Abstract
Aim
This study compared the effectiveness of several techniques in restoring compromised bonding to recently bleached enamel.
Methods
Seventy-five healthy bovine incisors were divided into five groups (n = 15). Fifteen teeth (Group 1) remained intact, whereas 60 (Groups 2 to 5) underwent at-home bleaching with 16% carbamide peroxide. The bonding procedures were as follows: Group 1: Bonding of resin composite to unbleached enamel; Group 2: Bonding immediately after bleaching; Group 3: Application of a 10% sodium ascorbate solution for 10 min before bonding; Group 4: Enamel removal to the depth of 0.5 mm; and Group 5: Increased curing time of the bonding agent to 80 instead of 20 s. After 24 h, the specimens were subjected to micro-shear testing, and the failure mode was determined.
Results
ANOVA revealed a significant difference in bond strength among the groups (P < 0.001). The mean bond strength was significantly lower in group 2 than in other groups (P < 0.05), which showed comparable bond strength to each other (P > 0.05). Adhesive failure was the most predominant failure type in all groups. The mixed failure occurred with a frequency of 26.7% in groups 3 and 5. The Fisher’s exact test revealed a significant difference in failure modes among the groups (P = 0.047).
Conclusions
The three experimental procedures used in this study, including the application of 10% sodium ascorbate before bonding, enamel removal to the depth of 0.5 mm, and increasing the curing time of the bonding agent to 80 s, were effective in restoring the compromised bonding to recently bleached enamel.
Similar content being viewed by others
Introduction
In contemporary dental practice, a growing number of patients seek dental treatment to achieve whiter teeth. Among the various techniques available, tooth bleaching has emerged as a popular, cost-effective, and minimally invasive method for improving dental and smile aesthetics [1, 2]. It is often employed either as a standalone treatment or as a preliminary step before conducting more aggressive restorative procedures, such as composite resin or porcelain laminate veneers.
Tooth whitening relies on an oxidation reaction. The breakdown of hydrogen peroxide (H2O2) generates oxygen free radicals that penetrate the enamel surface and fragment large pigment molecules into small ones, resulting in tooth whitening. However, the bleaching products may have detrimental effects on dental structures. The severity of these effects is dependent on the whitening technique applied, the concentration and the pH of the whitening agent used and the duration of treatment.
It has been demonstrated that the bleaching process can lead to morphological alterations in the tooth surface, diminish calcium content, and increase enamel surface roughness [3,4,5,6,7]. Another side effect of tooth whitening is the reduction in bond strength of restorations applied immediately after the bleaching treatment [4]. This reduction in adhesion strength is primarily attributed to the residual peroxide within the dental substrate, which hinders the polymerization of resin-based materials and interferes with resin infiltration [4, 8,9,10]. Others believe that the changes in morphological, compositional, and mechanical properties of bleached enamel may contribute to the decline in bond strength [4, 11].
Several strategies have been proposed to mitigate the adverse impact of bleaching on the bond strength of composite resin restorations. One widely advocated strategy involves postponing the bonding process for a period ranging from 1 to 3 weeks following bleaching [4, 12,13,14]. This delay allows for the removal of residual materials through the buffering action of saliva. However, this approach may not be feasible for all patients, particularly those with time constraints [15]. Another option is the application of antioxidants to react with the remaining oxygen radicals on the tooth surface, thereby restoring the adhesion interface [16,17,18,19]. Although various anti-oxidants have been proposed in the literature, sodium ascorbate is a commonly utilized choice among clinicians [20].
An alternative approach that can be employed in certain patients to enhance bond strength is the removal of the outermost layer of bleached enamel to a depth of 0.5–1 mm. This technique is suitable for individuals who require tooth reduction before undergoing esthetic procedures such as laminate or composite veneers, or those requiring extensive correction for abnormally shaped teeth. Since most of the changes induced by bleaching are believed to occur on the tooth surface, flattening the enamel may yield sufficient bond strength for adhesive restorations by eliminating the affected enamel [4].
Another possible option to restore compromised adhesion to bleached enamel is increasing the polymerization duration of dental adhesives. A previous study demonstrated that the extent of polymerization of adhesives on recently bleached dentin was enhanced by extending the curing period to 40–60 s as compared to 20 s [21]. It can be assumed that increasing the extent of polymerization would enhance bond strength to recently bleached enamel.
There are a few studies concerning the effect of enamel removal on enhancing bond strength to bleached substrate. Furthermore, little information is available on the impact of prolonged curing of adhesive on the bond strength of composite resin to bleached enamel. Therefore, this study aimed to compare the effectiveness of several techniques, including the application of a 10% sodium ascorbate solution before bonding, enamel removal to the depth of 0.5 mm, and an extended polymerization time of the bonding agent, in enhancing the micro-shear bond strength (µSBS) between composite resin and recently bleached enamel.
Materials and methods
The research protocol was approved by the ethics committee of Mashhad University of Medical Sciences (IR.MUMS.DENTISTRY.REC.1398.025). Seventy-five extracted bovine incisors were used in this in vitro study. They were obtained from sacrificed animals in a slaughterhouse. After cleaning off the tissue remnants and debris with a scaler, the teeth were evaluated under a stereomicroscope to discard those showing cracks, enamel detects, or caries. The selected teeth were kept in a 0.1% thymol solution for 1 week, then stored in normal saline until the time of the experiment.
Sample preparation
The roots were cut just below the cementoenamel junction by a diamond disk attached to a dental headpiece. Then, the crowns were mounted in polyvinyl chloride molds using self-curing acrylic resin, so that the enamel surface was faced upward and aligned with the horizon. The enamel surfaces were further flattened by wet grinding using 600, 1200, and 2500 grit silicon carbide papers.
Grouping and bonding procedure
The specimens were randomly divided into 5 groups of 15 each. In group 1 (positive control), no bleaching treatment was performed, whereas the teeth in groups 2 to 5 underwent at-home bleaching with 16% carbamide peroxide (Whiteness simple; FGM, Joinville, SC, Brazil). The bleaching gel was applied in 2–3 mm thickness over the enamel surface, for 8 h a day over 14 consecutive days. After each bleaching cycle, the teeth were thoroughly rinsed under running tap water and then kept in 100% humidity inside an incubator at 37 °C. The teeth in the control group were kept in deionized water at 37 °C for 14 days. After the last application of the bleaching gel, the teeth were thoroughly rinsed and underwent the bonding process, as explained in the following:
Group 1 (positive control): In this group, composite resin was bonded to unbleached enamel. The tooth surface was pumiced, etched with 37% phosphoric acid gel for 30 s, thoroughly rinsed with water, and dried. Then, a coat of bonding agent (Peak Universal Bond; Ultradent Products, Inc., South Jordan, UT, USA) was rubbed on the enamel surface and light-polymerized for 20 s using an LED unit at the intensity of 650 mW/cm2 (Bluephase C8; Ivoclar Vivadent, Liechtenstein). Afterwards, a tigon tube (internal diameter of 0.7 mm and a height of 1.5 mm) was placed on the enamel surface and filled with Filtek Z100 resin composite (A3 shade; 3 M ESPE, St Paul, MN, USA). The excess material surrounding the tube was removed and the specimen was photopolymerized for 40 s at the intensity of 650 mW/cm2.
Group 2 (negative control): In group 2, the composite resin was bonded to the enamel surface immediately after bleaching. The bonding procedure was performed as explained in group 1.
Group 3 (10% sodium ascorbate solution): The bleached specimens were treated with a 10% sodium ascorbate solution for 10 min before the bonding process. The solution was refreshed several times over the enamel surface. The samples were then thoroughly rinsed and subjected to the bonding process, as explained in group 1.
Group 4 (removal of bleached enamel): The bleached enamel surface was removed up to the depth of 0.0.5 mm using a diamond bur. The surface was then ground flat by 600, 1200, and 2500 grit silicon carbide papers under water irrigation and rinsed to remove any debris. The extent of enamel reduction was continuously measured by a micrometer (Mitutoyo, Kyoto, Japan). The specimens were then bonded, as explained previously.
Group 5 (increased curing time of the bonding agent): The bleached enamel surface was immediately bonded with resin composite, but the curing time of the bonding agent was extended to 80 s. The other procedures were similar to that explained in group 1.
Measurement of micro-shear bond strength (µSBS)
After the bonding process, the composite cylinders were stored in deionized water at 37ºC for 24 h. Then the tubes were separated with a blade and the specimens were subjected to micro shear testing in a universal machine (Santam, Tehran, Iran). A 0.2 mm diameter stainless steel wire loop was placed around the composite cylinder at the enamel interface, and the debonding force was applied at a cross-head speed of 1.0 mm/min. The maximum failure load was recorded in Newton (N) and divided by the surface area of the composite cylinder (mm2) to give the bond strength in megapascals (MPa).
Failure mode analysis
After the bond strength test, the fracture surface was examined at x 20 magnification to determine the type of failure. Bond failures were classified as adhesive (fracture at the interface of enamel and composite resin), cohesive (fracture within the enamel or the composite resin), or mixed (the simultaneous occurrence of adhesive and cohesive fractures).
Statistical analysis
The results were analyzed by the SPSS software (version 16.0, SPSS Inc, Chicago, IL, USA). The assumption of normality was confirmed using the Shapiro-Wilk test (P>0.05). One-way analysis of variance (ANOVA) was run to detect any significant difference in bond strength among the study groups, followed by the post hoc Tukey test for pairwise comparisons. The difference in the type of failure was assessed by Fisher’s exact test. A P-value<0.05 was considered significant.
Results
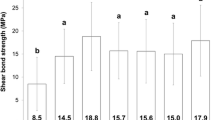
Table 1 presents the mean, standard deviation (SD), minimum (Min), and maximum (Max) values of micro-shear bond strength (MPa) in the study groups. The lowest bond strength (4.83 ± 3.28) was observed in the negative control group (group 2), in which the specimens underwent bonding immediately after bleaching. The highest µSBS value was observed in group 4 (removal of bleached enamel) and group 3 (10% sodium ascorbate), showing values of 11.87 ± 4.02 MPa and 11.79 ± 2.96 MPa, respectively. Bond strength in group 5 (increased curing time of the bonding agent) was 8.93 ± 2.92 MPa, which was lower than that of the unbleached control group (10.91 ± 3.82 MPa).
ANOVA revealed a significant difference in bond strength among the study groups (p<0.001; Table 1). Tukey test revealed that the mean bond strength was significantly lower in group 2 than in all the other groups (P>0.05; Table 1). Bond strength in the three experimental groups (groups 3, 4, and 5) was comparable to each other and to the positive control group (P>0.05; Table 1). Figure 1 illustrates a comparison of bond strength values in the study groups.
Table 2 presents the frequency and percentage of different failure modes in the study groups. Adhesive failure was the most predominant failure type and occurred in 82.7% of all specimens, whereas cohesive failure was observed in only 3 teeth (4%). Fisher’s exact test revealed a significant difference in the type of failure among the study groups (P = 0.047; Table 2). In the positive control group, all teeth showed adhesive failure. The mixed type of failure was observed in 26.7% of the specimens in groups 3 and 5.
Discussion
The present investigation examined various techniques to enhance the adhesive strength between resin composite and bovine enamel immediately following a bleaching procedure. Bovine incisors were employed due to their availability, flat surface, and morphological and chemical similarities to human teeth [4, 22, 23]. At-home bleaching was selected in this study employing a 16% carbamide peroxide gel for two weeks. This bleaching method is associated with several advantages including low cost, ease of application, desirable efficacy, and good stability, making it a popular choice among both patients and dental professionals [5, 24]. Bond strength was measured by the µSBS test, as it provides a more consistent distribution of stress at the bonded interface than the macro-shear bond test [4, 22].
The outcomes of this study revealed a significantly lower bond strength (4.83 ± 3.28 MPa) in specimens bonded immediately after the bleaching process, as compared to the other study groups. The three experimental procedures were effective in enhancing the bond strength of resin composite to bleached enamel and yielded bond strength values that were comparable to that of the unbleached specimens (10.91 ± 3.82 MPa). Among these procedures, enamel removal (11.87 ± 4.02 MPa) and the application of a 10% sodium ascorbate solution before the bonding process (11.79 ± 2.96 MPa) demonstrated the highest bond strength among the groups. Extending the curing time of the bonding agent resulted in slightly lower but comparable adhesion (8.93 ± 2.92 MPa) when compared to the unbleached control specimens.
Numerous studies exhibited a decrease in bond strength immediately following a bleaching procedure [14, 25,26,27,28,29]. This decrease is attributed to the presence of peroxide within the bleached substrate, which can inhibit resin polymerization and diminish the formation and penetration of resin tags into bleached enamel [4, 8,9,10]. Additionally, alterations in the mineral and organic composition of enamel, as well as surface morphological changes, can contribute to reduced adhesion to recently bleached enamel [4, 11].
In the present study, the bleached teeth treated with a 10% sodium ascorbate solution showed comparable bond strength to that of the unbleached specimens. The ascorbic acid salt is well known as a powerful anti-oxidant agent and there is ample evidence supporting its ability to counteract the oxidizing effect of peroxide-containing materials [15, 20, 28]. However, the efficacy of sodium ascorbate in mitigating the effects of bleaching agents depends on various factors such as its concentration and the duration of application, as well as the concentration and application time of the bleaching agent [30, 31].
In this study, a 10% sodium ascorbate solution was applied to the tooth surface for 10 min. Previous investigations have explored different concentrations of sodium ascorbate, ranging from 2.5 to 35% [20]. There is evidence suggesting that a minimum concentration of 10% is necessary to provide an antioxidant effect [20, 32]. Regarding the duration of sodium ascorbate application, prior studies have employed intervals ranging from 2 min to 180 min [20, 33]. However, it has been noted that prolonged intervals do not necessarily lead to enhanced bond strength, as the majority of reactions occur within the initial minutes after antioxidant application. Furthermore, long application intervals can be impractical in a clinical setting. Given the rapid loss of antioxidant efficacy, it is assumed that the frequency of antioxidant application is more critical than the duration of contact with tooth structure [15]. For this reason, the sodium ascorbate solution was frequently reapplied to the tooth surface in this study. Although some studies have advocated for the use of sodium ascorbate in gel form as opposed to liquid [15, 20], Kimyai and Valizadeh [34] indicated that both hydrogel and solution forms of sodium ascorbate effectively enhance the bond strength of resin composite to bleached enamel.
Enamel reduction to a depth of 0.5 to 1 mm is commonly performed in dental procedures involving veneers and other restorative treatments. The outcomes of this study revealed that removing 0.5 mm of enamel is an effective approach to enhance the bond strength of resin materials to teeth immediately after the bleaching treatment. Cheng et al. [4] also indicated that reducing the enamel surface by 0.5 mm resulted in micro shear bond strength (µSBS) values similar to those of the unbleached group. They argued that the release of free oxygen radicals and the resulting structural changes primarily occur at the surface, making the enamel reduction very effective in neutralizing the detrimental effects of bleaching procedures [4]. Surmelioglu et al. [35] reported that both enamel surface reduction and Er, Cr: YSGG laser conditioning were effective in eliminating the undesirable effects of the bleaching treatment, resulting in bond strength values comparable to that of the control specimens.
Based on the findings of this research, extending the curing time of the bonding agent to 80 s, as opposed to the standard 20 s, resulted in comparable bond strength to that of the unbleached control group. This finding supports the hypothesis that the extended curing time enhances the degree of polymerization. This can neutralize to some extent the inhibitory effects of oxygen free radicals trapped in the enamel structure on polymerization of dental adhesives, thereby improving adhesion to bleached enamel. It is also possible that increasing the curing time leads to raising the temperature of the enamel surface, which helps to release oxygen-free radicals from the tooth structure. However, further research is required to confirm this assumption. The outcomes of this study confirm the study of Cadenaro et al. [21]. They noted reduced polymerization (degree of monomer conversion) in adhesive systems bonded to dentin immediately after the whitening process when curing was limited to 20 s. However, longer curing periods (40–60 s) led to a significantly higher rate of monomer conversion for all the tested adhesive formulations [21]. The findings of the present study also demonstrate that incomplete polymerization is probably the main reason for decreased bond strength immediately after bleaching, as prolonged polymerization resulted in improved adhesion to bleached enamel. In contrast to the findings of this study, our previous investigation [22] indicated that extending the polymerization time of adhesive systems did not effectively counteract the inhibition of polymerization induced by the bleaching procedure on dentin. The disparities in these results may be attributed to variations in the substrates used (dentin versus enamel) or differences in the methodology across these studies.
In the present study, the mode of bond failure was predominantly adhesive in all groups, with an overall frequency of 82.7%, whereas cohesive failure was rarely observed in the sample. A mixed mode of failure was observed in 26.7% of specimens within groups 3 (10% sodium ascorbate) and 5 (prolonged curing time of adhesive). All specimens in the positive control groups showed adhesive type of failure. The type of bond failure exhibited significant variations among the study groups. However, teeth bonded immediately after bleaching exhibited a failure mode similar to that observed in groups with substantially higher bond strength values, suggesting a lack of correlation between the failure mode and bond strength in bleached enamel.
Consistent with the findings of this study, Kılınç et al. [12] reported a higher incidence of adhesive failure across all groups, with other failure modes being rare. In contrast, Türkün et al. [32] and Cheng et al. [4] noted a mixed type of failure occurring more frequently in all groups. Miranda et al. [36] reported that 52% of failures were of a mixed nature, whereas 28% were adhesive. These discrepancies between our results and those of previous studies may be attributed to variations in concentrations and contact durations of bleaching agents, and differences in immediate versus delayed bonding procedures.
The in vitro setting of this study should be considered as its main limitation. Moreover, the investigation examined a single type of bleaching material and one specific adhesive system. It is worth noting that the efficacy of sodium ascorbate may diminish when applied to teeth treated with greater concentrations of bleaching products, as they release an increased amount of oxygen into the dental substrate [20]. It is essential to exercise caution when extrapolating the findings of this study to office-based bleaching, which employs higher hydrogen peroxide concentrations. Further research is recommended to explore the effects of enamel removal and extended curing times of bonding agents within a clinical scenario, using various adhesive systems, and bleaching products and protocols.
Conclusion
Within the limitations of this study, the following conclusions can be established:
-
1.
A significant reduction in bond strength was observed when bonding to enamel was performed immediately after the bleaching treatment.
-
2.
The three experimental procedures employed in this study, including the application of 10% sodium ascorbate solution, enamel removal to a depth of 0.5 mm, and extension of adhesive curing time to 80 s, proved effective in restoring the compromised bonding to recently bleached enamel.
-
3.
There was a significant difference in the mode of failure among the groups. However, it appeared that the mode of bond failure did not exhibit any correlation with the bond strength of resin composite to bleached enamel.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Mohammadipour HS, Bagheri H, Khorshid M, Akbari M, Akhlaghi S. Tooth sensitivity and whitening effect of an in-office bleaching gel containing sodium hexametaphosphate: a randomized triple-blind clinical trial. J Dent Mater Tech. 2023;12(1). https://doi.org/10.22038/JDMT.2023.66578.1527.
Ahrari F, Akbari M, Mohammadpour S, Forghani M. The efficacy of laser-assisted in-office bleaching and home bleaching on sound and demineralized enamel. Laser Ther. 2015;24(4):257–64. https://doi.org/10.5978/islsm.15-OR-15.
Poorni S, Kumar RA, Shankar P, Indira R, Ramachandran S. Effect of 10% sodium ascorbate on the calcium: Phosphorus ratio of enamel bleached with 35% hydrogen peroxide: an in vitro quantitative energy-dispersive X-ray analysis. Contemp Clin Dent. 2010;1(4):223–6. https://doi.org/10.4103/0976-237x.76388.
Cheng YL, Musonda J, Cheng H, Attin T, Zheng M, Yu H. Effect of surface removal following bleaching on the bond strength of enamel. BMC Oral Health. 2019;19(1):50. https://doi.org/10.4103/0975-7406.11328510.1186/s12903-019-0742-4.
Ghanbarzadeh M, Ahrari F, Akbari M, Hamzei H. Microhardness of demineralized enamel following home bleaching and laser-assisted in office bleaching. J Clin Exp Dent. 2015;7(3):e405–9. https://doi.org/10.4317/jced.51705.
Ahrari F, Akbari M, Mohammadipour HS, Fallahrastegar A, Sekandari S. The efficacy and complications of several bleaching techniques in patients after fixed orthodontic therapy. A randomized clinical trial. Swiss Dent J. 2020;130(6):493–501.
Moosavi H, Arjmand N, Ahrari F, Zakeri M, Maleknejad F. Effect of low-level laser therapy on tooth sensitivity induced by in-office bleaching. Lasers Med Sci. 2016;31(4):713–9. https://doi.org/10.1007/s10103-016-1913-z.
Titley KC, Torneck CD, Smith DC, Chernecky R, Adibfar A. Scanning electron microscopy observations on the penetration and structure of resin tags in bleached and unbleached bovine enamel. J Endod. 1991;17(2):72–5. https://doi.org/10.1016/s0099-2399(06)81611-0.
Briso AL, Toseto RM, Rahal V, dos Santos PH, Ambrosano GM. Effect of sodium ascorbate on tag formation in bleached enamel. J Adhes Dent. 2012;14(1):19–23. https://doi.org/10.3290/j.jad.a21492.
Wilson D, Xu C, Hong L, Wang Y. Effects of different preparation procedures during tooth whitening on enamel bonding. J Mater Sci Mater Med. 2009;20(4):1001–7. https://doi.org/10.1007/s10856-008-3657-1.
Hussain M, Wang Y. Influence of prolonged light-curing time on the shear bonding strength of resin to bleached enamel. Oper Dent. 2010;35(6):672–81. https://doi.org/10.2341/10-095-l.
Kılınç H, Aslan T, Kılıç K, Er Ö, Kurt G. Effect of delayed bonding and antioxidant application on the bond strength to Enamel after Internal Bleaching. J Prosthodont. 2016;25(5):386–91. https://doi.org/10.4103/ccd.ccd_234_1710.1111/jopr.12303.
de Almeida AA, Lima DM, Pereira AF, Sousa SF, Alves CM. Influence of delay between dental bleaching with 35% hydrogen peroxide and orthodontic brackets on the bond strength at the enamel/adhesive interface. J Clin Exp Dent. 2019;11(5):e447–51. https://doi.org/10.4317/jced.55719.
Boccuzzi M, Nota A, Cosola S, De Simone G, Iozzo R, Pittari L, et al. Effect of bleaching treatments on the adhesion of orthodontic brackets: a systematic review. BMC Oral Health. 2023;23(1):758. https://doi.org/10.1186/s12903-023-03418-9.
Murad CG, de Andrade SN, Disconzi LR, Munchow EA, Piva E, Pascotto RC, et al. Influence of 10% sodium ascorbate gel application time on composite bond strength to bleached enamel. Acta Biomater Odontol Scand. 2016;2(1):49–54. https://doi.org/10.3109/23337931.2016.1152901.
Olmedo D, Kury M. Use of antioxidants to restore bond strength after tooth bleaching with peroxides. Eur J Oral Sci. 2021;129(2):e12773. https://doi.org/10.1111/eos.12773.
Feiz A, Mosleh H, Nazeri R. Evaluating the effect of antioxidant agents on shear bond strength of tooth-colored restorative materials after bleaching: a systematic review. J Mech Behav Biomed Mater. 2017;71:156–64. https://doi.org/10.1016/j.ortho.2023.10077710.1016/j.jmbbm.2017.03.010.
Nari-Ratih D, Widyastuti A. Effect of antioxidants on the shear bond strength of composite resin to enamel following extra-coronal bleaching. J Clin Exp Dent. 2019;11(2):e126–32. https://doi.org/10.4103/2278-0203.16023910.4317/jced.55359.
Lopes ALC, Ribeiro MES. Does the Elapsed Time from Bleaching and the Use of Sodium Ascorbate Influence the Bond Strength of Resin Cement to bleached Enamel? Materials. 2023;16(18). https://doi.org/10.1111/jopr.1230310.3390/ma16186328.
Pathak K, Kumar P, Choudhary A, Shekh TM, Gosai P, Patnana AK. Comparative Analysis of Shear Bond Strength of Composites to the Sodium Ascorbate Hydrogel-treated bleached Enamel surfaces: an in Vitro Analysis. Int J Clin Pediatr Dent. 2021;14(6):741–7. https://doi.org/10.5005/jp-journals-10005-2068.
Cadenaro M, Breschi L, Antoniolli F, Mazzoni A, Di Lenarda R. Influence of whitening on the degree of conversion of dental adhesives on dentin. Eur J Oral Sci. 2006;114(3):257–62. https://doi.org/10.1111/j.1600-0722.2006.00351.x.
Moosavi H, Nemati-Karimooy A, Rezaei F, Yavari Z, Ahrari F. Does the application of whitening dentifrices during at-home bleaching affect the bond strength of resin composite to dentin? BMC Oral Health. 2022;22(1):644. https://doi.org/10.1186/s12903-022-02680-7.
Ahrari F, Hasanzadeh N, Rajabi O, Forouzannejad Z. Effectiveness of sodium bicarbonate combined with hydrogen peroxide and CPP-ACPF in whitening and microhardness of enamel. J Clin Exp Dent. 2017;9(3):e344–50. https://doi.org/10.4317/jced.53108.
Irusa K, Abd Alrahaem I, Ngoc CN, Donovan T. Tooth whitening procedures: a narrative review. Dent Rev. 2022;100055. https://doi.org/10.1016/j.dentre.2022.100055.
Sardarian A, Malekpour B, Roshan A, Danaei SM. Bleaching during orthodontic treatment and its effect on bracket bond strength. Dent Res J (Isfahan). 2019;16(4):245–50. https://doi.org/10.1016/j.jmbbm.2017.03.010.
Kunt GE, Yılmaz N, Sen S, Dede D. Effect of antioxidant treatment on the shear bond strength of composite resin to bleached enamel. Acta Odontol Scand. 2011;69(5):287–91. https://doi.org/10.3109/00016357.2011.568958.
Topcu FT, Erdemir U, Ozel E, Tiryaki M, Oktay EA, Yildiz E. Influence of bleaching regimen and time elapsed on Microtensile Bond Strength of Resin Composite to Enamel. Contemp Clin Dent. 2017;8(3):451–8. https://doi.org/10.4103/ccd.ccd_234_17.
Mena-Serrano A, Aldás Fierro E, Estrada X, Boada A, Wendlinger M, Favoreto MW, et al. Effect of Sodium Ascorbate, grape seed extract, and Aloe Vera Application after In-Office bleaching on the bond strength of Enamel: a 3-Year evaluation. Int J Dent. 2023;2023:4625818. https://doi.org/10.1155/2023/4625818.
Moosavi H, Mohammadipour HS, Ghavamnasiri M, Alizadeh S. Effect of bleaching and thermocycling on Resin-Enamel Bond Strength. Int J Biomater. 2015;2015:921425. https://doi.org/10.1155/2015/921425.
Zaki SS, Ghorab SM, Shamaa MS. Antioxidant effect on shear bond strength of orthodontic brackets after tooth bleaching: a scoping review of in vitro studies. Eur J Oral Sci. 2023;21(3):100777. https://doi.org/10.1111/eos.1277310.1016/j.ortho.2023.100777.
Baia JCP, Oliveira RP, Ribeiro MES, Lima RR. Influence of prolonged Dental Bleaching on the Adhesive Bond Strength to Enamel surfaces. Int J Dent. 2020;2020:2609359. https://doi.org/10.1155/2020/2609359.
Türkün M, Celik EU, Kaya AD, Arici M. Can the hydrogel form of sodium ascorbate be used to reverse compromised bond strength after bleaching? J Adhes Dent. 2009;11(1):35–40. https://doi.org/10.3290/j.jad.a14730.
Freire A, Durski MT, Ingberman M, Nakao LS, Souza EM, Vieira S. Assessing the use of 35% sodium ascorbate for removal of residual hydrogen peroxide after in-office tooth bleaching. J Am Dent Assoc. 2011;142(7):836–41. https://doi.org/10.14219/jada.archive.2011.0273.
Kimyai S, Valizadeh H. Comparison of the effect of hydrogel and a solution of sodium ascorbate on dentin-composite bond strength after bleaching. J Contemp Dent Pract. 2008;9(2):105–12.
Surmelioglu D, Ozdemir ZM, Atilan S, Yeniceri NE. Effect of surface flattening and phototherapy on shear bond strength immediately after bleaching with different modes of universal adhesive. Niger J Clin Pract. 2020;23(1):110–5. https://doi.org/10.4103/njcp.njcp_337_19.
Miranda TA, Moura SK, Amorim VH, Terada RS, Pascotto RC. Influence of exposure time to saliva and antioxidant treatment on bond strength to enamel after tooth bleaching: an in situ study. J Appl Oral Sci. 2013;21(6):567–74. https://doi.org/10.1590/1679-775720130035.
Acknowledgements
The authors would like to thank the vice-chancellor for research of Mashhad University of Medical Sciences for the financial support of this project (grant number 971524). The results presented in this work have been taken from a student thesis (thesis number 3111).
Funding
The study was supported by a grant from the vice-chancellor for research at Mashhad University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
HM and HH developed the project, helped in data analysis, and edited the manuscript. FR and ZSZM collected the data and edited the manuscript. FA helped with data collection, analyzed the data, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research protocol was approved by the ethics committee of Mashhad University of Medical Sciences (IR.MUMS.DENTISTRY.REC.1398.025).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Moosavi, H., Hajizadeh, H., Mamaghani, Z.S.Z. et al. Comparison of various methods of restoring adhesion to recently bleached enamel. BMC Oral Health 24, 942 (2024). https://doi.org/10.1186/s12903-024-04656-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04656-1