Abstract
Background
Palliative radiotherapy for gastric cancer bleeding has been reported to be a safe and effective treatment, but predictive factors for achievement of hemostasis and overall survival have not been established.
Methods
In this retrospective study, 120 courses of palliative radiotherapy for gastric cancer bleeding in 117 patients in 4 institutes in Japan were reviewed with approval of the ethical committee in each institute. The rate of achieving hemostasis was evaluated by 50% or more reduction of red blood cell transfusion before and after the start of radiotherapy, elevation of blood hemoglobin concentration in a period of 4 weeks from the start of radiotherapy or improvement of subjective or objective clinical symptoms in a period of 4 weeks from the start of radiotherapy. Predictive factors for overall survival and achieving hemostasis were investigated with the Cox hazards model.
Results
The median overall survival period was 3.7 months. Multivariate analysis showed that absence of metastatic disease, higher biological effective dose, higher serum albumin level, lower blood urea nitrogen level and lower neutrophil-to-lymphocyte ratio (NLR) were associated with longer overall survival. Elevation of hemoglobin concentration in a period of 4 weeks from the start of radiotherapy (mean concentration: 8.2 g/dL vs. 8.9 g/dL, p = 0.006) and decrease in the amount of red cell transfusion from a 4-week period before to a 4-week period after the start of radiotherapy (mean amount: 716 mL vs. 230 mL, p < 0.0001) were observed. The overall rate of achievement of hemostasis was 59.6%. In multivariate analysis, higher biological effective dose was associated with achievement of hemostasis. Grade 2 or higher acute adverse effects related to radiotherapy were observed in 17.5% of cases in 120 treatment courses. Six cases (5.0%) had grade 3 or 4 adverse effects including gastric penetration in 1 patient and anorexia requiring total parental nutrition in 3 patients. No grade 5 adverse effects were observed.
Conclusions
Palliative radiotherapy for gastric cancer bleeding seems to be an effective and safe treatment strategy. Higher treatment dose was associated with longer overall survival and a hemostatic effect. Some hematological parameters may predict overall survival, and they would be helpful for deciding the treatment strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Gastric cancer is the fifth most common malignancy and the second-most common cause of cancer death worldwide. The incidences of gastric cancer are particularly high in East Asian and South American countries. In Japan, 126,009 people were diagnosed with gastric cancer and 44,192 people died from gastric cancer in 2018 [1].
Radiotherapy for gastric cancer is used in preoperative, postoperative, and palliative situations. Palliative radiotherapy for tumor bleeding has been reported to be a safe and effective treatment [2]. Some studies showed that a higher treatment dose is associated with a better clinical response [3], whereas another study showed that a short course and low dose of radiotherapy were adequate for palliative treatment of bleeding [4]. There has been no randomized controlled trial to determine the optimal treatment dose. In clinical practice, treatment dose is often decided based on the estimated patient’s prognosis with consideration of knowledge about pain control radiotherapy for bone metastases where a smaller dose is known to be related to a higher rate of symptom recurrence. However, there is no established method for predicting the prognosis of patients receiving palliative radiotherapy for gastric bleeding.
In this study, we collected data from multiple centers and investigated the effectiveness of radiotherapy in patients with locally advanced gastric cancer and the prognostic factors in such patients.
Methods
Study design
This study was a multi-institutional retrospective study.
Patients
In this study, the medical records of 117 patients who underwent palliative radiotherapy for bleeding from a gastric neoplasm from September 2014 to March 2021 in 4 institutes in Japan were reviewed. In principle, anticoagulants were stopped before start of radiation therapy.
Radiotherapy
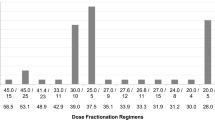
All patients underwent 3-dimensional conformal radiotherapy (3D-CRT) using computed tomography (CT)-based planning. Measures for respiratory motion using four-dimensional CT were used if possible with consideration of institutional equipment and status of the patient. The definitions of treatment volumes were as follows. Clinical target volume (CTV) was defined as gross tumor volume (GTV) plus a margin of 0 to 1 cm, but setting the whole stomach as CTV was also allowed. Internal target volume (ITV) was defined as CTV plus a margin of 0 to 2 cm with consideration of tumor respiratory motion and available countermeasures. Planning target volume (PTV) was defined as ITV plus a margin of 1 cm. Modification of target volumes with consideration of the status of the patient was allowed. Treatment was delivered with 2 to 4 beams of 6 to 10MV X-rays generated in a linear accelerator. Multiple-field irradiation was used if it was necessary to reduce the dose to the left kidney and bowel. Intensity-modulated radiotherapy (IMRT) was not used in any of the patients. Thirty Gy in 10 fractions delivered over a period of 2 weeks was considered as standard treatment, but physicians decided the treatment dose for each patient with consideration of the patients’ condition, tumor pathology, and treatment history.
Follow-up and evaluation
Hospital patient records were retrospectively reviewed. Data were collected from patient records up to September 30, 2021. To determine the effectiveness of treatment for tumor bleeding, the amount of red blood cell transfusion, blood hemoglobin concentration, and changes in symptoms were assessed. In Japan, 1 unit of red blood cell transfusion is 140 mL, which is prepared with 200 mL of donated blood. Hemostasis induced by radiotherapy was defined as follows: (1) 50% or greater reduction in the amount of red blood cell transfusion in a period of 4 weeks before and a period of 4 weeks after the start of radiotherapy if the patient received transfusion before the start of radiotherapy, (2) elevation in blood hemoglobin concentration of 1 g/dl or more at 4 weeks after the start of radiotherapy if the patient did not receive red blood cell transfusion in a period of 4 weeks before and after the start of radiotherapy, and (3) any improvement in objective clinical symptoms (e.g., disappearance of melena) and subjective clinical findings (e.g., confirmation of hemostasis by endoscopy). Acute adverse effects associated with radiotherapy in a period of 4 weeks after the end of radiotherapy were recorded according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
All statistical analyses were conducted using JMP Pro version 16.0 (SAS Institute Inc.). Predictive factors for hemostasis were investigated using logistic regression. Overall survival was estimated by the Kaplan-Mayer method, and the Cox hazards model was used for univariate and multivariate analyses. Student’s t-test or Dunnett’s test was used for evaluation of differences in mean values in two groups or multiple groups. A p value less than 0.05 was considered to be statistically significant for each analysis.
Results
A total of 117 patients were enrolled in this study. Three patients underwent two courses of radiotherapy, and 120 courses of treatment were therefore reviewed. Among the 3 patients who underwent a re-irradiation, median interval of two courses of radiotherapy was 141 days and median total BED10 was 67 Gy. Patients’ characteristics are shown in Table 1.
Thirty-one patients (26.5%) were alive at the time of data cut-off. The median survival period of the patients was 3.7 months. Survival rates at 6 months and 12 months were 37.3% and 18.6%, respectively (Fig. 1A). We conducted further analyses for prognostic factors for helping the decision of treatment intensity. Table 2 shows the results of univariate and multivariate analyses to determine predictive factors of overall survival. Parameters with p values < 0.05 were included into multivariate analysis, in which lymphocyte count and calcium-albumin ratio were excluded as possible confounding factors. In multivariate analysis, absence of metastatic disease, higher biological effective dose, higher serum albumin level, lower blood urea nitrogen level and lower neutrophil-to-lymphocyte ratio (NLR) were associated with longer overall survival. Figure 1B – E show Kaplan-Meyer survival curves according to median values of parameters that were significant in multivariate analysis. As an exploratory analysis, we constructed a formula to predict patients’ survival based on regression coefficients of the pre-treatment parameters.
Survival curves plotted by the Kaplan-Meyer method for (a) all patients and according to (b) existence of metastatic disease, c biological effective dose, d median value of serum albumin, e median value of blood urea nitrogen and f median value of neutrophil-to-lymphocyte ratio. Each p value was calculated by the log-rank test
When patients are divided into 2 groups by the median value of 1.56, median survival periods were 7.1 and1.8 months in higher and lower score group, respectively (p < 0.0001 by log-rank test).
In 120 treatment courses, 85 (70.8%) cases could be followed up for 4 weeks after the start of radiotherapy. In those cases, the mean amounts of red blood cell transfusion before and 4 weeks after the start of radiotherapy were 716 mL and 230 mL, respectively (p < 0.0001, Fig. 2A). A decrease of more than 50% in the amount of transfusion was achieved in 71% of the 85 cases. The mean hemoglobin concentration at the start of radiotherapy was 8.2 g/dL. Significant decline in the mean hemoglobin concentration was observed in 4 weeks before the start of radiotherapy and significant recovery was observed in 4 weeks after the start of radiotherapy (p = 0.002 and 0.006, respectively, Fig. 2B). Subjective and objective improvements of clinical symptoms were accessed in all of the 120 treatment courses, and improvement was achieved in 26 cases (21.7%). Hemostasis was achieved in 59.6% of the 120 cases. Table 3 shows the results of univariate and multivariate analyses to predict achievement of hemostasis. In multivariate analysis, higher biological effective dose was associated with achievement of hemostasis. The rates of hemostasis were 71.1% in patients who received 39 Gy in BED10 or a higher dose and 32.4% in patients who received less than 39 Gy (p < 0.0001, Fig. 3).
Grade 2 or higher acute adverse effects related to radiotherapy occurred in 17.5% of the cases. 6 cases (5.0%) had grade 3 or higher acute adverse effects, including grade 3 anorexia requiring total parenteral nutrition in 5 patients and grade 4 gastric perforation treated with emergency surgery in 1 patient. The patient with grade 4 gastric perforation had a T4 primary region invading the liver and treated with 30 Gy in 10 fractions of radiotherapy. The patient presented a gastric perforation on the next day of completion of the radiotherapy, which resulted in an emergent surgery. No grade 5 adverse effects were observed. Among 3 patients who underwent two courses of gastric irradiation, no grade 2 or higher acute adverse effects were observed.
Discussion
In this multicenter retrospective study, patients who underwent palliative radiotherapy for gastric neoplasm bleeding were reviewed. Hemostasis was achieved in 59.6% of treatment courses after palliative radiotherapy, and 5.0% of the cases had grade 3 or higher acute adverse effects. One of the largest retrospective studies on palliative radiotherapy for gastric cancer bleeding was carried out by Tey et al. [5]. They analyzed data for 115 advanced symptomatic gastric cancer patients and reported that the response rate to radiotherapy for bleeding was 80.6%, where treatment response was defined as no further blood transfusion or endoscopic treatment in 1 month after treatment. Viani et al. conducted a meta-analysis including 11 studies and reported that the overall response rate for bleeding was 77.7% [3], which is higher than that in the present study. In the present study, some of the patients were transferred to other hospitals immediately after radiotherapy and could not be followed up. This may be associated with the lower rate of hemostasis. In fact, the response rate was 77.8% when we included only 85 patients who could be followed up for more than 4 weeks, and that rate is consistent with the response rates in previous studies. Saito et al. recently published a prospective observational study and evaluated the hemostatic effect in multiple timepoints, where they defined bleeding response as hemoglobin level ≥ 8.0 g/dL, 7 consecutive days without blood transfusion and no salvage therapy for bleeding [6]. They reported that the overall response rate at 4 weeks being 53% and the per-protocol response rate at 4 weeks being 78%, showing the similar tendency to our data [6].
Multivariate analysis in our study showed that higher treatment dose was an independent predictor for achieving a hemostatic effect. For gastric cancer patients, a meta-analysis has shown that 30 Gy or a higher dose in BED10 was associated with bleeding response [3]. If the patient is tolerable, higher dose of radiotherapy seems to be beneficial in terms of hemostasis. On the other hand, the efficacy of a low dose and a short course of radiotherapy has been established by randomized control trials in patients with inoperable non-small cell lung cancer [7, 8] and patients with bladder carcinoma [9]. One study showed that a single course or multiple courses of radiotherapy at a dose of 6 Gy in 3 fractions was safe and effective for bleeding gastric carcinoma [4]. Considering these reports, it seems to be reasonable to consider a short course of radiotherapy for some patients with limited prognosis. In the present study, we provided a formula to predict patients’ prognosis. Although this formula needs to be evaluated in another cohort, it might help physicians choose treatment dose and fractionation.
In survival analysis, Treatment BED10 was a significant predictor for patients’ survival. However, treatment dose and fractionation are often decided based on physicians’ estimation (i.e. patients with better performance status is tended to receive higher dose of radiotherapy) and this selection bias should be noted. We also revealed some predictive factors besides lower BED10 for shorter overall survival including presence of metastatic disease, lower serum albumin concentration, higher blood urea nitrogen level and higher neutrophil-to-lymphocyte ratio. Recently, some hematological parameters have been shown to be related to prognosis in cancer patients treated with radiotherapy [10]. In the present study, among 4 patient parameters (metastatic disease, serum albumin, blood urea nitrogen and NLR), NLR showed the most significant difference in overall survival between two groups. High NLR is regarded to represent systemic inflammatory status and relatively weakened lymphocyte-mediated immune response to tumor. A relationship between high NLR and poor prognosis has recently been shown in various types of cancer [11]. NLR has been reported to be prognostic factor in gastric cancer patients treated with chemotherapy, chemoradiotherapy and surgery [12, 13], but there has been no report for patients treated with palliative radiotherapy. The results of the present study suggest that NLR predicts prognosis and might be helpful for choosing the treatment dose. Serum albumin level was another predictor for patients’ survival in our analysis. The relationship between lower serum albumin level and poor prognosis has been reported in various types of cancer including gastric cancer [14]. Lower serum albumin level is thought to reflect both malnutrition and systemic inflammatory status. This study also revealed that higher BUN level was related to shorter overall survival. BUN is not a common prognostic factor in cancer patients, but it is reported that increased BUN is related to high mortality rate in patient with gastrointestinal bleeding in emergency department [15]. High BUN level may indicate an advanced primary tumor through increased nitrogen intake caused by gastrointestinal bleeding.
In the present study, grade 3 or higher acute adverse effects occurred in 6 patients (5.0%). Five of those patients had grade 3 anorexia and 1 patient had grade 4 gastric perforation. Considering the site of disease, perforation seems to be an inevitable adverse effect to some extent. Overall, the rate of acute adverse effects seemed to be acceptable.
We recognize some limitations in our study. First, there were data defects because of the study being a retrospective multicenter study. We could not acquire follow-up data for some patients who were transferred to other hospitals soon after completion of radiotherapy. Secondly, the treatment dose depended on the physicians’ choice, and that bias makes it difficult to evaluate the relationship between treatment dose and hemostatic effect. Thirdly, most of the patients were treated with a dose of 30 Gy in 10 fractions (64.2%) or a dose of 20 Gy in 5 fractions (19.2%), and it was therefore difficult to examine the efficacy of single-dose radiotherapy or high-dose radiotherapy over 39 Gy in BED10.
Conclusions
In conclusion, palliative radiotherapy for gastric cancer bleeding seems to be an effective and relatively safe treatment strategy. A dose of 30 Gy in 10 fractions or a higher dose might be associated with a better hemostatic effect.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 3D-CRT:
-
3-Dimensional conformal radiotherapy
- CT:
-
Computed tomography
- CTV:
-
Clinical target volume
- GTV:
-
Gross tumor volume
- ITV:
-
Internal target volume
- PTV:
-
Planning target volume
- IMRT:
-
Intensity-modulated radiotherapy
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- NOS:
-
Not otherwise specified
- BED10 :
-
Biological effective dose calculated with α/β = 10 Gy
References
Cancer Statistics. Cancer Information Service, National Cancer Center, Japan. 2021. https://ganjoho.jp/reg_stat/statistics/data/dl/en.html. Accessed 1 Dec 2021.
J Tey YY Soon WY Koh CN Leong BA Choo F Ho 2015 Palliative radiotherapy for gastric cancer: a systematic review and meta-analysis Oncotarget 5 25797 25805
GA Viani CV Arruda AC Hamamura AC Faustino AFB Danelichen FK Matsuura 2020 Palliative radiotherapy for gastric cancer: Is there a dose relationship between bleeding response and radiotherapy? Clinics 75 e1644
H Kawabata K Uno K Yasuda M Yamashita 2017 Experience of Low-Dose, Short-Course Palliative Radiotherapy for Bleeding from Unresectable Gastric Cancer J Palliat Med 20 177 180
J Tey BA Choo CN Leong EY Loy LC Wong K Lim 2014 Clinical Outcome of Palliative Radiotherapy for Locally Advanced Symptomatic Gastric Cancer in the Modern Era Medicine 93 e118
Saito T, Kosugi T, Nakamura N, Wada H, Tonari A, Ogawa H, et al. Treatment response after palliative radiotherapy for bleeding gastric cancer: a multicenter prospective observational study (JROSG 17–3). Gastric Cancer. 2022;25:411–21.
N Bleehen D Girling D Machin R Stephens 1992 A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status Brit J Cancer 65 934 941
Bleehen NM, Girling DJ, Fayers PM, Aber VR and Stephens RJ. Inoperable non-small-cell lung cancer (NSCLC): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Brit J Cancer. 1991;63:265–70.
GM Duchesne JJ Bolger GO Griffiths JT Roberts JD Graham PJ Hoskin 2000 A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09 Int J Radiat Oncol Biology Phys 47 379 388
Takeda K, Umezawa R, Takahashi N, Matsushita H, Kozumi M, Ishikawa Y, et al. Impact of change in serum albumin level during and after chemoradiotherapy in patients with locally advanced esophageal cancer. Esophagus. 2018;15:190–7.
MA Cupp M Cariolou I Tzoulaki D Aune E Evangelou AJ Berlanga-Taylor 2020 Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies Bmc Med 18 360
T Arigami Y Uenosono S Ishigami K Okubo T Kijima S Yanagita 2016 A Novel Scoring System Based on Fibrinogen and the Neutrophil-Lymphocyte Ratio as a Predictor of Chemotherapy Response and Prognosis in Patients with Advanced Gastric Cancer Oncology 90 186 192
R Miyamoto S Inagawa N Sano S Tadano S Adachi M Yamamoto 2018 The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients Eur J Surg Oncol 44 607 612
A Brewczyński B Jabłońska K Pawlicki 2017 Associations Between Nutritional Parameters and Clinicopathologic Factors in Patients with Gastric Cancer: A Comprehensive Study Nutrition Cancer 69 1 10
E Kaya MA Karaca D Aldemir MM Ozmen 2016 Predictors of poor outcome in gastrointestinal bleeding in emergency department World J Gastroentero 22 4219 4225
Acknowledgements
Not applicable.
Funding
This study was not funded by any sources.
Author information
Authors and Affiliations
Contributions
KT proposed the study and was responsible for the overall study coordination including data collection, transcription of the data, statistical analysis and writing of the initial draft of the manuscript. TS, MK and YK reviewed the study concept and critically revised the manuscript. RU, TY, YI, NT, YS and KK revised the manuscript. KJ supervised the study and participated in the revision of the initial concept and the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Tohoku University Hospital Institutional Review Board (2021–1-452). Informed consent for each patient was waived because this is a retrospective observatory study. Information on the study was presented on the institutional web site, and the right to decline participating in this study was indicated for each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Takeda, K., Sakayauchi, T., Kubozono, M. et al. Palliative radiotherapy for gastric cancer bleeding: a multi-institutional retrospective study. BMC Palliat Care 21, 52 (2022). https://doi.org/10.1186/s12904-022-00943-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12904-022-00943-2