Abstract
Objective
Clinical studies have demonstrated the effectiveness of arthrocentesis in managing temporomandibular joint disorders (TMDs). However, there is a lack of consensus among these studies regarding the selection of injectables. Furthermore, an increasing number of drugs have been tested for TMDs in recent years, complicating the decision-making process for clinicians. This study conducted a network meta-analysis of randomized controlled trials (RCTs) to compare the clinical efficacy of different arthrocentesis treatment regimens.
Methods
We conducted a comprehensive search of Embase, PubMed, Cochrane Library, and Web of Science to gather articles on RCTs pertaining to the management of TMDs using arthrocentesis. This search spanned from inception of these databases up to July 29, 2024. We then performed a network meta-analysis using Stata 17.0 software. The outcome indicators used were VAS scores and changes in unassisted maximum opening. To determine the efficacy of each regimen, we employed surface-under the cumulative ranking curve (SUCRA) ranking.
Result
Forty RCTs were included, encompassing 1904 temporomandibular joints (TMJs) cases. Treatment options encompass platelet-rich plasma (PRP), hyaluronic acid (HA), corticosteroids (CS), bone marrow concentrate (BMAC), injectable platelet-rich fibrin (i-PRF), concentrated growth factor (CGF), Tenoxicam (TX), microfragmented adipose tissue (FAT), and their combination regimens. The SUCRA ranking revealed that the most effective treatment options at 1-, 3-, and 6-months post-arthrocentesis were HA + PRP, i-PRF, and BMAC, respectively.
Conclusion
HA + PRP, i-PRF and BMAC may represent the optimal arthrocentesis agents for the management of TMDs symptoms and restoration of TMJ function in the short, medium, and long term, respectively. Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024563975.
Similar content being viewed by others
Introduction
The temporomandibular joint (TMJ) plays a crucial role in coordinating various daily functions such as chewing, articulation, and breathing. It is one of the most frequently used and complex joints in the human body. Temporomandibular joint disorders (TMDs) are musculoskeletal disorders, affect 5–12% of the global population, with an average incidence of 34%, 47% in South America, 33% in Asia, 29% in Europe, and 26% in North America [1]. TMDs rank as the second most prevalent chronic musculoskeletal condition, trailing only behind chronic low back pain [2].
TMDs are a group of painful disorders that affect the masticatory muscles, temporomandibular joint and related structures. The etiology of TMDs is multifaceted, encompassing factors such as trauma, chronic pain syndromes, autoimmune diseases, sleep apnea, and psychiatric disorders. Notably, the potential for certain dental interventions to precipitate TMDs is frequently underemphasized. Diagnosis is achieved through a variety of methods including patient history, screeners, physical examination, and imaging. When gathering patient history, it is important for physicians to be empathetic and provide a comfortable environment with ample time to ensure a comprehensive understanding of the disease is achieved [3]. The original Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) has demonstrated reliability in numerous studies. However, its screening sensitivity is suboptimal. Consequently, Schiffman et al. made revisions to enhance its applicability in clinical settings and universality, resulting in the newly recommended Diagnostic Criteria for TMD (DC/TMD) [4]. Furthermore, physical indicators that support the diagnosis of TMDs encompass, but are not confined to, irregular jaw movements, a diminished range of motion, tenderness during mastication, evidence of bruxism, and joint noise. In instances where occlusal misalignment or intra-articular anomalies are suspected, supplementary imaging techniques may aid in the diagnosis of TMDs. Modalities such as magnetic resonance imaging (MRI), computed tomography (CT), X-ray plain film, and MRI can serve as supplementary diagnostic tools. Notably, MRI is deemed the most effective method for a comprehensive evaluation of joint conditions, albeit it may yield false positive results [5].
Patients diagnosed with TMDs often encounter a range of symptoms including joint pain, muscle discomfort, disc displacement, and restricted mouth movement [6].According to statistical data, the prevalence of muscular disorders, disc displacement, and other joint conditions among TMDs patients in Poland was 56.9%, 48.9%, and 31% respectively [7]. Besides causing significant physical and psychological distress to patients, TMDs also negatively impact social and economic development. Research [7] indicates that the average cost of treating and rehabilitating TMDs patients is 7,890 USD, with an annual global expenditure of 4 billion USD on TMDs management [8].
The vast majority of TMDs can be successfully managed with reversible, safe, and effective treatment modalities. Treatment options for TMDs can be broadly classified into non-pharmacological therapy and pharmacological therapy. Non-pharmacological treatment options typically encompass functional exercises, occlusal splint therapy, massage, acupuncture, biofeedback, ultrasound, and Transcutaneous Electrical Nerve Stimulation (TENS). Among these, ultrasound therapy is regarded as one of the most effective methods to alleviate pain and enhance muscle function [9]. Drug treatment for chronic TMDs typically includes muscle relaxants, non-steroidal anti-inflammatory drugs, analgesics, tricyclic antidepressants, benzodiazepines, corticosteroids, and various biologics. However, there is limited evidence supporting the efficacy of oral and topical drug treatments for chronic TMDs. In contrast, research on drug therapy combined with joint puncture is relatively abundant and provides a reasonable level of evidence. Consequently, Tran proposed a treatment pathway that progresses from conservative to invasive approaches [10]. There is increasing evidence to suggest that the initial treatment of TMDs with minimally invasive therapy may be more effective in alleviating clinical symptoms in patients compared to conservative treatments [11]. The procedure involves flushing and expanding the upper joint space with either normal saline or Ringer’s solution under local anesthesia. This process aims to dissolve and clear the inflammatory fibrous tissue within the joint cavity through joint irrigation and injection, thereby addressing joint adhesions and significantly enhancing the mobility of the affected joint. Additionally, it alleviates muscle and bone pain, as well as abnormal joint closure and locking [12].
Clinicians have always been faced with a complex array of treatment options regarding the treatment of TMDs, and recent findings regarding TMJ puncture have not simplified this dilemma, but rather added to its complexity [13]. Currently, clinics widely utilize drugs such as platelet-rich plasma (PRP), hyaluronic acid (HA), corticosteroid (CS), among others. In previous retrospective studies, Turosz [14] suggested that injectable platelet-rich fibrin (i-PRF) was the preferred drug for arthrocentesis in TMDs. Conversely, Ulmner [15] concluded that PRP, used as an injection for arthrocentesis, was the most effective in managing TMDs patients. However, there were studies [8, 16] that concluded intra-articular injections of PRP, HA and CS had no effect on improving TMJ pain and functional outcomes compared to placebo. Given the existing retrospective studies, it’s challenging to determine the optimal drug for TMJ injection. Therefore, we undertook an innovative network meta-analysis to discern ambiguities in the management of TMDs.
Materials and methods
This systematic review and network meta-analysis were conducted in accordance with the A Measurement Tool to Assess Systematic Reviews and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. Go to the International Systematic Review Registration Platform website to enroll in the study and receive the registration number: CRD42024563975.
Database and search
RCTs investigating various arthrocentesis regimens for TMDs were identified through computer searches of PubMed, Embase, Cochrane Library, and Web of Science databases. The RCTs included in this study involved TMD patients who received intra-articular injections of PRP, HA, CS, bone marrow aspirate concentrate (BMAC), i-PRF, concentrated growth factor (CGF), tenoxicam (TX), microfragmented adipose tissue (FAT) or placebo. These interventions were compared independently. The primary outcome measure was pain management and TMJ function, assessed during follow-up. The main outcome was TMJ pain, while the secondary outcome was the patient’s unassisted maximum opening. The search period extended from the creation of the databases to July 29, 2024. The search strategy employed a combination of Medical Subject Headings (MeSH) and free terms tailored to the characteristics of each database to ensure comprehensive and accurate results. Additionally, literature cited in reviews or meta-analyses on relevant topics was searched to supplement the information gathered. The keywords searched included platelet-rich plasma, hyaluronic acid, steroids, tenoxicam, morphine, granisetron, temporomandibular joint, and injections, intra-articular. For specific search formulas, see Supplement 1.
Inclusion and exclusion criteria
Inclusion criteria were as follows: Participants were diagnosed with TMDs through the Research Diagnostic Criteria for TMDs (RDC/TMD) or Diagnostic Criteria for TMD (DC/TMD) combined with a physical examination. The age and gender of the patients were not differentiated in detail. Moreover, the patients did not show a clear curative effect on conservative treatment methods such as soft food, wet hot compresses, analgesics, anti-inflammatory drugs, and splint treatment for TMDs. Patients with a history of TMJ surgery, polyarthritis or other rheumatic diseases and neurological diseases were excluded. Interventions and controls: Included randomized controlled trials should involve intra-articular injection of PRP, HA, CS, BMAC, i-PRF, CGF, TX, FAT or placebo (normal saline or Ringer’s lactic acid solution), but the number of injections is not restricted during the experiment. Included studies did not necessarily need to include placebo injections or irrigation. Included RCTs must report pain improvement in patients with primary outcome (visual analogue scale, VAS), secondary outcome (unassisted maximum opening, UMO) and at least 1 month of follow-up.
Study selection
The EndNote 21 software facilitated the identification of duplicate title information among the documents considered for inclusion. Two independent researchers (JMZ and YZ) conducted a literature screening, extracted relevant data, and verified it through cross-checking. Discrepancies were resolved either through discussion or by seeking arbitration from a third researcher (JY). The initial screening involved reviewing article titles; irrelevant literature was excluded. Subsequently, abstracts and full texts were scrutinized to determine if an article met the inclusion criteria. In cases where data was incomplete, original study authors were contacted via email or phone to obtain the missing information.
Data extraction
Two researchers (JMZ and YZ) independently extracted and cross-verified the following information: (1) Basic study details: first author, publication date, country of study, and sample size. (2) Baseline characteristics of the study population: mean age, whether arthroarthrosis was performed, type of drugs (including drug name, dose, frequency, etc.) and follow-up duration. (3) Outcome measures (VAS, UMO). Any discrepancies in the data extraction process were resolved through discussion or mediation by a third researcher (JY). All data were sourced from the original studies included in the analysis.
Risk of bias assessment
The quality of RCTs were evaluated using the Cochrane Risk of Bias Tool 2.0 (ROB 2.0). Two authors (JMZ/ YZ) used the Cochrane collaborative “risk of bias” tool sequence to generate results from six aspects: allocation hiding, blinding (or masking), incomplete data evaluation, evaluation report, and other sources of biased selective results. The methods of the articles included in the trial were independently evaluated. Each study was classified as low risk, high risk, or undefined risk, and differences in the evaluation process were resolved through discussion or negotiation with a third researcher (JY).
Data analysis
The efficacy of treatments was gauged by improvements in patients’ pain status and UMO. These were assessed through changes in the VAS scores and precise tools before and after intervention. A significant score difference denotes a pronounced treatment effect. Since the outcome measure is continuous, we calculated the mean and its standardized mean difference (SMD) based on VAS scores and UMO. Consequently, SMD and its 95% confidence interval (95% CI) served as effect size indicators. Studies that used line charts for patient condition changes without providing exact data were excluded to ensure research accuracy.
In cases of missing data, authors were contacted via email to obtain complete datasets; otherwise, such articles were excluded. When raw data was sufficient, outcome variables were measured following original studies, weighted by sample size, adhering to the Cochrane Handbook [18]. Network Meta-Analysis (NMA) was conducted using the “network” package in Stata 17.0, resulting in a network diagram. Each node represented an intervention, with its size indicating the sample size involved. Lines between nodes signified direct comparisons, with line thickness denoting the number of these comparisons.
Transitivity and consistency are NMA prerequisites. Overall inconsistency was assessed using the inconsistency model; non-significant differences (p > 0.05) indicated consistency. Local inconsistencies were examined using the “node splitting method.” Non-significant differences (p > 0.05) between split points warranted the use of the consistency model. For graphs with closed loops, the cyclic loop inconsistency determined inconsistencies. An inconsistency factors (IF) value’s 95% CI lower bound close to or at 0 implied consistency between direct and indirect evidence. Intervention rankings were determined by comparing the surface under the cumulative ranking curve (SUCRA). Higher SUCRA values correlated with increased intervention effectiveness and higher rankings [19]. Top-ranked drugs at each stage were further analyzed to elucidate potential therapeutic mechanisms and impacts on treatment outcomes. Funnel plots, corrected for comparisons and created using Stata17, were used to detect inter-study biases. Symmetrical funnel plots suggested no significant publication bias; asymmetry indicated potential bias [20].
Results
Study selection
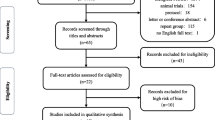
A total of 1664 articles were retrieved from the database. The EndNote 21 software was used to eliminate duplicates, resulting in 663 articles being removed. Of the remaining 1001 articles, 714 were excluded after reading the title and abstract as they did not meet the inclusion criteria. For the remaining 287 articles, a full text reading and screening process was conducted. This resulted in the exclusion of 111 articles for meeting and trial registration, 36 for systematic review or review, 41 for research methods that did not meet the requirements, 9 for data that could not be extracted, 40 for animal experiments, and 10 for unreasonable intervention measures. After this process, a final total of 40 articles [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] were included in the study. The study included a total of 1904 TMJs, with 839 in the control group and 1065 in the experimental group. The literature screening process is illustrated in Fig. 1. Among the 40 studies, 2 were four-arm trials, 6 were three-arm trials, and the remainder were two-arm trials. The basic information of the included literature is presented in Table 1.
Risk of bias assessment
A total of 24 studies [21, 23,24,25,26,27,28,29,30, 32, 35, 38,39,40,41,42,43,44, 47, 49, 52, 55, 57, 59] reported specific randomized methods, with 14 being double-blind [26, 27, 29, 30, 39,40,41,42, 44, 47, 49, 52, 57, 60], 10 single-blind [21,22,23, 25, 28, 32, 33, 37, 38, 59], and 1 not mentioning blindness [53]. No bias from established interventions or missing outcome data was found in all studies, and no significant bias in selective reporting of results was found in any study. Overall, the quality of the included literature was generally high, and the risk of bias was low. Bias risk results were plotted using ROB 2.0, as shown in Fig. 2.
NMA results
The network relationships between the various treatments are depicted in Fig. 3. The letters denote the respective interventions, the circle size indicates the number of individuals utilizing the intervention, and the thickness of the lines connecting the different letters represents the number of studies.
Network plot. Network plots at 1-month follow-up, (A) (VAS), (D) (UMO); At 3-month follow-up, (B) (VAS), (E) (UMO); At 6-month follow-up, (C) (VAS), (F) (UMO); PRP, platelet-rich plasma; HA, hyaluronic acid; CS, corticosteroid; BMAC, bone marrow aspirate concentrate; i-PRF, injectable platelet rich fibrin; CGF, concentrated growth factor; TX, tenoxicam; FAT, microfragmented adipose tissue; VAS, visual analog scale; UMO, unassisted maximum opening
Improvement of TMDs curative effect (1-month follow-up)
Studies utilizing VAS as an outcome measure encompassed 30 RCTs, incorporating 12 interventions and a cumulative total of 1436 TMJs. The evaluation of UMO as an outcome measure encompassed 24 RCTs, 11 interventions and a collective total of 1192 TMJs. Consistency testing and NMA were executed on the incorporated data. All inconsistent results yielded a p-value greater than 0.05, indicating an absence of overall inconsistency within the study. Node segmentation methodology revealed that the p-value was greater than 0.05 among each node, thereby suggesting generally favorable local consistency. The IF values derived from the cyclic inconsistency test results ranged from 0.02 to 2.24, with the lower limit of the 95% CI equal to or nearing zero, signifying an absence of substantial inconsistency between the closed loops (A and D in Fig. 4). The network relationships are centralized around PRP, HA, CS, and PLA, forming 8 and 9 trilateral closed loops, respectively (A and D in Fig. 3). NMA was conducted using the VAS as an outcome indicator. The results demonstrated that among the 66 two-on-two comparisons, only HA + PRP [MD = 2.19, 95% CI (3.50, 0.88)], HA [MD = 0.76, 95% CI (1.46, 0.06)] and PRP [MD = 0.72, 95% CI (-1.42, -0.02)] were statistically significant when compared with PLA. There was no significant difference found when other drugs were used. The efficacy of the SUCRA is in Fig. 5A. NMA results using UMO as an outcome indicator showed that among 55 2-on-2 comparisons, i-PRF [MD = 1.20, 95% CI (0.27, 2.12)], HA + PRP [MD = 1.05, 95% CI (0.22, 1.88)], PRP [MD = 0.52, 95% CI (0.07,0.98)] was statistically significant when compared with PLA, but no significant difference was found when using other drugs. The efficacy of SUCRA is in Fig. 6A.
Inconsistency factor values of the meta-analysis. A: A-L (BMAC, CS, FAT, HA, HA + CS, HA + PRP, MOR, PLA, PRP, TRAM, TX, i-PRF); B: A-L (BMAC CS HA HA + CGF HA + CS HA + PRP MOR PLA PRP TRAM TX i-PRF); C: A-M (BMAC CS FAT HA HA + CGF HA + CS HA + PRP MOR PLA PRP TRAM TX i-PRF); D: A-K (BMAC CS FAT HA HA + PRP MOR PLA PRP TRAM TX i-PRF); E: A-J (CS HA HA + CS HA + PRP MOR PLA PRP TRAM TX i-PRF); F A-L (BMAC CS FAT HA HA + CS HA + PRP MOR PLA PRP TRAM TX i-PRF)
Network meta-analysis of VAS. SUCRA of VAS at A, 1-month follow-up; B, 3-month follow-up; C, 3-month follow-up; PRP, platelet-rich plasma; HA, Hyaluronic acid; CS, corticosteroid; BMAC, bone marrow aspirate concentrate; i-PRF, injectable platelet rich fibrin; CGF, concentrated growth factor; TX, tenoxicam; FAT, microfragmented adipose tissue
Network meta-analysis of UMO. SUCRA of UMO at A, 1-month follow-up; B, 3-month follow-up; C, 3-month follow-up; PRP, platelet-rich plasma; HA, Hyaluronic acid; CS, corticosteroid; BMAC, bone marrow aspirate concentrate; i-PRF, injectable platelet rich fibrin; CGF, concentrated growth factor; TX, tenoxicam; FAT, microfragmented adipose tissue
Improvement of TMDs curative effect (3-month follow-up)
Studies utilizing VAS as an outcome measure encompassed 23 RCTs, 12 interventions, and a cumulative total of 1062 TMJs cases. Conversely, research focusing on UMO as an outcome measure comprised 20 RCTs, 10 interventions and a combined total of 898 TMJs instances. Consistency testing and NMA were executed on the incorporated data. All inconsistent results yielded p-values greater than 0.05, suggesting an absence of overarching inconsistency within the study. Node segmentation methodology revealed that only one node produced a p-value less than 0.05, indicating generally acceptable local overall consistency. The IFs values from the cyclic inconsistency test fluctuated between 0.22 and 4.46. Notably, the lower threshold of the 95% CI in most closed loops either equaled or approached zero, signifying no substantial inconsistency among these loops. Nonetheless, two closed loops indicated loop inconsistency. Further details will be elucidated in the discussion section (B and E in Fig. 4). The network associations primarily revolve around PRP, HA and PLA, forming 8 and 6 trilateral closed loops respectively. In this context, the VAS network also incorporates 2 quadrilateral closed loops (B and E in Fig. 3). NMA results, using VAS as the outcome index, revealed that among 66 2-on-2 comparisons, only the efficacy of i-PRF [MD = -1.10, 95% CI (-2.12, -0.08)] was significantly superior to that of PLA, with the difference being statistically significant at the time of comparison. The efficacy of SUCRA is in Fig. 5B. The NMA results with the UMO as the outcome indicator showed that in 45 2-on-2 comparisons, only the efficacy of i-PRF [MD = 1.30, 95% CI (0.69, 1.91)] was significantly superior to that of PLA, with the difference being statistically significant at the time of comparison. The difference was statistically significant. The efficacy of SUCRA is in Fig. 6B.
Improvement of TMDs curative effect (more than 6-month follow-up)
Studies using VAS as an outcome measure included 31 RCTs, 13 interventions, and a total of 1465 TMJs. The study using UMO as an outcome measure included 28 RCTs, 12 interventions, and a total of 1315 TMJs. Consistency testing and NMA were conducted on the included data. All inconsistent results had a p-value greater than 0.05, indicating no overall inconsistency in the study. Node segmentation analysis revealed that no inter-node p-values were less than 0.05, indicating generally good local consistency. IFs values from the cyclic inconsistency test ranged from 0.01 to 7.51. Most closed loops had a lower limit of the 95% CI equal to or nearing zero, suggesting no significant inconsistency. However, one loop exhibited inconsistency. Details will be discussed in the discussion section (C and F in Fig. 4). Network relationships primarily involve PRP, HA, and PLA, forming 9 and 8 trilateral closed loops, respectively (C and F in Fig. 3). The results of the NMA using VAS as the outcome index revealed that out of 91 2-on-2 comparisons, HA + CGF [MD = 9.53, 95% CI (14.10, 4.97)], HA + PRP [MD = 8.66, 95% CI (13.25, 4.06)], FAT [MD = 9.03, 95% CI (13.60, 4.47)], HA [MD = 7.91, 95% CI (11.85, 3.97)], PRP [MD = 8.26, 95% CI (12.83, 3.70)], HA + CS [MD = 7.32, 95% CI (12.46, 2.18)], TX [MD = 7.60, 95% CI (12.21, 2.18)], CS [MD = 4.73, 95% CI(-9.04,-0.41)] were superior to that of PLA, and the differences were statistically significant. The specific efficacy differences of other treatment schemes are illustrated in Fig. 5C. The results of the NMA using the UMO as the outcome indicator demonstrated that, among 66 2-on-2 comparisons, the effectiveness of i-PRF MD = 1.85, 95%CI (1.24,2.47)] and FAT [MD = 1.81, 95%CI (0.37,3.24)] were significantly different compared to that of PLA. The SUCRA ranking for efficacy in Fig. 6C.
SUCRA for all interventions are summarized in Table 2.
Publication bias
Funnel plots were constructed for the included literature based on outcome indicators. The majority of scatter points in these plots were symmetrically distributed. However, a few outcome indicators exhibited asymmetrical scatter points, indicating potential publication bias that could influence the results, as illustrated in Fig. 7.
Funnel plots. A: A-L (BMAC, CS, FAT, HA, HA + CS, HA + PRP, MOR, PLA, PRP, TRAM, TX, i-PRF); B: A-L (BMAC CS HA HA + CGF HA + CS HA + PRP MOR PLA PRP TRAM TX i-PRF); C: A-M (BMAC CS FAT HA HA + CGF HA + CS HA + PRP MOR PLA PRP TRAM TX i-PRF); D: A-K (BMAC CS FAT HA HA + PRP MOR PLA PRP TRAM TX i-PRF); E: A-J (CS HA HA + CS HA + PRP MOR PLA PRP TRAM TX i-PRF); F A-L (BMAC CS FAT HA HA + CS HA + PRP MOR PLA PRP TRAM TX i-PRF)
Discussion
TMJ is a unique synovial joint essential for jaw movement and function. Distinct from most synovial joints, the TMJ’s joint surface is enveloped by a dense layer of fibrocartilage, rather than the standard hyaline cartilage. Consequently, it is less vulnerable to age-related bone mineral density loss, which could diminish TMJ functionality. Moreover, the TMJ possesses an enhanced capacity for self-repair compared to other synovial joints [61]. When the mouth and face experience direct or indirect trauma, an occlusal relationship disorder or extracellular matrix degradation, the TMJ may exhibit dysfunction. The influence of TMDs on an individual’s overall quality of life is substantial, potentially resulting in functional impairments such as difficulties in eating, speaking, sleeping, and, for some patients, tinnitus [62]. Furthermore, the symptom complex associated with TMDs can induce psychological distress, encompassing depression, anxiety, and social isolation. Apart from the physical and mental impacts on individuals, TMDs may also impose a considerable financial burden due to medical expenses and absenteeism from work [63]. Existing research indicates that treatments for TMDs vary in effectiveness, from conservative approaches to surgical procedures. There are several treatment options for TMDs, most of which aim to alleviate the patient’s painful experience. However, their effectiveness has been a subject of debate. Physicians in the United States and Australia typically employ a comprehensive combination of treatments, including various conservative approaches, joint puncture, and open surgery [64]. A consensus of 24 experts suggests that conservative treatment should be the initial approach, even for advanced TMDs, although this proposal lacks substantial research support [65]. Besides medication for moderate to severe pain, multidisciplinary conservative treatments such as educational counseling, exercise therapy, manual reduction, non-invasive physical therapy, and occlusal splints are deemed effective for many mild cases [66]. Ultrasound therapy, a prime example of non-invasive physical therapy, can mitigate symptoms by enhancing blood circulation and modulating inflammatory responses [67]. Currently, the management of TMDs also encompasses the neurological domain, where neural regulation and biofeedback through appropriate electrical or magnetic stimulation can significantly alleviate the pain and tension in the masticatory muscle [68]. With the advent of precision medicine and minimally invasive techniques, joint injection strategies that promote regeneration have gained prominence over alternatives like PRP, HA and mesenchymal stem cells (MSCs). These methods have demonstrated notable advancements in managing TMDs [69]. This study analyzed follow-up data at 1-, 3- and 6-months post-arthrocentesis, identifying HA + PRP, i-PRF and BMAC as the most effective treatments for postoperative recovery. All three are classified as regenerative therapies.
HA is a crucial component of the extracellular matrix that contributes to the viscoelastic properties of joints. Moreover, HA has been demonstrated to support cell growth and chondrogenic differentiation of stem cells, as well as provide binding sites for growth factors [69], which are beneficial for tissue healing. Following the onset of TMDs, the friction coefficient of synovial fluid in the joint cavity increases by 3.5 times compared to normal conditions [70], and the volume of synovial fluid decreases, thereby affecting the nutritional support of TMJ cartilage. The combined effects of these changes accelerate TMJ wear, leading to tissue damage and functional decline. Consequently, the injection of HA into the TMJ lumen can not only lubricate and alleviate pain but also nourish articular cartilage to promote self-repair. PRP is a platelet concentrate derived from autologous whole blood following centrifugation. Its platelet concentration is at least twice the baseline concentration, rich in numerous growth factors and proteins, with its growth factor content reaching up to eight times the normal blood level. Numerous studies [71, 72] have shown that PRP may promote the proliferation of chondrocytes and MSCs, and it can serve as a biomolecular framework for tissue regeneration in cartilage defects to expedite damaged tissue repair. Therefore, intra-articular injection of PRP is widely employed in various diseases associated with joint tissue injury. Our study concluded that HA + PRP may be the most effective short-term management for TMDs. Among existing meta-analysis results, Al-Moraissi [73] and Li [74] concluded that HA and PRP had almost equivalent efficacy, but only in managing patients’ pain, with no significant improvement in patients’ temporomandibular activity and physiological function. However, neither of these two studies made a detailed distinction regarding follow-up time. Therefore, it remains unclear how well the drug is maintained. The combination of HA + PRP represents a highly innovative treatment program that maintains the normal physiological environment in the TMJ cavity through the physical and chemical properties of HA, reduces tissue friction loss, and relies on the regenerative capacity of PRP to repair damaged tissue structures, further alleviating TMDs symptoms and aiding patients in restoring normal TMJ physiological function. Unfortunately, no meta-analysis has been found to report on the combination of HA + PRP for TMDs.
Compared to PRP, the i-PRF offers several advantages including ease of processing, cost-effectiveness, and a higher concentration of regenerative cells, growth factors, and interleukin-10 at the same volume [75]. Furthermore, i-PRF can induce the proliferation and migration of MSCs. The use of i-PRF in regenerative dentistry has been extensively studied in both in vitro and in vivo studies. As a novel injectable biomaterial, i-PRF has been widely utilized in regenerative dentistry as a carrier for various biomolecules or in combination with other biomaterials. However, there are limited reports related to TMDs. Sielski’s review [76] obtained similar results as this paper, but their study did not conduct further subgroup analysis based on the follow-up period, leaving the phased efficacy of i-PRF uncertain. Additionally, due to the limitation of search terms, the drugs included were not comprehensive, which may have led to accidental results. Currently, no definitive meta-analysis of arthrocentesis combined with i-PRF in the treatment of TMDs has been published.
Bone marrow (BM) is located in the cancellous space of bone and the BM cavity of long bones, consisting of various cell types and reticular connective tissue. BM plays a crucial role in hematopoiesis, immunity, and defense mechanisms. BMAC is derived from the extraction of BM, which is then centrifuged and separated. Compared to BM, BMAC contains a higher concentration of MSCs, Hematopoietic Stem Cells and a variety of common blood cells at different stages of differentiation per unit volume. It is also rich in Transforming Growth Factor-β, Platelet-Derived Growth Factor and a range of bone morphogenetic proteins [77]. Among the many substances found in BMAC, the high concentration of MSCs and various growth factors significantly influence its regenerative capabilities. MSCs possess robust self-renewal and regeneration abilities, capable of differentiation into chondrocytes, bone cells, and adipocytes under specific conditions. Growth factors can protect cartilage tissue by reducing apoptosis, controlling inflammatory responses, and minimizing the degradation of cartilage matrix [78]. The combined effects of these components may positively impact injured TMJ. While there are limited reports on the application of BMAC in TMDs, Belk [79] has demonstrated that BMAC has at least similar effects to PRP in knee osteoarthritis studies. Notably, most current meta-analyses [79, 80] suggest PRP as the optimal long-term treatment for knee osteoarthritis, indicating significant potential for BMAC in TMD management.
In addition to prior studies, we examined various meta-analyses, including both paired and network meta-analyses. We identified that the research by Christidis [81] and Xu [82] exhibited certain similarities with our study. Christidis’s research employed a NMA to assess the efficacy and ranking of different medications for managing pain symptoms associated with TMDs. However, it did not address improvements in TMJ activity among TMDs patients, hence excluded medications such as HA, PRP or CS. In contrast, Xu’s study utilized NMA to evaluate the efficacy of HA, PRP, and i-PRF in managing TMDs pain and unassisted maximum opening (UMO). This approach closely resembled our methodology. Nonetheless, our study offers broader applicability by incorporating recently introduced medications for TMDs management and considering multi-drug combinations as separate interventions, which facilitates comparison between singular and combined drug efficacy. Notably, while Xu’s findings paralleled ours, our comprehensive analysis—encompassing a larger array of study protocols and demonstrating superior efficacy for certain treatments—led us to conclude that i-PRF was most effective three months post-arthrocentesis surgery. Conversely, Xu posited that i-PRF’s efficacy spanned the entire duration. Aggregating our results, we discerned no significant overall score differences among HA + PRP, i-PRF, and BMAC when evaluating intervention efficacy across all stages. However, the absence of UMO data at the three-month follow-up for BMAC precluded further comparative analysis of these interventions. Future research necessitates multi-center participation, extensive sampling and prolonged follow-ups to derive more precise conclusions.
In this study, three inconsistent results were observed during the ring inconsistency test, indicating discrepancies between direct and indirect intervention comparisons. This finding was initially disconcerting; however, upon re-analysis of the original data and a meticulous review of each article, potential explanations for these inconsistencies emerged. Notably, at the third and sixth month follow-ups using VAS as the outcome measure, three inconsistent closed loops were identified (B and C in Fig. 4): C - F - I (HA - HA + PRP - PRP), E - F - H - I (CS - HA + PRP - PLA - PRP) and A - D - I (BMAC - HA - PLA). A comprehensive review of the literature revealed that the inconsistencies might stem from our classification approach to interventions, where specific drugs and their injection frequencies were not distinctly specified, nor were the sources and preparation methods of certain drugs clearly defined. For instance, the molecular weight of HA was not differentiated in detail, leading to a uniform categorization of all HA types under a single umbrella. Research [83] suggests that HA with a molecular weight less than 5 kDa could possess pro-inflammatory effects, while HA exceeding 800 kDa demonstrates anti-inflammatory properties and the efficacy of moderate molecular weight HA remains ambiguous. Although Nardini and Ferreira’s study [84, 85] indicated no significant difference between medium and high molecular weight HA comparisons and varying injection frequencies of high molecular weight HA, the extensive sample size included might amplify minor differences, potentially skewing therapeutic outcomes. Similarly, CS such as methylprednisolone, dexamethasone and betametasone were grouped together without delineating specific types and injection frequencies. In the PRP studies, consistency in preparation processes was also not mandated. Given the variability and lack of unified nomenclature in PRP, along with poor standardization in evidence-based guidelines, differences in gravity and centrifugation times during preparation could result in varied PRP content concentrations across studies, indirectly impacting outcome measures [86]. Furthermore, the specific intervention content for PLA was not confined, encompassing possibilities such as isolated arthrocentesis, arthrocentesis combined with irrigation (normal saline or Ringer’s lactate solution), supplementation with placebo injections or even splint fixation assistance. Tsui and Moraissi’s meta-analysis [87, 88] confirmed that both arthrocentesis and splint-assisted approaches can somewhat improve TMDs. These factors contributing to inconsistencies also represent limitations of this study. Regarding BMAC within the closed loop, our assessment showed similarities in both preparation schemes and puncture operations among original studies, with variations solely in the volume of solution used for irrigation. Moreover, BMAC’s influence on VAS and unassisted maximum opening degree appeared relatively stable upon original data review. Hence, we provisionally conclude that BMAC is not a principal source of ring inconsistencies. Initially, the study aimed to distinguish various interventions minutely to pinpoint the most effective treatment regimen for TMDs via arthrocentesis, including dosage, injection frequency, and preparation methods. However, pre-grouping based on this premise revealed two main issues: an excess of indirect evidence, theoretically diminishing the level of evidence, and an inability to form complete network diagrams for some drugs, hindering comparisons. Consequently, we opted to aggregate drugs of similar categories.
Limitations
This study is not without its limitations. Initially, a preliminary investigation was undertaken to establish the definitive methodology. However, during the analysis of results, certain drugs were omitted due to discrepancies in study types or incomplete data, potentially leading to an inadequate representation of the drugs included in our study. Other issue may be the fact that some drugs are currently being tested or remain in clinical trials for TMDs, resulting in a paucity of research and insufficient evidence. Furthermore, we highlighted the limitations associated with the classification of interventions in our discussion as well.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Abbreviations
- TMJ:
-
Temporomandibular joint
- TMDs:
-
Temporomandibular joint disorders
- PRP:
-
Platelet-rich plasma
- HA:
-
Hyaluronic acid
- CS:
-
Corticosteroid
- BMAC:
-
Bone marrow aspirate concentrate
- i-PRF:
-
Injectable platelet rich fibrin
- CGF:
-
Concentrated growth factor
- TX:
-
Tenoxicam
- FAT:
-
Microfragmented adipose tissue
- VAS:
-
Visual analogue scale
- UMO:
-
Unassisted maximum opening
- SMD:
-
Standardized mean difference
- CI:
-
Confidence interval
- NMA:
-
Network Meta-Analysis
- IF:
-
Inconsistency factors
- SUCRA:
-
Surface under the cumulative ranking curve
- MSCs:
-
Mesenchymal stem cells
- BM:
-
Bone marrow
- RDC/TMD:
-
Research Diagnostic Criteria for Temporomandibular Disorders
- DC/TMD:
-
Diagnostic Criteria for TMD
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- TENS:
-
Transcutaneous Electrical Nerve Stimulation
References
Zieliński G, Pająk-Zielińska B, Ginszt M. A Meta-analysis of the global prevalence of Temporomandibular disorders. J Clin Med 2024, 13(5).
Ahmad M, Schiffman EL. Temporomandibular Joint disorders and Orofacial Pain. Dent Clin North Am. 2016;60(1):105–24.
Beaumont S, Garg K, Gokhale A, Heaphy N. Temporomandibular disorder: a practical guide for dental practitioners in diagnosis and management. Aust Dent J. 2020;65(3):172–80.
Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, et al. Diagnostic criteria for Temporomandibular disorders (DC/TMD) for clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache. 2014;28(1):6–27.
Lamot U, Strojan P, Šurlan Popovič K. Magnetic resonance imaging of temporomandibular joint dysfunction-correlation with clinical symptoms, age, and gender. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(2):258–63.
Alowaimer HA, Al Shutwi SS, Alsaegh MK, Alruwaili OM, Alrashed AR, AlQahtani SH, Batais MS. Comparative efficacy of non-invasive therapies in Temporomandibular Joint Dysfunction: a systematic review. Cureus. 2024;16(3):e56713.
Osiewicz MA, Lobbezoo F, Loster BW, Loster JE, Manfredini D. Frequency of temporomandibular disorders diagnoses based on RDC/TMD in a Polish patient population. Cranio. 2018;36(5):304–10.
Xie Y, Zhao K, Ye G, Yao X, Yu M, Ouyang H, PLATELET-RICH PLASMA ON TEMPOROMANDIBULAR JOINT OSTEOARTHRITIS. A SYSTEMATIC REVIEW AND NETWORK META-ANALYSIS OF RANDOMIZED CONTROLLED TRIALS. J Evid Based Dent Pract. 2022;22(3):101720.
Wieckiewicz M, Boening K, Wiland P, Shiau YY, Paradowska-Stolarz A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J Headache Pain. 2015;16:106.
Tran C, Ghahreman K, Huppa C, Gallagher JE. Management of temporomandibular disorders: a rapid review of systematic reviews and guidelines. Int J Oral Maxillofac Surg. 2022;51(9):1211–25.
Tang YH, van Bakelen NB, Gareb B, Spijkervet FKL. Arthroscopy versus arthrocentesis and versus conservative treatments for temporomandibular joint disorders: a systematic review with meta-analysis and trial sequential analysis. Int J Oral Maxillofac Surg. 2024;53(6):503–20.
Siewert-Gutowska M, Pokrowiecki R, Kamiński A, Zawadzki P, Stopa Z. State of the art in Temporomandibular Joint Arthrocentesis-A systematic review. J Clin Med 2023, 12(13).
Mauro G, Verdecchia A, Suárez-Fernández C, Nocini R, Mauro E, Zerman N. Temporomandibular Disorders Management-What’s New? A Scoping Review. Dent J (Basel) 2024, 12(6).
Turosz N, Chęcińska K, Chęciński M, Lubecka K, Bliźniak F, Chlubek D, Olszowski T, Sikora M. Temporomandibular Joint injections and Lavage: an overview of reviews. J Clin Med 2024, 13(10).
Ulmner M, Bjørnland T, Rosén A, Berge TI, Olsen-Bergem H, Lund B. Evidence for minimally invasive treatment-A systematic review on surgical management of disc displacement. J Oral Rehabil. 2024;51(6):1061–80.
Abt E, INTRA-ARTICULAR PHARMACOLOGICAL, INJECTIONS FOR TEMPOROMANDIBULAR JOINT OSTEOARTHRITIS. ARE COMPARABLE TO PLACEBO. J Evid Based Dent Pract. 2024;24(2):101985.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Cha Z. Dif erentiation and handling of homogeneity in Network Meta-analysis. Chin J Evidence-Based Med 2014.
Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20(4):641–54.
Abbadi W, Kara Beit Z, Al-Khanati NM. Arthrocentesis, Injectable Platelet-Rich Plasma and combination of both protocols of Temporomandibular Joint Disorders Management: a single-blinded Randomized Clinical Trial. Cureus. 2022;14(11):e31396.
Ansar AS, Munna K, Iqbal A, Mohammad F, Naved A, Shamimul H. Prognostic criteria for the management of temporomandibular disorders using arthrocentesis with normal saline and arthrocentesis with normal saline and platelet-rich plasma. J Med Life. 2022;15(5):698–704.
Asadpour N, Shooshtari Z, Kazemian M, Gholami M, Vatanparast N, Samieirad S. Combined platelet-rich plasma and Hyaluronic Acid can reduce Pain in patients undergoing arthrocentesis for Temporomandibular Joint Osteoarthritis. J Oral Maxillofac Surg. 2022;80(9):1474–85.
Attia A, Awad SS. Hyaluronic acid and platelet-rich plasma mixture Versus Hyaluronic Acid and Corticosteroid in the treatment of Temporomandibular Joint Internal Derangement: a comparative Randomized Study. J Maxillofacial Oral Surg. 2024;23(2):271–7.
Bayramoglu Z, Yavuz GY, Keskinruzgar A, Koparal M, Kaya GS. Does intra-articular injection of tenoxicam after arthrocentesis heal outcomes of temporomandibular joint osteoarthritis? A randomized clinical trial. BMC Oral Health. 2023;23(1):131.
Bergstrand S, Ingstado HK, Moystad A, Bjornland T. Long-term effectiveness of arthrocentesis with and without hyaluronic acid injection for treatment of temporomandibular joint osteoarthritis. J Oral Sci. 2019;61(1):82–8.
Bjørnland T, Gjaerum AA, Møystad A. Osteoarthritis of the temporomandibular joint: an evaluation of the effects and complications of corticosteroid injection compared with injection with sodium hyaluronate. J Oral Rehabil. 2007;34(8):583–9.
Chandra L, Goyal M, Srivastava D. Minimally invasive intraarticular platelet rich plasma injection for refractory temporomandibular joint dysfunction syndrome in comparison to arthrocentesis. J Family Med Prim Care. 2021;10(1):254–8.
Dasukil S, Arora G, Boyina KK, Jena AK, Jose A, Das S. Intra-articular injection of hyaluronic acid versus platelet-rich plasma following single puncture arthrocentesis for the management of internal derangement of TMJ: a double-blinded randomised controlled trial. J Cranio-Maxillofacial Surg. 2022;50(11):825–30.
De Riu G, Vaira LA, Carta E, Meloni SM, Sembronio S, Robiony M. Bone marrow nucleated cell concentrate autograft in temporomandibular joint degenerative disorders: 1-year results of a randomized clinical trial. J Cranio-Maxillofacial Surg. 2019;47(11):1728–38.
Dharamsi R, Nilesh K, Mouneshkumar CD, Patil P. Use of Sodium Hyaluronate and Triamcinolone Acetonide following arthrocentesis in treatment of Internal Derangement of Temporomandibular Joint: a prospective randomized comparative study. J Maxillofacial Oral Surg. 2024;23(1):204–9.
Fayed HM, Khairy MA, Eldahshan D, Sabry D, Ahmed WA. Bone marrow aspirate concentrate - A novel approach to alter the course of temporomandibular joint osteoarthritis (a clinical study). J Stomatology Oral Maxillofacial Surg 2024, 125(1).
Ghoneim NI, Mansour NA, Elmaghraby SA, Abdelsameaa SE. Treatment of temporomandibular joint disc displacement using arthrocentesis combined with injectable platelet rich fibrin versus arthrocentesis alone. J Dent Sci. 2022;17(1):468–75.
Gorrela H, Prameela J, Srinivas G, Reddy BVB, Sudhir M, Arakeri G. Efficacy of Temporomandibular Joint Arthrocentesis with Sodium Hyaluronate in the management of Temporomandibular Joint disorders: a prospective Randomized Control Trial. J Oral Maxillofac Surg. 2017;16(4):479–84.
Gupta A, Ali I, Zeeshan M, Singh S, Kumar A, Adil A. Role of intra-articular Piroxicam in the Temporomandibular Joint after Arthrocentesis for Anterior Disc Displacement without reduction. Cureus J Med Sci 2023, 15(2).
Hanci M, Karamese M, Tosun Z, Aktan TM, Duman S, Savaci N. Intra-articular platelet-rich plasma injection for the treatment of temporomandibular disorders and a comparison with arthrocentesis. J Cranio-Maxillofacial Surg. 2015;43(1):162–6.
Harba AN, Harfoush M. Evaluation of the participation of hyaluronic acid with platelet-rich plasma in the treatment of temporomandibular joint disorders. Dent Med Probl. 2021;58(1):81–8.
Hegab AF, Hameed H, Hassaneen AM, Hyder A. Synergistic effect of platelet rich plasma with hyaluronic acid injection following arthrocentesis to reduce pain and improve function in TMJ osteoarthritis. J Stomatol Oral Maxillofac Surg. 2023;124(1s):101340.
Huddleston Slater JJ, Vos LM, Stroy LP, Stegenga B. Randomized trial on the effectiveness of dexamethasone in TMJ arthrocentesis. J Dent Res. 2012;91(2):173–8.
Isacsson G, Schumann M, Nohlert E, Mejersjö C, Tegelberg Å. Pain relief following a single-dose intra-articular injection of methylprednisolone in the temporomandibular joint arthralgia-A multicentre randomised controlled trial. J Rehabil. 2019;46(1):5–13.
Isik G, Kenç S, Koyuncu B, Günbay S, Günbay T. Injectable platelet-rich fibrin as treatment for temporomandibular joint osteoarthritis: a randomized controlled clinical trial. J Cranio-Maxillofacial Surg. 2022;50(7):576–82.
Isik G, Kenç S, Özveri Koyuncu B, Günbay S, Günbay T. Does the Use of Injectable platelet-rich fibrin after arthrocentesis for disc displacement without reduction improve clinical outcomes? J Oral Maxillofac Surg. 2023;81(6):689–97.
Jacob SM, Bandyopadhyay TK, Chattopadhyay PK, Parihar VS. Efficacy of platelet-rich plasma Versus Hyaluronic Acid following arthrocentesis for Temporomandibular Joint Disc disorders: a Randomized Controlled Trial. J Maxillofacial Oral Surg. 2022;21(4):1199–204.
Jia XY, Jing SL, Sun Y, Gong ZC, Guo ZC. A randomized controlled clinical trial of concentrated growth factor combined with sodium hyaluronate in the treatment of temporomandibular joint osteoarthritis. BMC Oral Health. 2024;24(1):540.
Kiliç SC, Güngörmüs M. Is arthrocentesis plus platelet-rich plasma superior to arthrocentesis plus hyaluronic acid for the treatment of temporomandibular joint osteoarthritis: a randomized clinical trial. Int J Oral Maxillofac Surg. 2016;45(12):1538–44.
Kutuk SG, Gökçe G, Arslan M, Özkan Y, Kütük M, Arikan OK. Clinical and radiological comparison of effects of platelet-rich plasma, Hyaluronic Acid, and corticosteroid injections on Temporomandibular Joint Osteoarthritis. J Craniofac Surg. 2019;30(4):1144–8.
Liu SS, Xu LL, Liu LK, Lu SJ, Cai B. Platelet-rich plasma therapy for temporomandibular joint osteoarthritis: a randomized controlled trial. J Craniomaxillofac Surg. 2023;51(11):668–74.
Marzook HAM, Razek AAA, Yousef EA, Attia A. Intra-articular injection of a mixture of hyaluronic acid and corticosteroid versus arthrocentesis in TMJ internal derangement. J Stomatology Oral Maxillofacial Surg. 2020;121(1):30–4.
Mathpati SK, Jain G, Mishra V, Singh AK, Mishra R, Yadav BK. Platelet-Rich plasma in the management of Temporomandibular Joint Pain in Young adults with Temporomandibular Disorder. Cureus J Med Sci 2024, 16(3).
Mohammed SM, Abusanna MMH, Daily ZA. Assessment the efficacy of arthrocentesis with corticosteroid and arthrocentesis with sodium hyaluronate in treatment temporomandibular joint disorders: a comparative study. Indian J Forensic Med Toxicol. 2020;14(2):361–6.
Onder ME, Tüz HH, Koçyiğit D, Kişnişci RS. Long-term results of arthrocentesis in degenerative temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(1):e1–5.
Ozdamar SM, Alev B, Yarat A. The impact of arthrocentesis with and without hyaluronic acid injection in the prognosis and synovial fluid myeloperoxidase levels of patients with painful symptomatic internal derangement of temporomandibular joint: a randomised controlled clinical trial. J Rehabil. 2017;44(2):73–80.
Rajput A, Bansal V, Dubey P, Kapoor A. A comparative analysis of intra-articular injection of platelet-rich plasma and arthrocentesis in Temporomandibular Joint disorders. J Maxillofacial Oral Surg. 2022;21(1):168–75.
Sembronio S, Tel A, Tremolada C, Lazzarotto A, Isola M, Robiony M: Temporomandibular Joint Arthrocentesis and Microfragmented Adipose Tissue Injection for the Treatment of Internal Derangement and Osteoarthritis: A Randomized Clinical Trial. 2021, 79(7):1447–1456.
Shi ZD, Yang F, Zhang JY, Shi B: [Randomized controlled trial of sodium hyaluronate for degenerative disorders of the temporomandibular joint]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2002, 16(1):11–15.
Singh AK, Sharma NK, Kumar PGN, Singh S, Mishra N, Bera RN. Evaluation of arthrocentesis with and without platelet-rich plasma in the management of Internal Derangement of Temporomandibular Joint: a Randomized Controlled Trial. J Maxillofacial Oral Surg. 2021;20(2):252–7.
Sipahi A, Satilmis T, Basa S. Comparative study in patients with symptomatic internal derangements of the temporomandibular joint: analgesic outcomes of arthrocentesis with or without intra-articular morphine and tramadol. Br J Oral Maxillofacial Surg. 2015;53(4):316–20.
Sousa BM, López-Valverde N, López-Valverde A, Caramelo F, Fraile JF, Payo JH, Rodrigues MJ. Different treatments in patients with Temporomandibular Joint disorders: a comparative Randomized Study. Med (Kaunas Lithuania) 2020, 56(3).
Tabrizi R, Karagah T, Arabion H, Soleimanpour MR, Soleimanpour M. Outcomes of arthrocentesis for the treatment of Internal Derangement Pain: with or without corticosteroids? J Craniofac Surg. 2014;25(6):E571–5.
Yapici-Yavuz G, Simsek-Kaya G, Ogul H. A comparison of the effects of methylprednisolone acetate, Sodium Hyaluronate and Tenoxicam in the treatment of non-reducing disc displacement of the temporomandibular joint. Med Oral Patologia Oral Y Cir Bucal. 2018;23(3):E351–8.
Chang CL, Wang DH, Yang MC, Hsu WE, Hsu ML. Functional disorders of the temporomandibular joints: Internal derangement of the temporomandibular joint. Kaohsiung J Med Sci. 2018;34(4):223–30.
Fabrizia A, Giuseppe M, Martina C, Patricia RR, Vincenzo G, Ludovica N. Treatment approaches, outcomes and prognostic indicators in patients with tinnitus and temporomandibular disorders evaluated with DC/TMD: a systematic review and Meta-analysis. J Oral Rehabil 2024.
Abdul NS, Minervini G. Prevalence of Temporomandibular disorders in Orthognathic surgery patients: a systematic review conducted according to PRISMA guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. J Oral Rehabil. 2023;50(10):1093–100.
Haddad D, Millican E, Maxwell L, Wirianski A. Treatment options used in the management of people with temporomandibular disorders by Australian dentists and physiotherapists. J Oral Rehabil 2024.
Monje Gil F, Martínez Artal P, Cuevas Queipo de Llano A, Muñoz Guerra M, González Ballester D, López Arcas JM et al. López Cedrún JL, Gutiérrez Pérez JL, Martín-Granizo R, Del Castillo Pardo de Vera JL : Consensus Report and Recommendations on the Management of Late-stage Internal Derangement of the Temporomandibular Joint. J Clin Med 2024, 13(11).
Brighenti N, Battaglino A, Sinatti P, Abuín-Porras V, Sánchez Romero EA, Pedersini P, Villafañe JH. Effects of an Interdisciplinary Approach in the management of Temporomandibular disorders: a scoping review. Int J Environ Res Public Health 2023, 20(4).
Almeida LE. Temporomandibular disorders and Physiotherapy. J Contemp Dent Pract. 2023;24(10):723–4.
Lu G, Du R. Temporomandibular Joint Disorder: an integrated study of the pathophysiology, neural mechanisms, and therapeutic strategies. Arch Oral Biol. 2024;164:106001.
Cardoneanu A, Macovei LA, Burlui AM, Mihai IR, Bratoiu I, Rezus II, Richter P, Tamba BI, Rezus E. Temporomandibular Joint Osteoarthritis: pathogenic mechanisms involving the cartilage and subchondral bone, and potential therapeutic strategies for joint regeneration. Int J Mol Sci 2022, 24(1).
Tanaka E, Iwabe T, Dalla-Bona DA, Kawai N, van Eijden T, Tanaka M, Kitagawa S, Takata T, Tanne K. The effect of experimental cartilage damage and impairment and restoration of synovial lubrication on friction in the temporomandibular joint. J Orofac Pain. 2005;19(4):331–6.
Tatsis D, Vasalou V, Kotidis E, Anestiadou E, Grivas I, Cheva A, Koliakos G, Venetis G, Pramateftakis MG, Ouzounidis N et al. The combined use of platelet-rich plasma and adipose-derived mesenchymal stem cells promotes Healing. A review of experimental models and future perspectives. Biomolecules 2021, 11(10).
Cecerska-Heryć E, Goszka M, Serwin N, Roszak M, Grygorcewicz B, Heryć R, Dołęgowska B. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84–94.
Al-Moraissi EA, Almaweri AA, Al-Tairi NH, Alkhutari AS, Grillo R, Christidis N. Treatments for painful temporomandibular disc displacement with reduction: a network meta-analysis of randomized clinical trials. Int J Oral Maxillofac Surg. 2024;53(1):45–56.
Li J, Chen H. Intra-articular injection of platelet-rich plasma vs hyaluronic acid as an adjunct to TMJ arthrocentesis: a systematic review and meta-analysis. J Stomatol Oral Maxillofac Surg. 2024;125(2):101676.
Farshidfar N, Jafarpour D, Firoozi P, Sahmeddini S, Hamedani S, de Souza RF, Tayebi L. The application of injectable platelet-rich fibrin in regenerative dentistry: a systematic scoping review of in vitro and in vivo studies. Jpn Dent Sci Rev. 2022;58:89–123.
Sielski M, Chęcińska K, Chęciński M, Sikora M. Injectable platelet-rich fibrin (I-PRF) administered to Temporomandibular Joint Cavities: a scoping review. J Clin Med 2023, 12(9).
Yamaguchi FSM, Shams S, Silva EA, Stilhano RS. PRP and BMAC for Musculoskeletal conditions via Biomaterial Carriers. Int J Mol Sci 2019, 20(21).
Kim GB, Seo MS, Park WT, Lee GW. Bone Marrow Aspirate Concentrate: its uses in Osteoarthritis. Int J Mol Sci 2020, 21(9).
Belk JW, Lim JJ, Keeter C, McCulloch PC, Houck DA, McCarty EC, Frank RM, Kraeutler MJ. Patients with knee Osteoarthritis who receive platelet-rich plasma or bone marrow aspirate Concentrate injections have better outcomes Than patients who receive Hyaluronic Acid: systematic review and Meta-analysis. Arthroscopy. 2023;39(7):1714–34.
Singh H, Knapik DM, Polce EM, Eikani CK, Bjornstad AH, Gursoy S, Perry AK, Westrick JC, Yanke AB, Verma NN, et al. Relative efficacy of intra-articular injections in the treatment of knee osteoarthritis: a systematic review and network Meta-analysis. Am J Sports Med. 2022;50(11):3140–8.
Christidis N, Al-Moraissi EA, Barjandi G, Svedenlöf J, Jasim H, Christidis M, Collin M. Pharmacological treatments of Temporomandibular disorders: a systematic review including a Network Meta-Analysis. Drugs. 2024;84(1):59–81.
Xu J, Ren H, Zhao S, Li Q, Li C, Bao G, Kang H. Comparative effectiveness of hyaluronic acid, platelet-rich plasma, and platelet-rich fibrin in treating temporomandibular disorders: a systematic review and network meta-analysis. Head Face Med. 2023;19(1):39.
Lippi L, Ferrillo M, Turco A, Folli A, Moalli S, Refati F, Perrero L, Ammendolia A, de Sire A, Invernizzi M. Multidisciplinary Rehabilitation after Hyaluronic Acid injections for Elderly with knee, hip, shoulder, and Temporomandibular Joint Osteoarthritis. Med (Kaunas) 2023, 59(11).
Guarda-Nardini L, Rossi A, Arboretti R, Bonnini S, Stellini E, Manfredini D. Single- or multiple-session viscosupplementation protocols for temporomandibular joint degenerative disorders: a randomized clinical trial. J Oral Rehabil. 2015;42(7):521–8.
Ferreira NR, Oliveira AT, Sanz CK, Guedes FR, Rodrigues MJ, Grossmann E, DosSantos MF. Comparison between two viscosupplementation protocols for temporomandibular joint osteoarthritis. Cranio 2022:1–9.
Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich plasma: New Performance understandings and therapeutic considerations in 2020. Int J Mol Sci 2020, 21(20).
Tsui HC, Lam CM, Leung YY, Li KY, Wong NSM, Li DTS. Lavage volume of arthrocentesis in the management of Temporomandibular disorders: a systematic review and Meta-analysis. Diagnostics (Basel) 2022, 12(11).
Al-Moraissi EA, Farea R, Qasem KA, Al-Wadeai MS, Al-Sabahi ME, Al-Iryani GM. Effectiveness of occlusal splint therapy in the management of temporomandibular disorders: network meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. 2020;49(8):1042–56.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
JMZ was responsible for the design of the article concept and the writing of the manuscript; JMZ and YZ were responsible for the literature search and screening of literature that fulfilled the inclusion criteria; JMZ, YZ and JY were responsible for the extraction of data and quality evaluation of the included literature; TQZ and CG were responsible for interpreting the key results of the NMA. All authors contributed to the article and approved the submitted version. All authors gave their final approval and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical approval
This study is a secondary literature study and does not require ethical approval. Therefore, Clinical Trial Number is not applicable to this study.
Human ethics and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, JM., Yun, J., Zhou, TQ. et al. Arthrocentesis for temporomandibular joint disorders: a network meta-analysis of randomised controlled trials. BMC Oral Health 24, 1108 (2024). https://doi.org/10.1186/s12903-024-04858-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04858-7