Abstract
Introduction
Orthodontic clear aligners and retainers have numerous advantages that is making them ever increasingly popular. However, they might, similar to any other oral appliance, contribute to biofilm formation and finally dental caries or white spot lesions or gingival inflammations. The literature on biofilm formation on orthodontic clear appliances is very scarce and limited to a few microorganisms and materials. Therefore, this experimental study evaluated the biofilm formation on 5 thermoformed and 3D printed CAD/CAM orthodontic retainers in 3 intervals.
Methods
In this in vitro study, 345 specimens (270 test discs and 45 negative controls) were created from fabricated retainers. Retainers included a 3D printed CAD/CAM material (Detax) and four thermoformed retainers [Erkodent (polyethylene terephthalate glycol [PETG]); EasyVac (polyethylene); DB (polyester based on terephthalic acid); and Clear Tech]. They were all 1 mm thick, and all completely fabricated, i.e., heated or printed. The discs were placed in 96-well plates. Microorganisms were cultured on 270 discs for 24 h (90 discs), 72 h (90 other discs), and 5 days or 120 h (90 other discs). Biofilm formation of the strains and negative controls was measured using the microtiter plate assay by ELISA reading. The microbes’ ability to produce biofilm was categorized based on the comparison of average optical density (OD) of tests versus a cut-off point OD (ODc) calculated as the average of the OD of corresponding negative controls plus 3× its standard deviation: non-biofilm former [OD ≤ ODc], weak biofilm former [ODc < OD ≤ (2 × ODc)], moderate biofilm former [(2 × ODc) < OD ≤ (4 × ODc)], and strong biofilm former [(4 × ODc) < OD]. These were also converted to ranked scores between zero (no biofilm) and 3. The difference between ODs with control ODs were calculated. These were analyzed using 3-way ANOVA, 2-way ANOVA, and Tukey tests (α = 0.05, α = 0.008).
Results
The 3-way ANOVA showed that the overall difference among the ΔODs of 5 retainers (all microorganisms and all intervals combined, n = 270) was not significant (F = 1.860, P = 0.119). Nevertheless, the difference among 3 intervals (F = 31.607, P = 0.0000) and the difference among the 6 microorganisms (F = 24.044, P = 0.0000) were significant. According to the Tukey test, the differences between the 1st interval with either of the other two intervals was significant (both P values = 0.000). There were significant differences between Candida albicans with all other organisms (all 5 P values = 0.0000). All other pairwise comparisons were insignificant (all 10 P values ≥ 0.1). After taking the averages of the 3 intervals, the order of the biofilm generation for different materials were as follows: Detax (average score: 1.56), Easyvac (1.67), Erkodent (1.78), Clear Tech (1.83), BD (2.28).
Conclusions
As far as these 6 microorganisms are of concern, there might not be a significant overall difference among the clear retainer materials tested in this study. A significant overall increase was observed between the first and third days, which later did not significantly increase more until day 5. The Candida albicans biofilm was more intense than the tested 5 bacteria, which themselves showed rather similar growth patterns to each other.
Similar content being viewed by others
Introduction
Clear orthodontic appliances (aligners or retainers) can be more convenient and aesthetically pleasing than conventional appliances or retainers, and thus are becoming more and more popular and in demand by adults and even children [1,2,3,4,5,6,7,8]. Among the alluring benefits of using these appliances for treatment are maintaining dental hygiene, smaller rates of white spots compared to fixed retainers, reasonable price, improved patient aesthetics and comfort, as well as their removable nature, which allows for their removal in specific social situations and an improved hygiene control [1, 2, 4,5,6, 8,9,10,11].
However, they can also have disadvantages such as biofilm accumulation, release of possibly cytotoxic or estrogenic chemicals, needing patient cooperation, appliance loss, or discoloration [7, 10]. Numerous infections of the mouth may stick to retentive appliances and create biofilms, which are made of cells that are more resistant to antimicrobial drugs. Once the biofilms have developed on the retainer, they are hard to remove or clean [12, 13], leading to white spot lesions, tooth decay, and periodontal diseases [2, 7, 10, 11, 14].
One of their major disadvantages is their capacity to unbalance the oral microflora, potentially leading to dental caries, white spot lesions, or gingivitis: The mouth is a home to a broad range of microorganisms that coexist in an equilibrium state [2, 14]. Oral diseases including caries and periodontitis are linked to changes to this microflora and development of the biofilm [11, 15]. Oral biofilm, another name for dental plaque, is defined as an intricate network of microorganisms encased by an extracellular substrate secreted from microorganisms that mostly adhere to dental surfaces or gingival epithelial cells [11, 15], through adhering to the pellicle, an acellular protein-based coating that covers the enamel surface [10, 11, 15,16,17]. An orthodontic appliance alters and increases the microflora because it offers additional areas for bacteria to congregate and make comprehensive cleaning difficult [2, 10, 11, 14, 17].
The adhesion and generation of biofilms is a function of surface characteristics, surface area, and chemical composition of dental materials [10, 18]. Besides, the salivary wash and buffering capacity on dental and periodontal tissues is reduced by the clear orthodontic appliances themselves. Furthermore, such appliances serve as fresh habitats for bacteria to attach to and create the biofilm [10, 19]. Since clear appliances stay in contact with oral tissues and teeth for a long period, if organisms like S. mutans or C. albicans colonize them, there is a strong probability that the oral microflora will become disturbed, leading to caries formation, gingival inflammation, and even the development of candidiasis [2, 7, 10, 11, 14].
Because microbial biofilm can be an indicator for oral disease, it would be of major clinical interest to know the extent of biofilm formation on clear aligners or retainers [11]. Certain important features of clear appliances still need more research. This includes their effects on the microflora of the oral cavity as the available literature about biofilm formation on clear appliance materials is scarce [11]. To the best of our knowledge, only 3 studies exist on retainers [10, 11, 17] and one on aligners [8], of course noting that the materials used for aligners and retainers might be the same, only with different thicknesses.
Furthermore, the microbial cell types tested in these studies were limited or unknown. For example, one of the studies only assessed Streptococcus mutans [8], while two others assessed a mixture of unknown microbes from stimulated saliva [10, 11]. There is also a study that evaluated biofilm formation on 3 retainers clinically and reported their results only as numbers for biofilm coverage and thickness, again without identifying the microbes [17].
When it comes to 3D printed CAD/CAM clear aligners or retainers, the microbial adhesion and growth on them are not assessed before except in a late 2023 article [8]. 3D printed clear appliances might be the future of this field, as they have numerous advantages such as reductions in production errors, costs, production time, and plastic waste as well as more control over thickness and design [6, 20,21,22,23]. Therefore, they are worth investigation.
The growing need for the use of clear aligners/retainers warrants a thorough evaluation of this well-liked and novel therapeutic method [7]. Moreover, results pertaining to dental materials from a particular formula or even brand are not generalizable to others. This calls for studies on brands and materials not assessed before. Hence, and also in light of the above-mentioned importance of the increase of oral microflora by clear aligners/retainers as well as the scarce literature in this regard (especially about 3D printed CAD/CAM ones in particular), this study was conducted on retainers not assessed before.
As mentioned above, the novelty of this study is the use of 5 materials not tested before as well as the inclusion of 6 distinct microorganisms in a controlled fashion. The goal was to comparatively evaluate the biofilm formation by 6 important oral microorganisms on 5 clear retainers at 3 intervals. The null hypotheses were the lack of any difference among the retainers in terms of any of the 6 microorganisms and also over time.
Materials and methods
This multifactorial experimental study was performed on 345 specimens. The protocol and its ethics were approved by the Research Committee of the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (No: IR.AJUMS.REC.1400.496), in accordance with the Declaration of Helsinki. There was no human or animal subject involved, except the person who allowed us to use the digital file obtained from his intraoral scan for the research. This intraoral scan was taken as a routine therapeutic step and not for research. This patient was briefed about the study and signed an informed consent form. Apart from that, any consent to participate was not applicable. All methods were performed in accordance with the relevant guidelines and regulations (including the Declaration of Helsinki).
Sample size
The sample size of this study was 345 specimens: 270 specimens distributed across 3 sets of groups: 3 exposure durations × 5 retainer brands × 6 microorganisms × 3 specimens within each subgroup = 3 × 5 × 6 × 3 = 270 specimens. The number of 3 specimens per subgroup was selected as a common sample size in microbiological research and in all the previous 3 studies on biofilm formation on clear orthodontic appliances [8, 11]. Thus, there were 3 sets of 90 discs of clear retainers in each of the 3 intervals, each made of 5 different brands each under the influence of 6 microorganisms.
There were also 45 negative controls: three negative controls per brand per time interval, totaling to 9 negative controls per each of the 5 material types, and 15 negative controls per time interval.
Preparing the retainers and retainer discs
In this study, an intraoral scan of a patient with class 1 malocclusion was performed and the prepared STL (Stereo Lithography) file was used to make a hollow resin cast using a 3D printer. Five different commercial brands of clear retainers were used in this study: Erkodent, Germany (polyethylene terephthalate glycol [PETG]); Detax Freeprint 3D, Germany (undisclosed formulation [only stated as MMA-free polymer]); EasyVac, Korea (polyethylene); DB, England (polyester based on terephthalic acid); Clear Tech, America (undisclosed formulation [no response to authors’ email]). Of these, Detax was CAD/CAM, while the rest were thermoformed. The thickness of all of these materials was 1 mm.
Thermoformed retainers
The method of making thermoformed retainers: after cleaning the surface of the designed cast, the cast is placed in the Erkoform 3D (Germany) thermoform machine. Then, a retainer sheet was placed in the ERKOFORM 3D device. The device heats the retainer sheets to the temperature set by the retainer manufacturer. Once the retainer is heated, it is placed and fitted on the cast. Discs with a diameter of 3 mm and a thickness of 1 mm were taken out from the thermoformed retainers using a leather punch.
CAD/CAM 3D printed retainer
Detax Freeprint 3D was made using Asiga Max 3D printer (Australia). Due to the fragile structure of this retainer, it was not possible to use a leather punch, so discs with a diameter of 3 mm and a thickness of 1 mm were extracted from the retainer made with a 3D printer; this was done using a trephine bur attached to the handpiece of the implant motor.
The discs were placed in 96-well plates. A coding system was used for blind evaluation.
Microbiology methods
Studied strains
A total of 6 microorganisms (5 bacterial strains and 1 fungal strain) were prepared in accordance with American Type Culture Collection (ATCC) to assess the extent of biofilm formation subsequent to their interaction with orthodontic clear retainers in an in vitro setting. These strains consisted of Streptococcus mutans (ATCC35668), Streptococcus sanguinis (ATCC10556), Staphylococcus epidermidis (ATCC14990), Staphylococcus aureus (ATCC29213), and Lactobacillus casei (ATCC39392). Additionally, an opportunistic pathogenic yeast strain known as Candida albicans (ATCC10231) was also included. All the standard strains studied were already available in stock in the Department of Microbiology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Culture
All specimens were prepared in the form of lyophilized vials taken from the Scientific and Industrial Research Center of Iran. They needed to be revived first. For this purpose, the lyophilized materials were inoculated into the sterile liquid environment of TSB (Trypticase Soy Broth, Merck, Germany) and incubated at 37 °C for 48 h. To obtain isolated colonies, all suspensions were cultured on the Mueller Hinton Agar (Merck, Germany) for 48 h. For C. albicans and L. casei, Sabouraud Dextrose Agar (SDA) and Man–Rogosa–Sharpe (MRS) medium (Merck, Germany) were used, respectively. S. mutans, S. sanguinis, and L. casei were kept in microaerophilic conditions using a candle jar.
Assessment of biofilm formation by the microtiter plate assay
Biofilm formation of the strains was investigated using the microtiter plate assay. Accordingly, retainer’s discs were prepared from 5 different brands of orthodontic retainers. In microbiology, the McFarland standard is used as a reference to match the turbidity caused by the bacterial suspension, so that the number of bacteria will be within a certain limit. All retainers’ discs of each brand were sterilized with 70% ethanol and placed separately in sterile 96-well microplates (Greiner bio-one, Frickenhausen, Germany). Afterward, 100 µl of TSB medium and 100 µl of bacterial and yeast strains suspensions equivalent to 0.5 McFarland’s standard were added to all wells except for the negative control well. The control negative well contains 200 µl of TSB culture medium without bacteria. Subsequently, to assess and compare the biofilm formation, the 96-well microplates were incubated at 37 °C in the aerobic and CO2 incubator conditions to calculated different time points including 24, 72, and 120 h [24]. After the incubation time, the suspensions of microplates were gently removed with a sampler and all wells were washed thrice with 200 µl of phosphate-buffered saline (PBS) to remove the non-adherent microorganisms. The microtiter plates were dried at room temperature. The bacterial biofilms were fixed by adding 150 µl of absolute methanol to each well for 15 min and dried out at 37 °C for 20 min. To quantify the biofilm formation of studied strains, the crystal violet stain was used. The microbial biofilms were stained with 200 µl of 0.1% crystal violet (CV) that added to each well for 20 min at ambient temperature. The extra dye was removed by washing the microtiter plate’s wells twice. After air drying, CV was solubilized by adding 200 µL of 33% glacial acetic acid (v/v) (Merck, Germany) was added to each well. After the solution was homogenized by pipetting, the absorbance was read at 570 nm by the ELISA reader machine [25]. The staining stage for the CV assay was performed in a separate plate.
There were also 45 negative controls: three negative controls per brand per time interval.
The experiments were conducted three times to ensure accuracy and consistency. In this way, the optical density (OD) of each specimen was reported as the average OD of three experiments and compared with ODc (OD cut-off): The microbes’ ability to produce biofilm was categorized based on the comparison of average optical density (OD) of tests versus a cut-off point OD (ODc) as the average of the OD of corresponding negative controls plus 3× its standard deviation, as follows: non-biofilm former [OD ≤ ODc], weak biofilm former [ODc < OD ≤ (2 × ODc)], moderate biofilm former [(2 × ODc) < OD ≤ (4 × ODc)], and strong biofilm former [(4 × ODc) < OD].
Statistical analysis
Descriptive statistics and 95% CIs were computed for the groups and subgroups for OD minus ODc (delta OD, ΔOD). The differences between retainer brands, microorganisms, and intervals (and their interactions) in terms of their ‘OD – ODc’ or ΔOD values were assessed using a 3-way analysis of variance (ANOVA) and a Tukey post hoc test. As subgroup analyses, the difference between ΔOD values of various orthodontic retainers and also the difference between different time points were analyzed using a 2-way ANOVA and Tukey post hoc test. Profiles for capacity of retainer materials in accumulating microbial biofilm was calculated for each material culturing each of the 6 microorganisms, at each of the 3 intervals. The ranks 0, 1, 2, and 3 were respectively assigned to the extents of biofilm formation ‘no biofilm former, weak biofilm former, moderate biofilm former, and strong biofilm former’. These ranks were used to comparatively determine the overall advantages and disadvantages of each of the retainers being used to culture each of the 6 microorganisms at each of the intervals. The SPSS software version 26.0 (IBM Corp., Armonk, NY, USA) was used to perform all statistical tests. The level of significance was set at 0.05 for the 3-way ANOVA and its Tukey post hoc test. For the follow-up 2-way ANOVAs and their Tukey tests, the level of significance was adjusted to 0.008, using the Bonferroni method.
Results
Table 1 shows the descriptive statistics and 95% CIs for the OD values of all organisms (adjusted for ODs of negative controls) within each brand and at each interval.
Retainer brands, time, and microorganisms
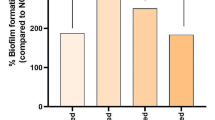
The 3-way ANOVA showed that the overall difference among the ΔODs of 5 retainers (all microorganisms and all intervals combined, n = 270) was not significant (F = 1.860, P = 0.119, Fig. 1). Nevertheless, the difference among 3 intervals (F = 31.607, P = 0.0000, Fig. 1) and the difference among the 6 microorganisms (F = 24.044, P = 0.0000, Fig. 1) were significant. All interactions were significant: retainer and time (F = 2.887, P = 0.004), microorganism and time (F = 8.968, P = 0.0000), retainer and microorganism (F = 2.384, P = 0.001). According to the Tukey test, the differences between the 1st interval with either of the other two intervals was significant (both P values = 0.000), but the difference between the 2nd and 3rd intervals was insignificant (P = 0.754, Fig. 1). There were significant differences between Candida albicans with all other organisms (all 5 P values = 0.0000). All other pairwise comparisons were insignificant (all 10 P values ≥ 0.1).
Candida albicans
The results of the 2-way ANOVA (α = 0.008) showed that both the variables ‘retainer brands and time intervals’ had a significant effect on the counts of Candida albicans adjusted for the negative control (Table 2, Fig. 2). The interaction of retainer types and time was significant as well (Table 2, Fig. 2). The Tukey post hoc comparisons conducted detected a significant pairwise comparison between the retainer brands Erkodent versus Detax (Table 3). According to the Tukey test, the changes over time points differed significantly or marginally significantly (Table 4).
Lactobacillus casei
There was no overall significant difference among the retainer types in terms of Lactobacillus casei counts adjusted for the negative control (Table 2; Fig. 3). The difference between time intervals was marginally significant (α = 0.008). The interaction between time and retainers was not significant (Table 2; Fig. 3). The Tukey test showed that the post hoc comparison between the 3rd and 5th days was marginally significant (Table 4).
Staphylococcus Aureus
The overall difference among the Staphylococcus aureus ΔOD of retainers was only borderline significant (Table 2; Fig. 4). The difference among time intervals was non-significant (Table 2; Fig. 4). The interaction between time and retainers was significant (Table 2; Fig. 4). The Tukey test was not performed.
Streptococcus sanguinis
In terms of Streptococcus sanguinis counts adjusted for the negative control, there was no significant difference among retainers, but the time intervals were significantly different from each other (Table 2; Fig. 5). The interaction of time and retainer was not significant (Table 2; Fig. 5). The Tukey test showed significant post hoc comparisons between the time interval 5th day versus time intervals 1st day and 3rd day; however, there was no significant difference between the 1st and 3rd days (Table 4).
Streptococcus mutans
Retainers marginally significantly and time significantly affected the ΔOD of Streptococcus mutans (Table 2; Fig. 6). Their interaction was not significant (Table 2; Fig. 6). The Tukey test showed that the post hoc difference between BD and Detax was marginally significant (Table 3). In terms of time points, the differences between the first day versus either the third day or the fifth day were significant; however, the levels of this microorganism were similar at the 3rd and 5th days (Table 4).
Staphylococcus epidermis
The difference among the retainers was not significant (Table 2; Fig. 7). However, the difference across the intervals was significant (Table 2; Fig. 7). The interaction of these variables was insignificant (Table 2; Fig. 7). According to the Tukey post hoc test, the difference between the 1st and 5th days was insignificant (Table 4). However, the difference between the 3rd day with either the 1st day or the 5th day was significant (Table 4).
Biofilm growth profiles
After converting the extents of biofilm formation (Table 5) into ranks and averaging the ranks for all evaluated microorganisms for each retainer, the following observations were made:
In the first day, Easyvac with an average score of 0.67 (as the average of the ranks for all 6 microbial types) was the least biofilm forming bran, followed by Detax (average score: 1.33), Clear Tech (average score: 1.5), BD (average score: 1.83), and Erkodent (average score: 2) as the one with the highest rank score.
In the third day, Erkodent (average score: 2.17) developed the least extent of biofilm. The brands BD (average score: 2.33) and Detax (average score: 2.33) were similar. They were followed by Clear Tech (average score: 2.5) and Easyvac (average score: 2.67).
In the fifth day, the CAD/CAM retainer Detax (average score: 1.0) showed the best and least extent of biofilm formation. It was followed by Erkodent (average score: 1.17), Clear Tech (average score: 1.5), Easyvac (average score: 1.67), and BD (average score: 2.67).
After taking the averages of the 3 intervals, the order of the biofilm generation for different materials were as follows: Detax (average score: 1.56), Easyvac (average score: 1.67), Erkodent (average score: 1.78), Clear Tech (average score: 1.83), BD (average score: 2.28).
Discussion
This study found no significant difference among the delta ODs of various retainer materials. A significant overall increase was observed between the first and third days, which later did not significantly increase more. The Candida albicans biofilm was more intense than the bacteria, which themselves showed rather similar growth patterns. Candida albicans is the most pathogenic member of the Candida spp genus; it is efficient at assimilating sugar and has a natural capacity to move upon healthcare plastics and acrylic resin areas, resulting in candidiasis [14, 26,27,28]. Candida species are thought to be a component of natural oral microflora that typically do not cause illnesses. Nevertheless, they are an opportunistic fungus that may begin a disease if the equilibrium of the environment is agitated [14, 26,27,28]. More important than C. albicans may be S. mutans; Streptococcus spp, particularly S. mutans, a gram-positive, carbohydrate-fermenting bacterium that has a system like that of Candida albicans, are the primary cause of cariogenic biofilm formation. This organism is acidogenic and generates polysaccharides outside the cell, which leads to the production of a lot of lactic acid and the eventual dissolution of dental surfaces. It may stick to tooth structures and survive in an acidic condition [14, 29,30,31,32]. Notably, Candida albicans and Streptococus mutans may coaggregate on tooth surfaces through a cooperative process, with Candida albicans acting as an adjunct in caries formation [14, 33, 34].
Chemical composition and free surface energy may matter [10, 11]. The materials used in clear orthodontic retainers include polymers like polypropylene, thermoplastic polyurethane, polycarbonate, polyethylene terephthalate, polyethylene terephthalate glycol, ethylene-vinyl acetate, or polyvinyl chloride [6, 7, 10, 11]. Besides, 3D printing in orthodontics is usually done using materials like epoxy resins (stereolithography materials), polylactic acid, glass-filled polyamide, nylon (polyamide), acrylonitrile-butadiene-styrene plastic, steel, silver, photopolymers, polycarbonate, wax, and titanium [6]. In the present study, no significant difference was observed among the retainers in terms of biofilm formation. This was in agreement with the results of Tektas et al. [11] who did not find a significant difference either. Chemical compositions along with surface roughness, surface area, surface wettability, and other surface properties are key to biofilm formation capacity [10, 18, 35, 36]. They attributed their results to the use of merely PETG-based samples in their investigation [11]. Nevertheless, in our study which tested different materials and even different methods of fabrication, the difference between retainers was insignificant. Investigations on aligners have shown that human saliva somewhat wetted every PETG aligner examined, with their CA-medium exhibiting the least amount of wetting [37]. The majority of producers use polyethylene terephthalate glycol (PETG), a transparent, amorphous copolymer that does not crystallize under tension, to make aligners [11]. Even while different PETF aligner material variants showed comparable surface morphology, surface roughness was observed to significantly impede wetting processes [11, 37]. The total work of adhesion, which measures the film generated on these materials’ potential for retention, varied between the investigated materials; a greater work value corresponds to a higher retention rate. Consequently, it is possible to hypothesize that variations in work of adhesion have a clinical bearing on the development of pellicle [11, 37]. Recent work from our lab has documented changes in mechanical characteristics and surface roughness associated with intraoral aligner services for a wide range of materials, including PETG and polyurethane-based polymers [11, 38]. The enforced modifications are restricted to the effects of water on the polymeric substance since aligners are only exposed to the intraoral environment for a brief period of time. The primary process of polymer breakdown in aqueous conditions has been characterized in the biomedical literature as hydrolytic breakdown and water attack on polyurethanes [11, 39].
The adhesion of microorganisms to abiotic surfaces is the initial stage of biofilm formation, especially following the use of orthodontic instruments or implants [10, 40]. Surface roughness, surface free energy, surface tension, and chemical composition of the surface can all have an impact on this stage of biofilm development, which in turn affects wettability and the adherence of saliva proteins [10, 41, 42]. Electrostatic and hydrophobic interactions can lead to early bacterial attachment to material surfaces [10, 41, 42]. The process of colonization by microbes in the mouth involves the transportation of microorganisms to the desired surface, the initial microbial adhesion, and subsequent microbial attachment that is dependent on certain interactions [11, 35, 43]. Microbial cells adhere to hard tissue, soft tissue, and material surfaces through numerous factors such as Van der Waals forces, electrostatic interactions [18] which may result in biofilm formation [11, 35, 36, 44]. Microorganisms can use several surfaces, each with its unique surface properties, for colonization in the oral cavity, including clear or fixed or removable orthodontic appliances, teeth, restorative materials, prosthodontic appliances, or dental implants [11, 35, 36, 43, 45].
Unlike some studies [10], in the present study, the flat plates were not assessed to better simulate the real conditions; instead, the tested samples were subjected to the usual process of aligner/retainer manufacturing. It was because the areas of plaque, such as cusps and embrasures, provide a better clinical simulation. In addition, it has been shown that the surface roughness of clear appliances after being placed in the thermoform machine is much higher than when they were initially delivered from the factory and no process is performed on them [37]. Although an in vitro test condition allows the researchers to control every other factor except the variable of interest, at the same time, they disallow them to simulate realistically the oral cavity environment with lots of changes in temperature or pH and with the existence of masticatory forces and wear as well as a long follow-up duration. A 72-hour experiment cannot reproduce clinical use of retainers that may last for weeks or months [11]. Although we extended this period to 120 h, this is still much shorted than clinical conditions. Therefore, clinical studies are needed to verify our results. The generalizability of this study is limited to the materials, brands, and microbes used.
Conclusions
As far as these 6 microorganisms are of concern, there might not be a significant overall difference among the clear retainer materials tested in this study. A significant overall increase was observed between the first and third days, which later did not significantly increase more until day 5. The Candida albicans biofilm was more intense than the tested 5 bacteria, which themselves showed rather similar growth patterns to each other. A few deviations were observed when examining each microorganism separately.
Data availability
The data are available from the corresponding author upon request.
References
Moradinejad M, Harrell RE, Mousavi SM, Alavi M, Basseri AD, Feiz A, et al. Effects of clear aligners on the vertical position of the molar teeth and the vertical and sagittal relationships of the face: a preliminary retrospective before-after clinical trial. BMC Oral Health. 2024;24(1):234. https://doi.org/10.1186/s12903-024-03972-w.
Rouzi M, Zhang X, Jiang Q, Long H, Lai W, Li X. Impact of clear aligners on oral health and oral microbiome during orthodontic treatment. Int Dent J. 2023;73(5):603–11.
Shahabuddin N, Kang J, Jeon HH. Predictability of the deep overbite correction using clear aligners. Am J Orthod Dentofac Orthop. 2023;163(6):793–801. https://doi.org/10.1016/j.ajodo.2022.07.019.
Castroflorio T, Sedran A, Parrini S, Garino F, Reverdito M, Capuozzo R, et al. Predictability of orthodontic tooth movement with aligners: effect of treatment design. Prog Orthod. 2023;24(1):2. https://doi.org/10.1186/s40510-022-00453-0.
Talens-Cogollos L, Vela-Hernández A, Peiró-Guijarro MA, García-Sanz V, Montiel-Company JM, Gandía-Franco JL, et al. Unplanned molar intrusion after invisalign treatment. Am J Orthod Dentofac Orthop. 2022;162(4):451–8.
Bichu YM, Alwafi A, Liu X, Andrews J, Ludwig B, Bichu AY, et al. Advances in orthodontic clear aligner materials. Bioactive Mater. 2023;22:384–403.
Yazdi M, Daryanavard H, Ashtiani AH, Moradinejad M, Rakhshan V. A systematic review of biocompatibility and safety of orthodontic clear aligners and transparent vacuum-formed thermoplastic retainers: Bisphenol-A release, adverse effects, cytotoxicity, and estrogenic effects. Dent Res J (Isfahan). 2023;20:41.
Taher BB, Rasheed TA. The impact of adding Chitosan nanoparticles on Biofilm formation, cytotoxicity, and certain physical and mechanical aspects of directly printed orthodontic clear aligners. Nanomaterials (Basel). 2023;13(19). https://doi.org/10.3390/nano13192649.
Phan X, Ling PH. Clinical limitations of invisalign. J Can Dent Assoc. 2007;73(3).
Hamdoon SM, AlSamak S, Ahmed MK, Gasgoos S. Evaluation of biofilm formation on different clear orthodontic retainer materials. J Orthod Sci. 2022;11:34. https://doi.org/10.4103/jos.jos_7_22.
Tektas S, Thurnheer T, Eliades T, Attin T, Karygianni L. Initial bacterial adhesion and biofilm formation on aligner materials. Antibiot (Basel). 2020;9(12):908–12. https://doi.org/10.3390/antibiotics9120908.
Chugh VK, Singh S, Chugh A, Tandon P. Survival rate of two orthodontic bonded retainer wires. Am J Orthod Dentofac Orthop. 2019;155(1):4–5. https://doi.org/10.1016/j.ajodo.2018.09.009.
Low B, Lee W, Seneviratne CJ, Samaranayake LP, Hägg U. Ultrastructure and morphology of biofilms on thermoplastic orthodontic appliances in ‘fast’ and ‘slow’ plaque formers. Eur J Orthod. 2011;33(5):577–83. https://doi.org/10.1093/ejo/cjq126.
Bonafé ACF, Oliveira D, Fernandes EE, Garcia MT, Dias I, Bressane A, et al. Microbiological evaluation in invisible aligner chemical cleaning methods against Candida albicans and Streptococcus mutans. Am J Orthod Dentofac Orthop. 2023;164(2):e43–50. https://doi.org/10.1016/j.ajodo.2023.05.014.
Xu Y, You Y, Yi L, Wu X, Zhao Y, Yu J, et al. Dental plaque-inspired versatile nanosystem for caries prevention and tooth restoration. Bioact Mater. 2023;20:418–33. https://doi.org/10.1016/j.bioactmat.2022.06.010.
Wong HM, Zhang YY, Li QL. An enamel-inspired bioactive material with multiscale structure and antibacterial adhesion property. Bioact Mater. 2022;7:491–503. https://doi.org/10.1016/j.bioactmat.2021.05.035.
Alla R, Telaprolu K, Surya BR, Kubavat AK, Ravuri P, Ali T. Evaluation of Biofilm formation on various clear orthodontic retainer materials: an original research. J Pharm Negat Results. 2022;13(10):6378–82. https://doi.org/10.47750/pnr.2022.13.S10.796
Türköz C, Canigür Bavbek N, Kale Varlik S, Akça G. Influence of thermoplastic retainers on Streptococcus mutans and Lactobacillus adhesion. Am J Orthod Dentofac Orthop. 2012;141(5):598–603. https://doi.org/10.1016/j.ajodo.2011.11.021.
Jongsma MA, Pelser FD, van der Mei HC, Atema-Smit J, van de Belt-Gritter B, Busscher HJ, et al. Biofilm formation on stainless steel and gold wires for bonded retainers in vitro and in vivo and their susceptibility to oral antimicrobials. Clin Oral Investig. 2013;17(4):1209–18. https://doi.org/10.1007/s00784-012-0807-0.
Edelmann A, English JD, Chen SJ, Kasper FK. Analysis of the thickness of 3-dimensional-printed orthodontic aligners. Am J Orthod Dentofac Orthop. 2020;158(5):e91–8.
Jindal P, Juneja M, Siena FL, Bajaj D, Breedon P. Mechanical and geometric properties of thermoformed and 3D printed clear dental aligners. Am J Orthod Dentofac Orthop. 2019;156(5):694–701.
Peeters B, Kiratli N, Semeijn J. A barrier analysis for distributed recycling of 3D printing waste: taking the maker movement perspective. J Clean Prod. 2019;241:118313.
Tartaglia GM, Mapelli A, Maspero C, Santaniello T, Serafin M, Farronato M, et al. Direct 3D printing of clear orthodontic aligners: current state and future possibilities. Mater (Basel). 2021;14(7). https://doi.org/10.3390/ma14071799.
Bozkurt AP, Ünlü Ö, Demirci M. Comparison of microbial adhesion and biofilm formation on orthodontic wax materials; an in vitro study. J Dent Sci. 2020;15(4):493–9. https://doi.org/10.1016/j.jds.2020.04.011.
Zhang D, Xia J, Xu Y, Gong M, Zhou Y, Xie L, et al. Biological features of biofilm-forming ability of acinetobacter baumannii strains derived from 121 elderly patients with hospital-acquired pneumonia. Clin Experimental Med. 2016;16:73–80.
da Silva PMB, Acosta EJTR, de Rezende Pinto L, Graeff M, Spolidorio DMP, Almeida RS, et al. Microscopical analysis of Candida albicans biofilms on heat-polymerised acrylic resin after chlorhexidine gluconate and sodium hypochlorite treatments. Mycoses. 2011;54(6):e712–7.
Ren Z, Cui T, Zeng J, Chen L, Zhang W, Xu X, et al. Molecule targeting glucosyltransferase inhibits Streptococcus mutans biofilm formation and virulence. Antimicrob Agents Chemother. 2016;60(1):126–35.
Jacobsen ID. The role of host and fungal factors in the commensal-to-pathogen transition of Candida albicans. Curr Clin Microbiol Rep. 2023;10(2):55–65.
Gao Z, Chen X, Wang C, Song J, Xu J, Liu X et al. New strategies and mechanisms for targeting Streptococcus mutans biofilm formation to prevent dental caries: a review. Microbiol Res. 2023:127526.
Iacopetta D, Ceramella J, Catalano A, D’Amato A, Lauria G, Saturnino C, et al. Diarylureas: new promising small molecules against Streptococcus mutans for the treatment of dental caries. Antibiotics. 2023;12(1):112.
Rezaei T, Mehramouz B, Gholizadeh P, Yousefi L, Ganbarov K, Ghotaslou R, et al. Factors associated with Streptococcus mutans pathogenicity in the oral cavity. Biointerface Res Appl Chem. 2023;13(4):368.
Ribeiro AA, Paster BJ. Dental caries and their microbiomes in children: what do we do now? J oral Microbiol. 2023;15(1):2198433.
Lu Y, Lin Y, Li M, He J. Roles of Streptococcus mutans-Candida albicans interaction in early childhood caries: a literature review. Front Cell Infect Microbiol. 2023;13:1151532.
Li Y, Huang S, Du J, Wu M, Huang X. Current and prospective therapeutic strategies: tackling Candida albicans and Streptococcus mutans cross-kingdom biofilm. Front Cell Infect Microbiol. 2023;13:1106231.
Kreve S, Dos Reis AC. Bacterial adhesion to biomaterials: what regulates this attachment? A review. Japanese Dent Sci Rev. 2021;57:85–96.
Kreve S, Dos Reis AC. Effect of surface properties of ceramic materials on bacterial adhesion: a systematic review. J Esthetic Restor Dentistry. 2022;34(3):461–72.
Suter F, Zinelis S, Patcas R, Schätzle M, Eliades G, Eliades T. Roughness and wettability of aligner materials. J Orthodont. 2020;47(3):223–31.
Papadopoulou AK, Cantele A, Polychronis G, Zinelis S, Eliades T. Changes in roughness and mechanical properties of invisalign® appliances after one-and two-weeks use. Materials. 2019;12(15):2406.
Mondal S, Martin D. Hydrolytic degradation of segmented polyurethane copolymers for biomedical applications. Polym Degrad Stab. 2012;97(8):1553–61.
Saldarriaga Fernández IC, Busscher HJ, Metzger SW, Grainger DW, van der Mei HC. Competitive time- and density-dependent adhesion of staphylococci and osteoblasts on crosslinked poly(ethylene glycol)-based polymer coatings in co-culture flow chambers. Biomaterials. 2011;32(4):979–84. https://doi.org/10.1016/j.biomaterials.2010.10.011.
Dittmer MP, Hellemann CF, Grade S, Heuer W, Stiesch M, Schwestka-Polly R, et al. Comparative three-dimensional analysis of initial biofilm formation on three orthodontic bracket materials. Head Face Med. 2015;11:10. https://doi.org/10.1186/s13005-015-0062-0.
Velliyagounder K, Ardeshna A, Koo J, Rhee M, Fine DH. The microflora diversity and profiles in dental plaque biofilms on brackets and tooth surfaces of orthodontic patients. J Indian Orthodontic Soc. 2019;53(3):183–8.
Bawazir M, Dhall A, Lee J, Kim B, Hwang G. Effect of surface stiffness in initial adhesion of oral microorganisms under various environmental conditions. Colloids Surf B. 2023;221:112952.
Tardelli JDC, Bagnato VS, Reis AC. Bacterial adhesion strength on titanium surfaces quantified by atomic force microscopy: a systematic review. Antibiotics. 2023;12(6):994.
Ozer NE, Sahin Z, Yikici C, Duyan S, Kilicarslan MA. Bacterial adhesion to composite resins produced by additive and subtractive manufacturing. Odontology. 2024;112(2):460–71.
Acknowledgements
NA (Not applicable).
Funding
The study was self-funded by the authors and their institution.
Author information
Authors and Affiliations
Contributions
Mehrnaz Moradinezhad searched the literature, conceived the study, designed the study, wrote the thesis, and critically reviewed the article. Effat Abbasi designed the study, performed the experiments, collected the data, and supervised the thesis. Alireza Hashemi Ashtiani searched the literature, designed the study, supervised the thesis, and critically reviewed the article. Reza Pourlotfi performed the experiments, collected the data, wrote the thesis, and contributed to the article. Vahid Rakhshan designed the study, performed the statistical analysis, prepared the figures and tables, and drafted the article. All authors viewed the final version of the article and agreed to submit it to this journal.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol and its ethics were approved by the Research Committee of the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (No: IR.AJUMS.REC.1400.496), in accordance with the Declaration of Helsinki. There was no human or animal subject involved, except the person who allowed us to use the digital file obtained from his intraoral scan for the research. This intraoral scan was taken as a routine therapeutic step and not for research. This patient was briefed about the study and signed an informed consent form. Apart from that, any consent to participate was not applicable. All methods were performed in accordance with the relevant guidelines and regulations (including the Declaration of Helsinki).
Consent for publication
NA (Not applicable).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Moradinezhad, M., Abbasi Montazeri, E., Hashemi Ashtiani, A. et al. Biofilm formation of Streptococcus mutans, Streptococcus sanguinis, Staphylococcus epidermidis, Staphylococcus aureus, Lactobacillus casei, and Candida Albicans on 5 thermoform and 3D printed orthodontic clear aligner and retainer materials at 3 time points: an in vitro study. BMC Oral Health 24, 1107 (2024). https://doi.org/10.1186/s12903-024-04893-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04893-4