.
Walter C, Al-Nawas B, Wolff T, Schiegnitz E, Grötz KA. Dental implants in patients treated with antiresorptive medication—a systematic literature review. Int J Implant Dent. 2016;2(1):9. https://doi.org/10.1186/s40729-40016-40041-40727.
Wancket LM. Animal models for evaluation of bone implants and devices: comparative bone structure and common model uses. Vet Pathol. 2015;52(5):842–50.
Acknowledgements
The authors thank the Straumann GmbH for the fabrication of the dental implants. Furthermore, the authors thank Nicole Bataille for her excellent technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the START funding program of the medical faculty of Aachen University. It was part of research project 105/18.
Author information
Authors and Affiliations
Contributions
KK: conception and design, acquisition of data, drafting the work, final approval. BH-S: acquisition of data, analysis and interpretation, revising the work, final approval. SCM: conception and design, revising the work, final approval. FP: acquisition of data, revising the work, final approval. MH: conception and design, revising the work, final approval. PW: analysis and interpretation, revising the work, final approval. FH: analysis and interpretation, revising the work, final approval. AM: conception and design, analysis and interpretation, drafting the work, final approval. AND to have approved the submitted version (and any substantially modified version that involves the author's contribution to the study); AND to have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
We confirm that all experiments were performed in accordance with relevant named guidelines and regulations that the authors complied with the ARRIVE guidelines. The study protocol was approved by the local ethics commission for animal studies. For this type of study, formal consent is not required.
Consent for publication
Not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kniha, K., Hermanns-Sachweh, B., Möhlhenrich, S.C. et al. Effect of systemic antiresorptive medication on the histopathological parameters of implant osseointegration in an in vivo rodent study. BMC Oral Health 23, 117 (2023). https://doi.org/10.1186/s12903-023-02763-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-02763-z