Abstract
Backgrounds
This study aimed to compare the effects of different energy drinks on the surface roughness, weight loss, and color change of various bioactive restorative materials.
Methods
Charisma Diamond One, Activa™ BioActive Restorative, Activa™ Presto™ and Equia Forte HT Fil samples were prepared using plastic molds (8 × 2 mm) (n = 10/groups). After polishing, the samples were weighed, their colors were recorded using a spectrophotometer according to the CIEDE2000 system, and their surface roughness was measured using a profilometer. The samples were immersed in Powerade, Burn, Monster and distilled water for 7 days. After immersion, all the measurements were repeated. Statistical analyses were performed using the Wilcoxon signed-rank test and the Mann‒Whitney U test (p < 0.05).
Results
All energy drinks roughened the surface of Equia Forte HT Fil (p < 0.05). Powerade and Monster increased the Ra of all materials after 7 days (p < 0.05). Burns affected all materials except the Activa Bioactive (p < 0.05). Significant weight loss was observed in the Equia Forte group after immersion in all the energy drinks, whereas no weight loss was observed in the other groups. According to the color measurements, ΔE00 values were greater in the Burn and Monster groups, except for the Equia Forte HT Fil group (p < 0.05).
Conclusion
Energy drinks affected bioactive materials to varying degrees. The glass hybrid material was the most affected, and the bioactive restorative materials based on the resin matrix were the least.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Dental caries remains one of the most common diseases worldwide.Under normal conditions, demineralization and remineralization are in balance. Before the demineralization process, remineralization is most desirable and does not cause caries. Disruption of the balance towards demineralization causes caries formation on the tooth surface [1]. In the past, professionals chose amalgam as a restorative material option. Due to its disadvantages, such as aesthetic problems, excessive loss of tooth tissue for its application, and mercury safety, this material has been used less and less over time [2]. Therefore, resin composites and glass-based dental materials have been introduced to the market as alternatives to amalgam [3].
Resin composites are often preferred by professionals due to their optical and physical properties, as they are suitable for minimally invasive procedures [3]. Although the long-term performance of resin composite restorations has been proven, their disadvantages, such as difficult application, the need for strict moisture control, polymerization shrinkage, secondary caries, degradation of interfacial adhesion and lack of biocompatibility with periodontal tissues, cannot be neglected [3, 4]. Due to the positive dynamic relationship between bioactive materials and the tissues in which they are applied, bioactive materials are also widely used in dentistry [5].
Bioactive restorative materials, although their primary purpose is to restore and replace missing tooth structure, they both may actively stimulate cellular or tissue responses and control the interactions of microbiological species [6]. Modern dentistry has introduced biomaterials designed to promote apatite formation and the remineralization of tooth structure [6]. In cariology, bioactive materials can control caries and biofilm formation due to their mineral release and antibacterial properties. With the development of nanotechnology, the current biomaterials used to control caries formation include fluoride-based, calcium-based, phosphate-based, graphane-based, metal oxide nanomaterials, and peptide-based materials. For some biomaterials in this group, there are no commercial products yet because the clinical evidence for these materials is still insufficient, and further research is needed [7].
Glass ionomer cement (GIC) is considered a bioactive material because it contains biologically active ions. In GIC, fluoride ions inhibit the formation of secondary caries and fluorapatites for the remineralization process. Calcium, strontium, sodium, phosphate and silicate ions are also biologically active ions in their structure. Due to their adhesion layer, they can form an active layer with the tooth tissue without forming a fibrous encapsulation. The acidic polyalkenoid structure in GIC forms chemical bonds with the tooth structure and this mechanism is affected by the ability of mineralization of calcium phosphates on a material’s surface in vivo [8].
Newly developed bioactive materials include bioactive ionic resin and bioactive ionomer glass. Bioactive ionic resins release calcium (Ca2+), fluoride (F−) and phosphate (PO43−). In addition, fluorine-containing glass fillers in acidic media help to increase the resistance of bioactive restorative materials to solubility [9]. Bioactive glass filler can improve the mechanical properties and surface roughness of materials [9]. According to a previous study, bioactive restorative materials have a better resin matrix than GIC, and their physical properties, such as flexural strength and elastic modulus, are comparable to those of resin composites [10]. However, further studies are still needed to determine the effects of bioactive restorative materials [10].
For long-term successful restoration, apart from related factors such as operators and patients, the choice of restorative material is important [11]. Restorative materials in the oral environment are exposed to many acidic conditions. One of them might be caused by the constant consumption of energy drinks [12]. The uptake of energy drinks has increased among adolescents and adults [12]. A study reported that the consumption of energy drinks was related to a lack of knowledge about their caffeine content, which exceeded the recommended limit. Consumers who were aware of the potential side effects consumed these drinks significantly less. A previous study reported that if a person consumes 2 to 3 energy drinks per day, the risk of dental erosion will increase [12]. After their consumption, the oral cavity becomes acidic, and unexpected changes occur on the surface of the teeth and restorations. A previous study reported that an acidic environment adversely affects the surface and optical properties of restorative materials [13].
At low pH, discoloration of restorative materials may also occur. According to a previous study, the optical properties of restorative materials are affected by the filler size and composition of the material [14]. A higher filler content and larger monomers positively influence the color stability under acidic conditions [14]. Additionally, the polymer structures present may absorb moisture in the oral environment, the siloxane bond chains may break, and the monomers may be washed away by the solvents as saliva. Therefore, the solubility of the material surface may cause surface irregularities and discoloration [15, 16].
The combined effect of newly developed bioactive restorative materials on these parameters has not been widely studied. Considering the clinical significance of the effect of storage media on the surface and optical parameters of restorative materials, the purpose of this study was to evaluate the effect of different energy drinks on the surface roughness, solubility/erosion, and color change of different bioactive restorative materials. The null hypothesis was that energy drinks have no impact on the surface roughness, weight loss, or color change of bioactive materials or resin composites.
Methods
Four restorative materials were investigated: a nanohybrid resin composite (Charisma Diamond One), bioactive restorative materials (Activa™ BioActive Restorative/AB and Activa™ Presto™/AP), and a bulk-fill glass hybrid material (Equia Forte HT Fil/EF). The materials, lot numbers, types, and compositions are presented in Table 1. These materials were tested for surface roughness, weight loss and color change after 7 days of energy drink immersion [17,18,19].
A total of 160 disc samples of 8 × 2 mm (n = 10) were prepared according to the manufacturer’s instructions in plastic molds. Charisma Diamond One (CD) was placed in the mold in one increment using Optra Sculp Pad (Ivoclar Vivadent AG, Schaan, Liechtenstein) modeling instrument. Activa Bioactive Restorative (AB) is a two-paste system dispensed directly from its automix syringe into the mold, while Activa Presto (AP) is a flowable bioactive restorative material that is placed in one increment. Equia Forte (EF) was encapsulated and mixed for 10 s with a mechanical vibrator device (Silvermix90, GC). After the capsule was mixed, the material was placed into the molds using an applicator gun.
Mylar strips and glass slides were placed on the top and bottom sides of all specimens. Specimens, except for EF, were photopolymerized for 20 s on both sides using a third-generation polywave LED light-curing device (ZenoLite, President Dental, Germany) under standard curing mode with an output wavelength of 430–490 nm and an intensity of 1300 mW/cm2 in continuous curing mode with a perpendicular angle. The output of the light intensity was measured with a radiometer after every 10 samples preparations. After polymerization, all specimens were polished using all sizes of Sof-Lex discs (3 M ESPE, St. Paul, MN, USA). Polishing was performed under dry conditions for 15 s/disc for each sample with a 10 000 rpm handpiece at low speed with a one-way rotation movement, and each disc was used only once for all samples. After each polishing step, the samples were rinsed with a water spray for 10 s and air-dried for 5 s. To avoid operator variation, all finishing and polishing procedures were performed by the same operator. Then, the specimens were immersed in distilled water for 24 h and stored at room temperature.
Commercially available energy drinks—Powerade (Coca-Cola Co. Atlanta GA, United States), Burn (Coca-Cola Co. Atlanta GA, United States) and Monster (Monster Energy Limited, Corona, California, United States)—were purchased and opened before the immersion procedure. The energy drinks in all the groups were regularly changed every 24 h for 7 days and stored at 37 °C. The pH of each energy drink was measured using a digital pH meter (Mettler Toledo digital pH meter, Greifensee, Switzerland) at room temperature every day before sample immersions. Powerade (pH:3.57) contains water, glucose, citric acid, sodium citrate, potassium citrate, gum arabic, glyceric esters of wood resin, aspartame, acesulfame-k, vitamin B6. Burn (pH:2.5) contains carbonated water, sucrose, citric acid, taurine (0.4%), sodium citrate, E163, E150d, potassium sorbate, sodium benzoate, flavor, caffeine (0.03%), inositol, vitamins [nicotinamide (B3), d-calcium pantothenate, pyridoxine hydrochloride (B6), cyanocobalamin (B12)], seed extract of guarana (0.005%), antioxidants (ascorbic acid). Monster (pH:2.7) contains carbonated water, sugar, glucose, apple juice concentrate, orange juice concentrate, taurine, citric acid, guava puree, sodium citrate, potassium sorbate, panax ginseng flavor, caffeine, maltodextrin, gum arabic, sodium benzoate, sucralose, natural flavors and niacinamide.
Before all the samples were randomly divided into subgroups according to the presence of distilled water and three different energy drinks, initial measurements of color, weight and roughness were performed. After being immersed in the indicated beverages for 7 days, all measurements were repeated and recorded.
The average surface roughness (Ra, µm) of the specimens was measured using a surface profilometer (SurfTest SJ-301, Mitutoyo, Tokyo, Japan). The measurements were made with a cutoff length of 0.25 mm, a tracing length of 0.8 mm, and a stylus speed of 0.25 mm/s. The average roughness (Ra) values were derived from three measurements at different locations for each sample.
For weight loss, an electronic balance with the nearest 0,0001 g (Pioneer PA64, Ohaus, Pine Brook, USA) was used to evaluate the weight of the samples at baseline and after 7 days.
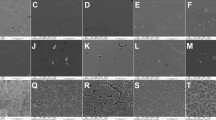
Color measurements according to the CIEDE2000 system were performed using a noncontact spectrophotometer (SpectroShade Micro, MHT, Milan, Italy) against a gray background under daylight conditions in air and at the same time of day to obtain initial values. The device was calibrated at the beginning of every five measurements according to the manufacturer’s recommendations using the white and dark calibration standard provided. The positioning of the device on the sample was achieved by an angle control system of the device, which calculates the optimal angle of incidence between the optic handpiece and a target sample. This angle was verified by a horizontal green line representing the accurate geometry. The spectral data obtained were translated into CIEDE2000 coordinates by software using the standard D65 illuminant and 2° observer angle as a reference. Three consecutive measurements were taken on the samples, and the mean value was recorded as the final value (ΔE00). The initial color measurement was performed after initial immersion in distilled water for 24 h. All the procedures are illustrated in Fig. 1.
For the calculation of the samples in this study, power analysis was performed for the Wilcoxon-Mann-Whitney test with the G*Power 3.1 (HeinrichHeine-Universitaet Duesseldorf, Germany) program and the number of samples was calculated as 30 for each group for power = 0.80, α error probability = 0.05 effect size (d) = 0.67. Each sample (n = 10) was evaluated for color measurements and surface roughness a total of 3 times.
All the statistical analyses were performed using statistical software (SPSS 25.0, IBM Corp., Armonk, NY, USA). Surface roughness values were analyzed using ANOVA and Tukey tests, while color change values were evaluated using the Wilcoxon signed-rank test and Mann‒Whitney U test. The p value was set to p < 0.05 for all tests.
Results
The surface roughness values (Ra, µm ) of the samples before and after immersion are shown in Table 2. The smoothest surfaces at the beginning were obtained with AB (0.29 μm ± 0.08) and AP (0.37 μm ± 0.12). Compared with the baseline values, the surfaces of all the groups, except the AB group, were roughened by distilled water storage after 7 days (p < 0.05).
All the energy drinks significantly roughened the surfaces of EF (p < 0.05). Powerade and Monster increased the Ra of all the bioactive materials after 7 days (p < 0.05). All groups, except the AB group, were roughened after 7 days.
Table 3 shows the mean values of weight loss after immersion in different energy drinks. Significant weight loss was observed in the EF groups after immersion in all energy drinks (230.35 mg in distilled water; 194.00 mg in Powerade; 107.20 mg in Burn; 153.00 mg in Monster; p = 0.042), while no loss was observed in the other groups. Significant weight loss was observed in the AB immersed in Monster group (p = 0.046).
The ΔE00 values of the different restorative materials included in the study are reported in Fig. 2. The least affected bioactive material from the process was the AB. Burns and Monster, which are lower pH materials, led to greater discoloration of all the bioactive materials (p < 0.05). After 7 days of immersion in Burn, the EF group was more affected than those of the other groups. After the repolishing procedures, the EF immersed in Burn still had the highest ΔE00 values.
Differences in the ∆E00 values of the materials immersed in different solutions (initial, after immersion and after polishing)
*C. Charisma Diamond One; A: Activa Bioactive; P: Activa Presto; E: Equia Forte HT Fil**The same letter in the same-colored boxes means no statistically significant differences
Discussion
In dentistry, bioactive restorative materials are very recent materials that must be both mechanically resistant and esthetically acceptable. However, the clinical behavior of this new group of materials is not yet well known. For this reason, the aim of this study was to investigate the surface roughness, weight loss, and color change of some of these bioactive materials. The hypothesis evaluated was rejected because the restorative materials tested, which were immersed in energy drinks, were affected differently in terms of surface roughness, weight loss and color change.
In oral environment, the restorative materials can be affected by beverages [13]. According to a previous study, significant changes were observed in the hardness values of the materials after 7 days of immersion in different beverages [17]. Another study has reported that the sorption and solubility of the resin-based materials containing bioactive glass are affected by being kept in distilled water for 7 days [18]. In another study using restorative materials, significant color change was observed after 7 days [19]. In the light of these informations, the materials tested were kept in energy drinks for a peripd of 7 days.
Surface irregularities of dental restorative materials lead to plaque accumulation, increased discoloration and eventual restoration failure. This variable is by the skill of the operator and the material structure [11]. However, in this study, all the materials were polished by the same operator with the same polishing system for the same period of time to obtain uniform surfaces, and different initial surface roughness values were observed among the tested materials. This observation showed that the material structure plays a major role in the final surface smoothness of the restoration.
Of the restorative materials tested, EF exhibited the highest surface roughness of all the immersed energy drinks. This result could be related to the internal porous structure of glass ionomer-based materials or to their mixing and application procedures, which may contain some bubbles [20]. To make glass ionomer and glass hybrid materials more wear resistant and to protect them until they fully mature, it is recommended to apply a resin-based coating material to their surfaces. A previous study showed that this coating material does not protect against water dissolution but contributes to the abrasion resistance of glass ionomer materials [21]. In addition, glass ionomers or glass hybrid materials combined with this resin coating reduce the surface roughness [21]. Since clinically, this top resin coating layer dissolves rapidly after a few months in the mouth and the material remains in direct contact with the oral environment, we deliberately did not use this layer in our glass hybrid groups. The objective was to test the pure dissolution and discoloration of the glass hybrid material exposed to energy drinks and not its effects on the resin coating layer, which will continue to disappear under clinical conditions.
According to a previous study, the consumption of low-pH energy drinks increased the risk of porosity in dental restorations [22]. Furthermore, the citric acid contained in energy drinks has a high erosive potential. In our study, all the energy drinks we tested contained citric acid. The increased values of surface roughness obtained for the tested restorative materials after immersion could be explained by their citric acid content, except for AB. This situation is consistent with the results of a recent study investigating the effects of surface roughness of restorative materials in HCl media [23]. According to the results of this study, the AB was not affected, but the EF was affected by the erosive challenge. The AB is a type of resin-modified glass ionomer material that undergoes double-bond reactions. However, these two groups of materials differ in the dimethacrylate-phosphate monomers that cross-bond due to the presence of aluminum cations [24]. For this reason, the dimethacrylate-phosphate monomers could be the cause of the surface roughness values obtained.
For the results of weight loss, the greatest change was observed with the EF compared to the other materials. This difference was attributed to the fact that EF was used without its coating. In a previous study, EF with its resin coating was found to have a lower weight loss than EF without its coating [25]. In addition, the process of inserting EF into the molds may have led to the formation of voids in the material, increasing the solubility by allowing more erosive fluids to penetrate the material structure [26].
According to the surface roughness results, all the materials were affected by the acidic environment. This clearly shows that the surface layer of the light-cured resin-based materials was affected by the acidic environment without further weight loss, i.e., dissolution of the material [25]. The scaling method we used to evaluate the weight loss of the materials is very simple. However, this approach provides reliable results very quickly and may indicate the need for more sophisticated analyses. Because the results we obtained were obvious and unambiguous, we did not use another evaluation method for material substance loss.
The quality of a restoration in dentistry is also reinforced by its long-term lasting aesthetic properties [27]. There are two main methods for evaluating the color of a restoration. One is the visual method, which is traditional but subjective and therefore depends mainly on the operator. The other is an instrumental method using spectrophotometers, spectroradiometers or colorimeters, which is more objective [27]. The Commission Internationale de l’Eclairage (CIE, International Commission on Illumination) introduced the CIElab color difference formula, expressed as ∆E values, to evaluate the color changes of materials at two different times. Based on the calculated and perceived color differences in the CIElab color space, the CIEDE2000 formula was subsequently developed [28]. Previous studies have reported that the CIEDE2000 system is more consistent with the visual assessment of color changes in dentistry [29, 30]. Based on these results, the CIEDE2000 formula was used in the present study.
The color change of dental restorative materials can be influenced by the material structure, storage temperature, and pH of the discoloring liquid. In the present study, energy drinks were refreshed daily to maintain their acidity, which was measured at baseline using a digital pH meter. The surface layer of dental materials, such as glass ionomers, which are cured by an acid‒base reaction, has an incomplete radical polymerization structure. This reaction could be responsible for a stronger color change on the surface of this group of materials [31]. In this study, EF was used as a glass hybrid material for this acid‒base reaction. This phenomenon might have led to the stronger color change of this group compared to the other restorative materials tested.
The AB showed less color change than the other groups. In a study, the color stability of bioactive restorative materials was compared after immersion in different media [32]. The color stability of the AB was greater than that of the other materials tested. This phenomenon can be attributed to the fact that the AB contains urethane dimethacrylate instead of bisphenol A-glycidyl methacrylate [32]. Materials containing urethane dimethacrylate with limited hydrophilic aliphatic chains and carbamate bonds have lower water sorption than materials containing bisphenol A-glycidyl methacrylate and therefore cause less color change in the long term [33]. Although the Charisma Diamond One resin composite tested in the current study also contains urethane dimethacrylate, it exhibited more color changes than did the AB. This difference can be attributed to the electropositive barium fillers contained in the resin composite, which have a high affinity for water. The absorption of water into the resin composite structure during the discoloration process degrades the silica structure in the dental composite [34].
After immersion in different energy drinks, all materials were repolished with the same discs. This process may partially contribute to the removal of the superficial discoloration of the materials [7]. Therefore, all materials had lower color change values after repolishing than after the staining process. However, these values were all lower than the baseline values.
Our results showed that CD, AB and AP were more resistant to energy drinks than was EF in terms of wear and discoloration. Since this study involved new bioactive restorative materials tested under specific conditions, we believe that the results will contribute to the literature.
One of the limitations of this study is that it was designed in vitro. Therefore, we could not include, as observed in clinical situations, the buffering capacity of saliva against the acid attacks to which the materials were exposed when consuming energy drinks. Additionally, dietary habits, oral hygiene, and other patient-related factors could not be considered or evaluated. The second limitation is that the glass hybrid material was used without a resin coating. This coating could protect the glass hybrid material from erosion and color changes. Therefore, further studies need to be performed with groups that also use glass hybrid materials covered with a resin coating.
Conclusion
Within the limitations of this study, the following conclusions can be drawn:
-
1.
The bioactive glass hybrid material EF was affected by all energy drinks in terms of surface roughness, weight loss and color change and should not be used as a restorative material in heavy energy drink consumers.
-
2.
The bioactive restorative material AB was the least affected by all energy drinks in terms of surface roughness, weight loss and color change and would be a good restorative material alternative for heavy energy drink consumers.
-
3.
CD and AP restorative materials may be used as second options while restoring heavy energy drink consumer’s teeth.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AB:
-
Activa™ BioActive Restorative
- AP:
-
Activa™ Presto™
- CD:
-
Charisma Diamond One
- EF:
-
Equia Forte HT Fil
- GIC:
-
Glass Ionomer Cement
References
Chen J, Duangthip D, Gao SS, et al. Oral health policies to tackle the burden of early childhood caries: a review of 14 countries/regions. Front Oral Health. 2021;9(2):670154. https://doi.org/10.3389/froh.2021.670154. eCollection 2021.
Spaveras A, Antoniadou M. Awareness of students and dentists on sustainability ıssues, safety of use and disposal of dental amalgam. Dent J. 2023;11(1):21. https://doi.org/10.3390/dj11010021
Rasines Alcaraz MG, Veitz-Keenan A, Sahrmann P, et al. Direct composite resin fillings versus amalgam fillings for permanent posterior teeth. Cochrane Database Syst Rev. 2014;3. https://doi.org/10.1002/14651858.CD005620.pub3
Favetti M, Montagner AF, Fontes ST, et al. Effects of cervical restorations on the periodontal tissues: 5-year follow-up results of a randomized clinical trial. J Dent. 2021. https://doi.org/10.1016/j.jdent.2020.103571
Ferracane JL, Sidhu SK, Melo MAS, et al. Bioactive dental materials: developing, promising, confusing. JADA Foundational Sci. 2023. https://doi.org/10.1016/j.jfscie.2023.100022. 2.
Schmalz G, Hickel R, Price RB, Platt JA. Bioactivity of dental restorative materials: FDI Policy Statement. Int Dent J. 2022. https://doi.org/10.1016/j.identj.2022.11.012
Zhang OL, Niu JY, Yin IX, et al. Bioactive materials for caries management: a literature review. Dent J. 2023;11(3):59. https://doi.org/10.3390/dj11030059
Makanjuola J, Deb S. Chemically activated glass-ionomer cements as bioactive materials in dentistry: a review. Prosthesis. 2023;5(1):327–45. https://doi.org/10.3390/prosthesis5010024
Ladino LG, Bernal A, Calderón D, Cortés D. Bioactive materials in restorative dentistry: a literature review. SVOA Dent. 2021;2:74–81.
Khan AS, Syed MR. A review of bioceramics-based dental restorative materials. Dent Mater J. 2019;38(2):163–76. https://doi.org/10.4012/dmj.2018-039
Demarco FF, Cenci MS, Montagner AF, et al. Longevity of composite restorations is definitely not only about materials. Dent Mater. 2022;39:1–12. https://doi.org/10.1016/j.dental.2022.11.009
Marinoni M, Parpinel M, Gasparini A, et al. Psychological and socioeducational correlates of energy drink consumption in children and adolescents: a systematic review. Eur J Pediatr. 2022;181:889–901. https://doi.org/10.1007/s00431-021-04321-7
Tavangar M, Bagheri R, Kwon TY, et al. Influence of beverages and surface roughness on the color change of resin composites. J Investig Clin Dent. 2018;9(3):e12333. https://doi.org/10.1111/jicd.12333
Ozdas DO, Kazak M, Cilingir A, et al. Color stability of composites after short-term oral simulation: an in vitro study. Open Dent J. 2016;10:431–7. https://doi.org/10.2174/1874210601610010431
de Brito O, de Oliveira I, Monteiro G. Hydrolytic and biological degradation of bulk-fill and self-adhering resin composites. Oper Dent. 2019;44(5):e223–33. https://doi.org/10.2341/17-390-L
Mundim FM, Garcia Lda F, Pires-de-Souza Fde C. Effect of staining solutions and repolishing on color stability of direct composites. J Appl Oral Sci. 2010;18(3):249–54. https://doi.org/10.1590/S1678-77572010000300009
Chan KC, Fuller JL, Hormati AA. The ability of foods to stain two composite resins. J Prosthet Dent. 1980;43:542–5. https://doi.org/10.1016/0022-3913(80)90328-5
Raszewski Z, Chojnacka K, Mikulewicz M, Alhotan A. Bioactive glass-enhanced resins: a new denture base material. Mater (Basel). 2023;16(12):4363. https://doi.org/10.3390/ma16124363
Ozkanoglu S, Akin EGG. Evaluation of the effect of various beverages on the color stability and microhardness of restorative materials. Niger J Clin Pract. 2020;23(3):322–8. https://doi.org/10.4103/njcp.njcp_306_19
Da Mata M, Santos-Pinto L, Cilense Zuanon AC. Influences of the insertion method in glass ionomer cement porosity. Microsc Res Tech. 2012;75(5):667–70. https://doi.org/10.1002/jemt.21109
Kanik Ö, Turkun LS, Dasch W. In vitro abrasion of resin-coated highly viscous glass ionomer cements: a confocal laser scanning microscopy study. Clin Oral Investig. 2017;21(3):821–9. https://doi.org/10.1007/s00784-016-1820-5
Tanthanuch S, Kukiattrakoon B, Thongsroi T, et al. In vitro surface and color changes of tooth-colored restorative materials after sport and energy drink cyclic immersions. BMC Oral Health. 2022;22(1):578. https://doi.org/10.1186/s12903-022-02624-1
Willers AE, Branco TB, Sahadi BO, et al. Effect of erosive challenge with HCl on restorative materials. Clin Oral Investig. 2022;26(8):5189–203. https://doi.org/10.1007/s00784-022-04487-w
Łagocka R, Skoczyk-Jaworska M, Mazurek-Mochol M. Self-adhesive, bulk-fill bioactive materials as an alternative to silver amalgam in restorative dentistry. Pomeranian J Life Sci. 2022;68(2):40–8. https://doi.org/10.21164/pomjlifesci.840
Colombo M, Gallo S, Chiesa M, et al. In vitro weight loss of dental composite resins and glass-ionomer cements exposed to a challenge simulating the oral intake of acidic drinks and foods. J Compos Sci. 2021;5(11):298. https://doi.org/10.3390/jcs5110298
Gavranović-Glamoč A, Ajanović M, Kazazić L, Strujić-Porović S, et al. Evaluation of solubility of Luting cements in different solutions. Acta Med Acad. 2020;49(1):57–66. https://doi.org/10.5644/ama2006-124.284
Ghinea R, Pérez MM, Herrera LJ, et al. Color difference thresholds in dental ceramics. J Dent. 2010;38:57–64. https://doi.org/10.1016/j.jdent.2010.07.008
International Commission on Illumination (CIE). Colorimetry—technical report, 3rd ed. Commission Internationale de L’Eclairage, Bureau Central de la CIE, Vienna, CIE Pub. No. 15; 2004.
Paravina RD, Pérez MM, Ghinea R. Acceptability and perceptibility thresholds in dentistry: a comprehensive review of clinical and research applications. J Esthet Restor Dent. 2019;31:103–12. https://doi.org/10.1111/jerd.12465
Gómez-Polo C, Portillo Muñoz M, Lorenzo Luengo MC, et al. Comparison of the CIELab and CIEDE2000 color difference formulas. J Prosthet Dent. 2016;115(1):65–70. https://doi.org/10.1016/j.prosdent.2015.07.001
Tekçe N, Tuncer S, Demirci M, et al. The effect of different drinks on the color stability of different restorative materials after one month. Restor Dent Endod. 2015;40(4):255–61. https://doi.org/10.5395/rde.2015.40.4.255
Sajini SI, Mushayt AB, Almutairi TA, Abuljadayel R. Color stability of bioactive restorative materials after ımmersion in various media. J Int Soc Prev Community Dent. 2022;12(4):418–25. https://doi.org/10.4103/jispcd.JISPCD_40_22
Çarıkçıoğlu B. Influence of various mouthrinses on color stability and whiteness of resin-based restorative materials. Color Res Appl. 2021;46(5):1142–50. https://doi.org/10.1002/col.22642
Khan AA, Siddiqui AZ, Mohsin SF, Al-Kheraif AA. Influence of mouth rinses on the surface hardness of dental resin nanocomposite. Pak J Med Sci. 2015;31(6):1485–9. https://doi.org/10.12669/pjms.316.7611
Acknowledgements
Not applicable.
Funding
The research does not have financial support.
Author information
Authors and Affiliations
Contributions
HDK performed material preparation and data collection, wrote the first draft. IG performed material preparation and data collection, wrote the first draft. HB performed statistical analysis, wrote the first draft. LST performed material preparation and data collection. All authors contributed to the study’s conception and design. All authors commented on previous versions. All authors read and approved the final manuscript before submission and agreed to be accountable for all aspects of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kose, H.D., Giray, I., Boyacioglu, H. et al. Can energy drinks affect the surface quality of bioactive restorative materials?. BMC Oral Health 24, 1011 (2024). https://doi.org/10.1186/s12903-024-04781-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04781-x